Abstract
Objective
Guillain-Barré Syndrome (GBS) is the leading cause of acute peripheral neuropathy worldwide, often associated with recent foodborne infection with Campylobacter jejuni. In this cross-sectional analysis of data from the Agricultural Health Study, we tested whether swine and poultry exposure were associated with increased prevalence of GBS-like neurologic symptoms.
Methods
Using multivariate analysis, we tested the symptoms such as numbness and weakness, relevant to inflammatory peripheral neuropathies, among farmers with self-reported occupational poultry or swine exposure compared with farmers who reported no occupational animal exposure.
Results
Among swine farmers/workers, prevalence of weakness and numbness were increased (P< 0.05). Among poultry farmers/workers, prevalence of weakness and numbness were increased, but increased prevalence of weakness was not statistically significant.
Conclusions
Occupational contact with live poultry or swine, potentially related to C. jejuni exposure, was associated with increased reporting of GBS-like symptoms.
Keywords: Guillain-Barré Syndrome, Campylobacter jejuni, peripheral neuropathy, farmer, Agricultural Health Study, swine, poultry
Workers in agriculture face a variety of occupational risks, including exposures to pathogens. These exposures can be associated not only with acute infection but also with long-term sequelae, such as neurologic disease. Guillain-Barré Syndrome (GBS), an autoimmune disorder, is the major cause of acute peripheral neuropathy in the world.1 Clinically, patients affected by GBS or GBS-like illness present with symptoms that range from weakness and tingling in affected limbs to a life-threatening, ascending paralysis.1,2 Worldwide, incidence of GBS ranges from 0.6 to 4 cases per 100,000 population.3 Men are 1.5 times more likely to develop the autoimmune neuropathy than women.3 GBS in the United States may carry an economic burden of up to $420 million.4
The major identified risk factor for GBS is infection by the bacterial pathogen Campylobacter jejuni.1,5,6 In a literature review, C. jejuni infection preceded 30% to 40% of cases of GBS.1 Other studies have demonstrated increased risk of GBS in the two months after an episode of C. jejuni infection.6,7 Clusters of GBS cases may occur after outbreaks of bacterial enteritis.3 Other risk factors for GBS include other prior infections, for example, with cytomegalovirus, Epstein-Barr virus, influenza, or Mycoplasma pneumoniae.3,5
The putative mechanism underlying this association is autoimmunity induced by a cross-reacting mimicry between surface antigens of C. jejuni and corresponding epitopes of the host peripheral nervous system.8,9 Antibodies to variant C. jejuni lipooligosaccharides cross-react with human neuronal gangliosides to cause the pathophysiological events that induce autoimmune peripheral neuropathy.10 Particular subtypes of C. jejuni, such as HS:19 and HS:041, are more closely linked to the development of GBS, which is associated with polymorphisms in specific genes that encode variant surface proteins.11–13 Variation in host immunity also plays a role in determining individual susceptibility for developing GBS after infection.8,9
Studies describing associations between GBS and C. jejuni infection primarily have examined infections associated with poultry consumption.14,15 The occupational risks for C. jejuni exposure among workers in food animal production have been well-described16,17 Because Campylobacter is carried commensally by poultry and often is isolated from swine, occupational contact with these animals is associated with increased risks of C. jejuni carriage, particularly under conditions of concentrated animal-feeding operations.18 In 2007, Price et al.19 reported on increased risks of neurologic symptoms indicative of Guillain-Barré--like peripheral neuropathy in a relatively small cross-sectional study of poultry workers. The present study builds on these results by examining prevalence of symptoms consistent with peripheral neuropathy in a large cohort of farmers: the Agricultural Health Study (AHS). Our motivation for using available data from the AHS came, in large part, from our interest in identifying a viable population for a future cohort study of inflammatory peripheral neuropathy.
MATERIALS AND METHODS
The AHS is the largest longitudinal cohort study of the agricultural workforce; it was designed to study exposures and health outcomes in licensed pesticide applicators and their spouses.20 Cohort members were enrolled between 1993 and 1997 in Iowa and North Carolina.20 The present analysis is restricted to the subset of private pesticide applicators, primarily farmers, with information on neurological symptoms and animal exposures. Neurologic symptoms were not collected from spouses. Commercial applicators, who as a group have little animal exposure, were not included.
A questionnaire completed at enrollment-collected information on demographics, baseline health, pesticide use, and animal exposure. The latter included data on major income-producing animals raised on the farm and participation in work activity in swine or poultry confinement areas. A second questionnaire completed at home by approximately 44% of the enrolled private applicators collected information on self-assessed cognitive and motor neurologic function.21 Questionnaires are available at www.aghealth.org/questionnaires.html.
The study design was a nested prevalence study within the AHS. The present analysis was restricted to 22,916 private applicators (44% of the cohort), who completed both enrollment and take-home questionnaires (Fig. 1). There were only minor differences, most notably for age, between those participants who did and those who did not complete the take-home questionnaire.22
FIGURE 1.
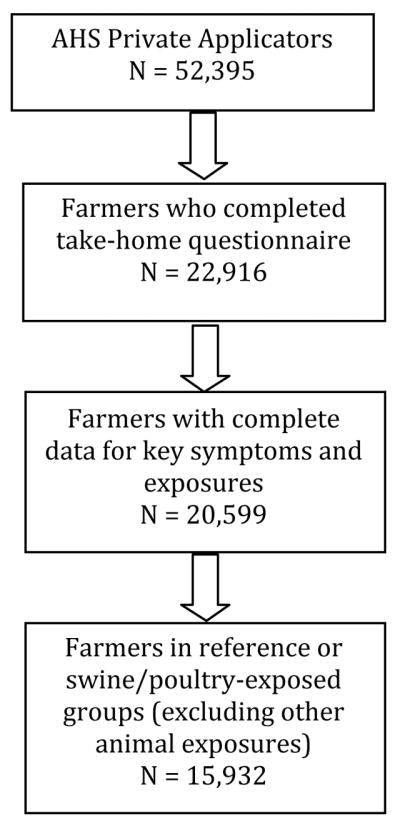
Study design for analysis of animal exposure and neurologic symptoms nested within the agricultural health study cohort.
Subjects with missing data for any of the key neurologic symptoms or exposures (see later) were excluded (approximately 11%). Of the remaining 20,599 subjects, those who reported either swine or poultry exposure were compared with those who reported no occupational animal exposure in the past year. This excluded farmers with nonswine, nonpoultry occupational animal exposure, because animals other than poultry and swine also may carry C. jejuni.23 This reduced the final study population to 15,932.
The study population was divided into four nonexclusive categories of occupational animal exposure and one reference category of 7610 farmers with no self-reported occupational animal exposure in the past year (Table 1). The exposure categories included 7079 farmers who reported hog/swine production in the past year (swine farmers); 5930 individuals who reported working in swine confinement areas (swine workers); 784 farmers who reported raising poultry on their farm in the past year (poultry farmers); and 713 individuals who reported working in poultry confinement areas (poultry workers). Although 306 farmers responded that eggs were a major income-producing crop or animal on their farms, we excluded these individuals if they did not also report that poultry were a major income-producing crop or animal due to a lack of information on live animal exposure. Individuals who reported working in swine or poultry confinement areas by definition worked in an environment with large numbers of animals and were likely to experience higher or more frequent exposure to Campylobacter.24,25 For this reason, swine or poultry farmers were analyzed separately from swine or poultry confinement workers. These four categories of animal exposure---swine farmers, swine confinement workers, poultry farmers, and poultry confinement workers---were not mutually exclusive. Table 2 shows that 7566 farmers (87%) had exclusive exposure to swine, 587 (7%) had exclusive exposure to poultry, and 513 (6%) were exposed to both. Therefore, additional analysis was performed examining exclusive categories of animal exposure.
TABLE 1.
Demographic and Farming Characteristics of 15,932 Agricultural Health Study Private Pesticide Applicators, 1993–1997
| Swine Farmers | Swine Confinement Workers | Poultry Farmers | Poultry Confinement Workers | Reference* | |
|---|---|---|---|---|---|
| Number | 7079 | 5930 | 784 | 713 | 7610 |
| Gender, N (%) | |||||
| Male | 6992 (99) | 5859 (99) | 762 (97) | 696 (98) | 7357 (97) |
| Female | 87 (1) | 71 (1) | 22 (3) | 17 (2) | 253 (3) |
| Race, N (%) | |||||
| White | 6953 (98) | 5841 (99) | 761 (97) | 694 (97) | 7350 (97) |
| Nonwhite | 117 (2) | 83 (1) | 20 (3) | 17 (3) | 244 (3) |
| State, N (%) | |||||
| North Carolina | 573 (8) | 512 (9) | 400 (51) | 424 (59) | 3829 (50) |
| Iowa | 6506 (92) | 5418 (91) | 384 (49) | 289 (41) | 3781 (50) |
| Age, mean (yr) | 45.2 | 45.0 | 46.6 | 45.7 | 51.2 |
| Education, N (%) | |||||
| ≤ High school | 3837 (55) | 3072 (52) | 469 (60) | 409 (57) | 4188 (55) |
| >High school | 3159 (45) | 2781 (47) | 293 (37) | 284 (40) | 3258 (43) |
| Smoking: ever, N (%) | 2581 (37) | 2173 (37) | 361 (46) | 349 (49) | 4093 (54) |
| Alcoholic drinks: ever, N (%) | 5186 (73) | 4398 (74) | 420 (54) | 378 (53) | 4284 (56) |
| Food frequency in last year | |||||
| Chicken, mean | 2–3 times/mo | 2–3 times/mo | Once/week | Once/week | Once/week |
| Pork, mean | Once a week | Once a week | 2–3 times/mo | 2–3 times/mo | 2–3 times/mo |
| Bacon/sausage, mean | 2–3 times/mo | 2–3 times/mo | 2–3 times/mo | Once a week | 2–3 times/mo |
| Pesticides: ever use, N (%) | |||||
| Insecticide | 6692 (95) | 5630 (95) | 734 (94) | 664 (93) | 6830 (90) |
| Herbicide | 6990 (99) | 5859 (99) | 775 (99) | 704 (99) | 7345 (97) |
| Fungicide | 1425 (20) | 1285 (22) | 302 (39) | 301 (42) | 3303 (43) |
| Fumigant | 732 (10) | 671 (11) | 201 (26) | 196 (28) | 2166 (29) |
Reference group has no self-reported animal farming exposure.
TABLE 2.
Overlapping Animal Exposure by Occupational Category Among 15,932 Private Pesticide Applicators in the Agricultural Health Study, 1993–1997*
| Total | Swine Confinement Workers | Poultry Farmers | Poultry Confinement Workers | |
|---|---|---|---|---|
| Swine farmers | 7079 | 4958 (70) | 97 (1) | 11 (0.2) |
| Swine confinement workers | 5930 | --- | 5 (0.04) | 58 (1) |
| Poultry farmers | 784 | --- | --- | 305 (39) |
| Poultry confinement workers | 713 | --- | --- | --- |
Farmers reporting exposure in all four categories: 112; Farmers reporting exposure in any three categories: 181.
Values are N (%).
Motor and sensory symptoms, specifically weakness in limbs and numbness in extremities, are noted frequently in persons with GBS-like disease.26 Two symptom queries were selected: “numbness or pins-and-needles in your hands or feet” and “weakness in your arms or legs.” Prior studies of GBS have used queries that differentiate among symptoms that are more or less typical of GBS. Since speech and vision impairments are not common in cases of GBS, three additional symptom queries were selected: “difficulty speaking,” “blurred vision or double vision,” and “difficulty seeing at night.” Thus, five self-reported neurologic symptom outcomes were assessed: numbness, weakness, difficulty speaking, blurred vision, and night blindness (Table 3). Outcomes were assigned as positive if any symptom episode was reported in the previous year.
TABLE 3.
Prevalence of Neurologic Symptoms Among 15,932 Private Pesticide Applicators in the Agricultural Health Study, 1993–1997*
| Numbness | Weakness | Difficulty Speaking | Blurred Vision | Night Blindness | |
|---|---|---|---|---|---|
| Swine farmers | 2077 (29) | 1105 (16) | 356 (5) | 711 (10) | 818 (12) |
| Swine confinement workers | 1795 (30) | 968 (16) | 315 (5) | 620 (11) | 720 (12) |
| Poultry farmers | 242 (31) | 128 (16) | 34 (4) | 78 (10) | 88 (11) |
| Poultry confinement workers | 219 (31) | 122 (17) | 31 (4) | 84 (12) | 86 (12) |
| Referents | 1914 (25) | 1057 (14) | 316 (4) | 800 (11) | 960 (13) |
| Overall | 4345 (27) | 2377 (15) | 736 (5) | 1653 (10) | 1921 (12) |
Values are N (%).
Prevalence ratios (PRs) were calculated to compare risk of self-reported neurologic symptoms within each of the categories of animal exposure relative to the referent group (no animal exposure). Univariate and multivariate regression analyses were performed using the P1REL0506.01 AHS P1 data release and the PROC GENMOD function for log-binomial models using SAS 9.1 software (Cary, NC), as described by Spiegelman and Hertzmark.27 Because of the small numbers of women and nonwhites in the study, additional analysis was performed excluding these two groups from the extended model.
A priori, demographic characteristics, pesticide use, alcohol consumption, frequency of eating chicken and pork products, and state of residence were considered potential confounders on the basis of medical and scientific understanding (Table 1). Because previous AHS studies have reported associations between pesticides and some of the same neurologic symptoms examined here,21,28,29 the covariate of “ever used/never used” pesticides was subdivided by category of pesticide: insecticides, herbicides, fungicides, and fumigants. An adjusted model was built to control the estimate of association for all potential, measured confounders.
The institutional review boards of NIH and its contractors approved the AHS. The Johns Hopkins University institutional review board determined that this secondary data analysis was exempted from further review. This work was supported in part by the intramural research program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES049030--11), and National Cancer Institute (Z01-CP010119).
RESULTS
Overall, 15,932 individuals were evaluated in this study. The percentage of missing data for covariates was small (<3%) and generally was comparable among exposed groups and referents. Subjects with missing data were excluded from the analysis. Poultry farmers, poultry confinement workers, swine farmers, swine confinement workers, and referents with no self-reported occupational animal exposure differed to a small but significant degree in sex, race, state of residence, education level, and pesticide use by chi-squared analysis (Table 1).
The observed, overall prevalence in this cohort for self-reported symptoms of weakness was 15%, numbness was 27%, difficulty speaking was 5%, blurred vision was 10%, and night blindness was 12% (Table 3). Prevalence ratios were estimated, comparing each exposure group to the referent group, using univariate and multivariate (adjusted) regression models to evaluate differences in risk of self-reported neurologic symptoms, as shown in Table 4. Adjusted models are discussed here. Overall, farmers and workers exposed to swine and/or poultry were more likely to report at least one episode of numbness in the past year and were more likely to report at least one episode of weakness in the past year compared with referents without animal exposure. The prevalence of difficulty speaking, blurred vision or night blindness did not differ between referents and any category of swine or poultry exposure.
TABLE 4.
Prevalence Ratio Estimates and 95% Confidence Intervals for Neurologic Symptoms by Exposure to Swine or Poultry Among 15,932 Farmers in the Agricultural Health Study, 1993–1997
| Numbness | Weakness | Difficulty Speaking | Blurred Vision | Night Blindness | |
|---|---|---|---|---|---|
| Swine farmers (n = 7079) | |||||
| Unadjusted model | 1.24 [1.15–1.33] P< 0.0001 |
1.15 [1.05–1.26] P = 0.003 |
1.22 [1.05–1.43] P = 0.01 |
0.95 [0.85–1.06] P = 0.35 (ns) |
0.91 [0.82–0.99] P = 0.05 |
| Adjusted model* | 1.18 [1.08–1.29] P = 0.0003 |
1.22 [1.09–1.37] P = 0.0007 |
0.96 [0.79–1.17] P = 0.69 (ns) |
1.06 [0.93–1.21] P = 0.41 (ns) |
0.99 [0.88–1.13] P = 0.98 (ns) |
| Swine confinement workers (n = 5930) | |||||
| Unadjusted model | 1.29 [1.20–1.39] P < 0.0001 |
1.21 [1.10–1.33] P < 0.0001 |
1.29 [1.10–1.52] P = 0.002 |
0.99 [0.89–1.11] P = 0.91 (ns) |
0.96 [0.86–1.06] P = 0.41 (ns) |
| Adjusted model* | 1.23 [1.12–1.35] P < 0.0001 |
1.28 [1.14–1.44] P < 0.0001 |
1.04 [0.86–1.27] P = 0.67 (ns) |
1.10 [0.96–1.26] P = 0.18 (ns) |
1.05 [0.93–1.20] P = 0.42 (ns) |
| Poultry farmers (n = 784) | |||||
| Unadjusted model | 1.33 [1.13–1.56] P = 0.0005 |
1.21 [0.99–1.48] P = 0.06 (ns) |
1.05 [0.73–1.50] P = 0.81 (ns) |
0.94 [0.74–1.20] P = 0.62 (ns) |
0.88 [0.70–1.10] P = 0.26 (ns) |
| Adjusted model* | 1.27 [1.07–1.51] P = 0.006 |
1.19 [0.97–1.48] P = 0.10 (ns) |
0.89 [0.61–1.30] P = 0.55 (ns) |
0.92 [0.71–1.20] P = 0.53 (ns) |
0.84 [0.66–1.08] P = 0.17 (ns) |
| Poultry confinement workers (n = 713) | |||||
| Unadjusted model | 1.32 [1.12–1.56] P = 0.001 |
1.28 [1.04–1.57] P = 0.02 |
1.05 [0.72–1.53] P = 0.80 (ns) |
1.14 [0.90–1.44] P = 0.29 (ns) |
0.95 [0.75–1.20] P = 0.67 (ns) |
| Adjusted model* | 1.25 [1.05–1.50] P = 0.01 |
1.22 [0.98–1.52] P = 0.07 (ns) |
0.87 [0.58–1.30] P = 0.49 (ns) |
1.11 [0.85–1.43] P = 0.45 (ns) |
0.90 [0.70–1.15] P = 0.39 (ns) |
| Total poultry/swine exposed farmers (n = 8666) | |||||
| Adjusted model* | 1.18 [1.08–1.28] P = 0.0002 |
1.25 [1.13–1.39] P < 0.0001 |
1.01 [0.84–1.20] P = 0.95 (ns) |
1.07 [0.94–1.21] P = 0.30 (ns) |
0.98 [0.87–1.10] P = 0.73 (ns) |
Adjusted model controlled for state, race, sex, age, education, alcohol consumption, smoking, consumption of chicken and pork products, and exposure to pesticides (insecticides, herbicides, fungicides and fumigants).
ns indicates nonsignificant.
Swine farmers and confinement workers were more likely to report at least one episode of numbness or at least one episode of weakness during the previous year compared with referents (Table 4). Additional analysis of exclusive categories of exposure demonstrated that farmers and workers who reported exposure only to swine and not poultry were more likely to report numbness (PR = 1.18 [95% Confidence Interval (CI), 1.08 to 1.29]) and more likely to report weakness (PR = 1.24 [95% CI, 1.11 to 1.39]) compared with referents. Of these, the 616 individuals who reported swine exposure only through working in confinement areas and reported no poultry exposure were at higher risk for reporting symptoms of numbness (PR = 1.26 [95% CI, 1.04 to 1.53]) and weakness (PR = 1.49 [95% CI, 1.18 to 1.87]) compared with referents. The 1708 swine farmers who reported only farming pigs and not working in swine confinement areas were not significantly more likely than referents to report symptoms of numbness (PR = 1.01 [95% CI, 0.88 to 1.16]) and weakness (PR = 1.11 [95% CI, 0.93 to 1.31]).
Poultry farmers and confinement workers were more likely than referents to report at least one episode of numbness in the past year (Table 4). Poultry farmers and confinement workers also were more likely to report weakness than referents, but this difference was not statistically significant (Table 4). Additional analysis of exclusive categories of exposure demonstrated that farmers and workers who reported exposure only to poultry had a small increased risk of numbness (PR = 1.12 [95% CI, 0.91 to 1.37]) and weakness (PR = 1.18 [95% CI, 0.92 to 1.51]) compared to referents, but this difference was not statistically significant. The 121 individuals who reported poultry exposure only through working in confinement areas and reported no swine exposure were more likely than referents to report symptoms of numbness (PR = 1.17 [95% CI, 0.77 to 1.76]) and weakness (PR = 1.43 [95% CI, 0.89 to 2.30]), but this was not statistically significant.
The 513 farmers who reported both poultry and swine exposure were more likely than referents to report numbness (PR = 1.37 [95% CI, 1.11 to 1.70]). This same group with dual exposure had increased risk of weakness (PR = 1.23 [95% CI, 0.94 to 1.61]) compared to referents, but this was not statistically significant.
Additional analysis excluding race and sex from the adjusted model did not change inference for any of the comparisons, nor did analysis comparing each exposure group to the referent group by ordinal degree of lifetime days of use of pesticide exposure instead of by ever--never categorization (see Supplemental Table at http://links.lww.com/JOM/A46). In this latter analysis, an ordinal variable was created for each of the four classes of pesticides on the basis of self-reported years of use and days per year of use. From this self-reported exposure, we calculated cumulative lifetime days of pesticide use by class and then categorized this use according to degree of self-reported exposure for each of the four variables used in the model. In addition, PRs generally were robust to a change in cutoff point for outcomes, that is, classifying outcomes as positive on the basis of self-report of two-or-more symptom episodes rather than one-or-more symptom episodes per year. Analysis by state did not change inference for associations between pig exposure and any of the outcomes. However, poultry farmers and workers in North Carolina were not at higher risk for numbness.
DISCUSSION
This is the first large study to examine risk of neurological symptoms among persons with exposure to animals potentially associated with C. jejuni. We demonstrated increased prevalence of two self-reported neurologic symptoms, numbness and weakness, with occupational exposure to swine or to poultry. Swine and poultry farmers and workers were more likely than nonanimal farmers to self-report numbness, and swine farmers and workers were more likely to self-report weakness. These self-reported symptoms were somewhat specific in that these farmers and workers were not more likely to self-report vision and speech problems. Of the neurologic symptoms assessed by self-report through Phase 1 AHS questionnaires, numbness and weakness are most likely to occur with a peripheral neuropathy like GBS.30,31 However, these symptoms are neither pathognomonic for GBS nor specific to the disease.
Animal production workers may contact Campylobacter and other pathogens via ingestion or inhalation.18 In concentrated animal-feeding operations in particular, confinement, animal density, and poor hygiene contribute to increased prevalence of pathogen carriage among animals and in the farm environment.18,25,32,33 Domesticated poultry are reservoirs for C. jejuni.34,35 Colonization of chickens in high-density houses occurs rapidly.36 Prevalence can reach 90% to 100% in commercial broiler flocks.37 In swine, Campylobacter coli colonization is most commonly found; the prevalence of C. jejuni colonization in swine varies widely and may reach 76%38–40. Multiple studies support occupational exposure as a route of transmission for Campylobacter.19,41 Exposure to contaminated environments and to colonized animals may facilitate transmission of the pathogen to humans.18,34 This study highlights a need for enhanced surveillance and reporting of neurologic and other chronic or delayed sequelae from pathogens such as C. jejuni among workers occupationally exposed to live animals.
Pesticide exposure, especially to insecticides such as organophosphates and organochlorines, has been shown to increase the likelihood of self-reported numbness and weakness in this cohort and may account partially for the elevated prevalence rates found in this group of farmers.21,28 Although adjustment for exposure to the four classes of pesticides attenuated most of the estimates, a significant association remained between animal exposure and these two neurologic symptoms. Interestingly, some symptoms associated with pesticide use---namely, vision and speech impairments---are less representative of GBS and were not associated with animal exposure in the adjusted models.
There are four limitations of this study: first, no direct information was collected on Campylobacter exposure; second, outcomes were subjective, self-reported symptoms rather than objective diagnoses based on medical testing; third, data are incomplete for nonoccupational sources of exposure to Campylobacter; and fourth, the AHS questionnaire was not designed specifically to capture medical diagnosis of GBS or other peripheral neuropathies. Further, we cannot rule out the possibility that another animal-related exposure (not Campylobacter) may contribute to the increased risk of numbness and weakness among farmers and confinement workers. Farmers who experience adverse effects in response to animal contact may self-select out of animal-related occupations, leading to selection bias and attenuation of estimates. Although these limitations and the cross-sectional nature of the study design prevent a conclusion of causality, the data suggest that farmers potentially occupationally exposed to C. jejuni through contact with swine or poultry may be at increased risk for development of symptoms consistent with GBS.
The finding that swine farmers and workers were at similar risk for numbness and weakness compared with poultry farmers and workers is interesting. An analysis removing all poultry farmers and workers from the swine models failed to negate the association. Furthermore, farmers exclusively contacting poultry did not demonstrate a statistically significant increase in likelihood of reporting symptoms of numbness or weakness. Unlike the results of Price et al.,19 this study found no statistically significant increased prevalence of self-reported numbness and weakness in poultry farmers or workers without swine contact. This may be due to differences in the populations sampled by Price compared to the AHS, related to the intensity of work-related exposures to C. jejuni. Price et al.19 enrolled chicken catchers and others hired by producers to work on multiple farms and did not collect information on pesticide use,20 whereas the AHS subjects were enrolled through pesticide licensing centers, reported extensive use of pesticides, and worked primarily on their own farms.
In conclusion, occupational exposure to poultry and swine farming may put workers at risk for symptoms of peripheral neuropathy. Exposure to the animal pathogen, Campylobacter jejuni, a known risk factor for GBS, is a possible mechanism for this association. Growth of concentrated poultry and swine production may increase the likelihood of these exposures, with implications for subsequent development of disease because confinement raising can lead to intensification and spread of bacteria within a flock or herd.18 Poultry and swine farmers, particularly confinement workers with intense animal contact, may serve as an important population for prospective study of neurologic sequelae associated with infections by zoonotic pathogens like Campylobacter.
Supplementary Material
Acknowledgments
Disclosure of Funding: The authors thank Dr Guy McKhann for his invaluable assistance. This work was supported in part by the intramural research program of the National Institutes of Health, the National Institute of Environmental Health Sciences (Z01-ES049030--11), and the National Cancer Institute (Z01-CP010119). Meghan Davis is supported by a Johns Hopkins Bloomberg School of Public Health Sommer scholarship.
Abbreviations
- GBS
Guillain-Barré Syndrome
- AHS
Agricultural Health Study
- PR
prevalence ratio
- CI
confidence interval
Footnotes
Supplemental digital content is available for this article. Direct URL citation appears in the printed text and is provided in the HTML and PDF versions of this article on the journal’s Web site (www.joem.org).
Competing interests declaration: Each author declares that he or she has no actual or potential competing financial interest.
References
- 1.Allos BM. Association between Campylobacter infection and Guillain-Barré syndrome. J Infect Dis. 1997;176(suppl 2):S125–S128. doi: 10.1086/513783. [DOI] [PubMed] [Google Scholar]
- 2.Hughes R. Campylobacter jejuni in Guillain-Barré syndrome. Lancet Neurol. 2004;3:644. doi: 10.1016/S1474-4422(04)00902-0. [DOI] [PubMed] [Google Scholar]
- 3.Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005;366:1653–1666. doi: 10.1016/S0140-6736(05)67665-9. [DOI] [PubMed] [Google Scholar]
- 4.Buzby JC, Allos BM, Roberts T. The economic burden of Campylobacter-associated Guillain-Barré syndrome. J Infect Dis. 1997;176(suppl 2):S192–S197. doi: 10.1086/513785. [DOI] [PubMed] [Google Scholar]
- 5.Tam CC, O’Brien SJ, Petersen I, Islam A, Hayward A, Rodrigues LC. Guillain-Barré syndrome and preceding infection with Campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE. 2007;2:e344. doi: 10.1371/journal.pone.0000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCarthy N, Giesecke J. Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni. Am J Epidemiol. 2001;153:610–614. doi: 10.1093/aje/153.6.610. [DOI] [PubMed] [Google Scholar]
- 7.Tam CC, Rodrigues LC, Petersen I, Islam A, Hayward A, O’Brien SJ. Incidence of Guillain-Barré syndrome among patients with Campylobacter infection: a general practice research database study. J Infect Dis. 2006;194:95–97. doi: 10.1086/504294. [DOI] [PubMed] [Google Scholar]
- 8.Magira EE, Papaioakim M, Nachamkin I, et al. Differential distribution of HLA-DQ beta/DR beta epitopes in the two forms of Guillain-Barré syndrome, acute motor axonal neuropathy and acute inflammatory demyelinating polyneuropathy (AIDP): identification of DQ beta epitopes associated with susceptibility to and protection from AIDP. J Immunol. 2003;170:3074–3080. doi: 10.4049/jimmunol.170.6.3074. [DOI] [PubMed] [Google Scholar]
- 9.Ho T, Griffin J. Guillain-Barré syndrome. Curr Opin Neurol. 1999;12:389–394. doi: 10.1097/00019052-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Hughes RA, Hadden RD, Gregson NA, Smith KJ. Pathogenesis of Guillain-Barré syndrome. J Neuroimmunol. 1999;100:74–97. doi: 10.1016/s0165-5728(99)00195-2. [DOI] [PubMed] [Google Scholar]
- 11.Nachamkin I, Engberg J, Gutacker M, et al. Molecular population genetic analysis of Campylobacter jejuni HS:19 associated with Guillain-Barré syndrome and gastroenteritis. J Infect Dis. 2001;184:221–226. doi: 10.1086/322008. [DOI] [PubMed] [Google Scholar]
- 12.Wassenaar TM, Fry BN, Lastovica AJ, Wagenaar JA, Coloe PJ, Duim B. Genetic characterization of Campylobacter jejuni O: 41 isolates in relation with Guillain-Barré syndrome. J Clin Microbiol. 2000;38:874–876. doi: 10.1128/jcm.38.2.874-876.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engberg J, Nachamkin I, Fussing V, et al. Absence of clonality of Campylobacter jejuni in serotypes other than HS:19 associated with Guillain-Barré syndrome and gastroenteritis. J Infect Dis. 2001;184:215–220. doi: 10.1086/322010. [DOI] [PubMed] [Google Scholar]
- 14.Usuki S, Taguchi K, Cawthraw SA, et al. Human and chicken antibodies to gangliosides following infection by Campylobacter jejuni. Exp Neurol. 2006;200:50–55. doi: 10.1016/j.expneurol.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Humphrey T, O’Brien S, Madsen M. Campylobacters as zoonotic pathogens: a food production perspective. Int J Food Microbiol. 2007;117:237–257. doi: 10.1016/j.ijfoodmicro.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 16.Cawthraw SA, Lind L, Kaijser B, Newell DG. Antibodies, directed towards Campylobacter jejuni antigens, in sera from poultry abattoir workers. Clin Exp Immunol. 2000;122:55–60. doi: 10.1046/j.1365-2249.2000.01349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson IG. Airborne Campylobacter infection in a poultry worker: case report and review of the literature. Commun Dis Public Health. 2004;7:349–353. [PubMed] [Google Scholar]
- 18.Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 19.Price LB, Roess A, Graham JP, et al. Neurologic symptoms and neuropathologic antibodies in poultry workers exposed to Campylobacter jejuni. J Occup Environ Med. 2007;49:748–755. doi: 10.1097/JOM.0b013e3180d09ec5. [DOI] [PubMed] [Google Scholar]
- 20.Alavanja MC, Sandler DP, McMaster SB, et al. The agricultural health study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed private pesticide applicators in the agricultural health study. Environ Health Perspect. 2005;113:877–882. doi: 10.1289/ehp.7645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tarone RE, Alavanja MC, Zahm SH, et al. The agricultural health study: factors affecting completion and return of self-administered questionnaires in a large prospective cohort study of pesticide applicators. Am J Ind Med. 1997;31:233–242. doi: 10.1002/(sici)1097-0274(199702)31:2<233::aid-ajim13>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 23.Horrocks SM, Anderson RC, Nisbet DJ, Ricke SC. Incidence and ecology of Campylobacter jejuni and coli in animals. Anaerobe. 2009;15:18–25. doi: 10.1016/j.anaerobe.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Von Essen SG, Auvermann BW. Health effects from breathing air near CAFOs for feeder cattle or hogs. J Agromedicine. 2005;10:55–64. doi: 10.1300/J096v10n04_08. [DOI] [PubMed] [Google Scholar]
- 25.Sapkota AR, Ojo KK, Roberts MC, Schwab KJ. Antibiotic resistance genes in multidrug-resistant enterococcus spp. and streptococcus spp. recovered from the indoor air of a large-scale swine-feeding operation. Lett Appl Microbiol. 2006;43:534–540. doi: 10.1111/j.1472-765X.2006.01996.x. [DOI] [PubMed] [Google Scholar]
- 26.McKhann GM, Cornblath DR, Ho T, et al. Clinical and electrophysiological aspects of acute paralytic disease of children and young adults in northern china. Lancet. 1991;338:593–597. doi: 10.1016/0140-6736(91)90606-p. [DOI] [PubMed] [Google Scholar]
- 27.Spiegelman D, Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 28.Kamel F, Engel LS, Gladen BC, Hoppin JA, Alavanja MC, Sandler DP. Neurologic symptoms in licensed pesticide applicators in the agricultural health study. Hum Exp Toxicol. 2007;26:243–250. doi: 10.1177/0960327107070582. [DOI] [PubMed] [Google Scholar]
- 29.Kamel F, Tanner C, Umbach D, et al. Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol. 2007;165:364–374. doi: 10.1093/aje/kwk024. [DOI] [PubMed] [Google Scholar]
- 30.Asbury AK. Diagnostic considerations in Guillain-Barré syndrome. Ann Neurol. 1981;9(suppl):1–5. doi: 10.1002/ana.410090703. [DOI] [PubMed] [Google Scholar]
- 31.Griffin JW, Li CY, Ho TW, et al. Pathology of the motor-sensory axonal Guillain-Barré syndrome. Ann Neurol. 1996;39:17–28. doi: 10.1002/ana.410390105. [DOI] [PubMed] [Google Scholar]
- 32.Silbergeld EK, Davis MF, Leibler JH, Peterson AE. One reservoir: redefining the community origins of antimicrobial resistant infections. Med Clin N Am. 2008;92:1391–1407. doi: 10.1016/j.mcna.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 33.Sapkota AR, Curriero FC, Gibson KE, Schwab KJ. Antibiotic-resistant enterococci and fecal indicators in surface water and groundwater impacted by a concentrated swine feeding operation. Environ Health Perspect. 2007;115:1040–1045. doi: 10.1289/ehp.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCrea BA, Tonooka KH, VanWorth C, Boggs CL, Atwill ER, Schrader JS. Prevalence of Campylobacter and salmonella species on farm, after transport, and at processing in specialty market poultry. Poult Sci. 2006;85:136–143. doi: 10.1093/ps/85.1.136. [DOI] [PubMed] [Google Scholar]
- 35.Gregory E, Barnhart H, Dreesen DW, Stern NJ, Corn JL. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 1997;41:890–898. [PubMed] [Google Scholar]
- 36.McCrea BA, Tonooka KH, VanWorth C, Atwill ER, Schrader JS. Colonizing capability of Campylobacter jejuni genotypes from low-prevalence avian species in broiler chickens. J Food Prot. 2006;69:417–420. doi: 10.4315/0362-028x-69.2.417. [DOI] [PubMed] [Google Scholar]
- 37.McCrea BA, Macklin KS, Norton RA, Hess JB, Bilgili SF. A longitudinal study of salmonella and Campylobacter jejuni isolates from day of hatch through processing by automated ribotyping. J Food Prot. 2006;69:2908–2914. doi: 10.4315/0362-028x-69.12.2908. [DOI] [PubMed] [Google Scholar]
- 38.Harvey RB, Young CR, Ziprin RL, et al. Prevalence of Campylobacter spp isolated from the intestinal tract of pigs raised in an integrated swine production system. J Am Vet Med Assoc. 1999;215:1601–1604. [PubMed] [Google Scholar]
- 39.Varela NP, Friendship RM, Dewey CE. Prevalence of Campylobacter spp isolated from grower-finisher pigs in Ontario. Can Vet J. 2007;48:515–517. [PMC free article] [PubMed] [Google Scholar]
- 40.Young CR, Harvey R, Anderson R, Nisbet D, Stanker LH. Enteric colonisation following natural exposure to Campylobacter in pigs. Res Vet Sci. 2000;68:75–78. doi: 10.1053/rvsc.1999.0335. [DOI] [PubMed] [Google Scholar]
- 41.Ellis A, Irwin R, Hockin J, Borczyk A, Woodward D, Johnson W. Outbreak of Campylobacter infection among farm workers: an occupational hazard. Can Commun Dis Rep. 1995;21:153–156. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


