Abstract
The vasculature of the CNS is structurally and functionally distinct from that of other organ systems and is particularly prone to developmental abnormalities and hemorrhage. Although other embryonic tissues undergo primary vascularization, the developing nervous system is unique in that it is secondarily vascularized by sprouting angiogenesis from a surrounding perineural plexus. This sprouting angiogenesis requires the TGF-β and Wnt pathways because ablation of these pathways results in aberrant sprouting and hemorrhage. We have genetically deleted Gpr124, a member of the large family of long N-terminal group B G protein-coupled receptors, few members of which have identified ligands or well-defined biologic functions in mammals. We show that, in the developing CNS, Gpr124 is specifically expressed in the vasculature and is absolutely required for proper angiogenic sprouting into the developing neural tube. Embryos lacking Gpr124 exhibit vascular defects characterized by delayed vascular penetration, formation of pathological glomeruloid tufts within the CNS, and hemorrhage. In addition, they display defects in palate and lung development, two processes in which TGF-β and/or Wnt pathways also play important roles. We also show that TGF-β stimulates Gpr124 expression, and ablation of Gpr124 results in perturbed TGF-β pathway activation, suggesting roles for Gpr124 in modulating TGF-β signaling. These results represent a unique function attributed to a long N-terminal group B–type G protein-coupled receptor in a mammalian system.
Keywords: tumor endothelial marker 5, orphan receptor, adhesion, forebrain
Blood vessel development is a critical feature of early embryogenesis. Mesenchymal cells in the developing embryo differentiate into vascular endothelial cells, which assemble into a primitive vascular network that remodels to provide a tissue-specific vasculature in most embryonic tissues. The developing CNS is unique in that it does not contain a primary network and is initially avascular; instead, the primary network forms a perineural plexus that surrounds the neural tube, which is then vascularized by secondary sprouts emanating from the plexus (1). This secondary sprouting is in some ways analogous to the angiogenic sprouting that leads to vascularization of initially avascular tumors. The unique manner of CNS vascularization may relate to the high frequency of developmental vascular abnormalities and hemorrhage seen within the CNS as well as to other unique features, such as the blood–brain barrier (2).
Several signaling pathways, such as the VEGF/VEGF receptor, Delta/Notch, Angiopoietin/Tie, and Ephrin/Eph pathways (reviewed in ref. 3), are required for vascularization throughout the embryo; these general angiogenic pathways are also required in the CNS. In addition, the Wnt/β-catenin and TGF-β pathways are specifically required for secondary angiogenic sprouting into the developing neural tube (4–6). Combined deletion of Wnt7a and Wnt7b in the neuroepithelium, or endothelium-specific deletion of β-catenin, lead to failed CNS angiogenesis and vascular hemorrhage (4, 6). Similar phenotypes result from the absence of various members of the TGF-β ligand or receptor families (7–10) or from the absence of integrin αv or β8 receptor subunits (11–14).
Here we explore the function of Gpr124, a member of the large family of long N-terminal group B (LNB) G protein-coupled receptors (GPCRs), few members of which have identified ligands or well-defined biologic functions in mammals (15, 16). A long N-terminal extracellular domain characterizes the LNB GPCRs, featuring functional motifs present in other proteins, where they appear to be important for protein–protein or adhesion-type interactions (15, 17). The vast majority of members of this family remain orphans, and whether these receptors signal through a conventional G protein mechanism is largely unknown (18).
Gpr124 was originally described as tumor endothelial marker 5 (TEM5), based on its discovery as a factor with enhanced expression in endothelial cells from colorectal malignancies (19). Recent in vitro studies suggest a function for Gpr124 in the regulation of endothelial cell survival and growth (20, 21) as well as endothelial cell migration (22). We report here that Gpr124 is specifically expressed in the developing CNS vasculature and is required for proper angiogenic sprouting into the developing neural tube.
Results
Targeted Global Deletion of Gpr124 Produces Developmental Abnormalities in Cerebral Vasculature, Palate, and Lungs and Results in Perinatal Lethality.
We genetically deleted Gpr124 by using VelociGene technology (23) to replace the majority of the protein-coding region with a LacZ reporter gene whose expression is driven by the endogenous Gpr124 promoter (SI Appendix, Fig. S1 A and B). Reporter gene expression in the developing embryo at embryonic day 11.5 (E11.5) was most notable in the vasculature of the head and body as well as in the snout, limbs, and heart (SI Appendix, Fig. S1C).
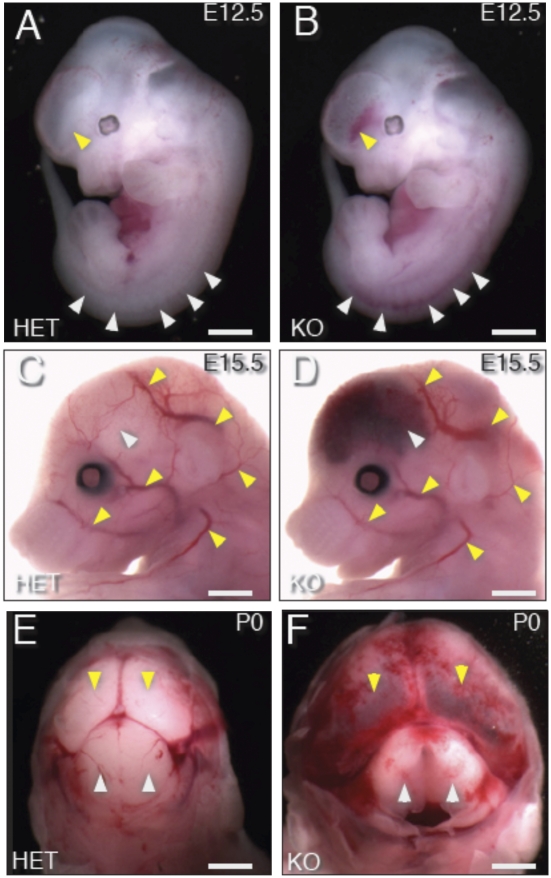
At E10.5, visual comparison of whole embryos did not reveal any differences between embryos homozygously deleted for Gpr124 (Gpr124Lz/Lz) and their WT (Gpr124WT/WT) or heterozygous (Gpr124Lz/WT) littermates. However, by E12.5, all homozygous null embryos (of >56 examined) displayed blood accumulation in the base of the forebrain and along the length of the spinal cord (Fig. 1 A and B, SI Appendix, Fig. S2, and Movie S1). At E15.5, blood accumulation was present within the entire forebrain and spinal cord but not elsewhere in the brain (Fig. 1 C and D, SI Appendix, Fig. S3, and Movie S2). At birth, Gpr124Lz/Lz neonates had enlarged heads caused by inflated forebrain ventricles, with blood accumulation in the forebrain and in the meninges overlying the brain (Fig. 1 E and F). In addition, all Gpr124Lz/Lz offspring examined at E15.5 or postnatal day 0 (P0) had a cleft palate (Fig. 2 A and B, SI Appendix, Fig. S3, and Movie S2), and E15.5 null embryos had reduced lung size (Fig. 2 C and D, SI Appendix, Figs. S3–S5, and Movies S2 and S3). Neonatal Gpr124Lz/Lz pups were never found alive.
Fig. 1.
Targeted deletion of Gpr124 results in profound CNS-specific vascular hemorrhage. Gpr124Lz/Lz (KO) mutants displayed prominent hemorrhage in the ventral forebrain and along the spinal cord at E12.5 [arrowheads indicate hemorrhage in Gpr124Lz/Lz mutants (B) and corresponding normal regions in heterozygous (Het) Gpr124Lz/WT embryos (A)]. The hemorrhage extended throughout the forebrain of KO mutants at E15 (white arrowhead in D) and through to birth (P0) (yellow arrowheads in F). (Compare with corresponding normal regions in Het embryos in C and E). Stereotypic, large superficial vessels of the head and forelimb had the same normal appearance in KO mutants as in Het embryos (yellow arrowheads in C and D).
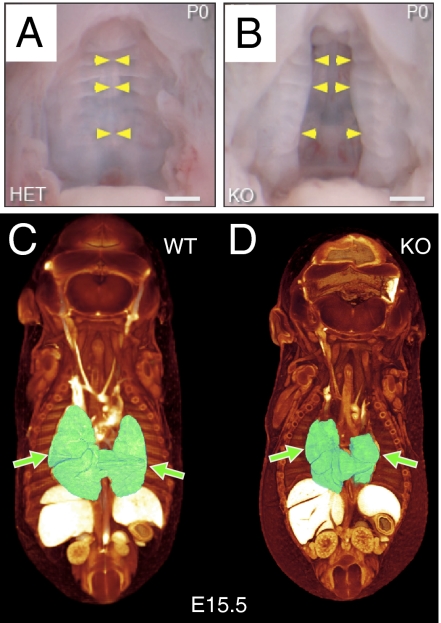
Fig. 2.
Failure in secondary palate formation and hypoplasia of lungs in Gpr124-deficient embryos. All Gpr124-deficient embryos had a large cleft of the secondary hard palate (B), which is normally fused (A), at P0. Yellow arrowheads point to the fused midline in A and to the unfused medial edges of the palatal shelves in B. Visualization of embryonic morphology by soft tissue–enhanced microcomputed X-ray tomography (μCT) at E15.5 revealed that, although other internal organs appeared normal, lungs (shaded green, arrows) were hypoplastic in Gpr124-deficient embryos (D) compared with control littermates (C).
Gpr124 Is Required for Normal Angiogenic Sprouting and Development of Vasculature Within the Forebrain and Spinal Cord.
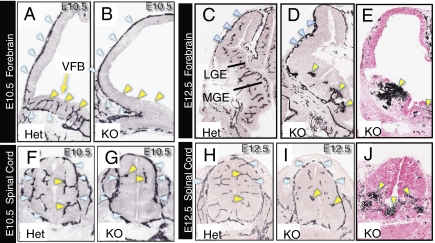
Sprouting angiogenesis from the perineural vascular plexus (PNVP) into the developing neural tube normally begins at E9–E10 (1). These sprouts penetrate radially through the ventral surfaces of the forebrain (Fig. 3A) and lateral aspects of the spinal cord (Fig. 3F). In striking contrast to normal embryos, the ventral forebrains and ventral spinal cords of Gpr124Lz/Lz embryos at E10.5 were almost entirely avascular, although the PNVP appeared normal (Fig. 3 B and G).
Fig. 3.
Gpr124 is required for normal angiogenic sprouting and development of vasculature within the forebrain and spinal cord. Endothelial immunostaining for platelet endothelial cell adhesion molecule 1 (PECAM-1) (A–D and F–I) showed that, although a PNVP formed in the forebrain of both control Gpr124Lz/WT (Het) embryos and Gpr124Lz/Lz (KO) embryos (blue arrowheads in A and B), angiogenic sprouting into the ventral forebrain was evident only in Het embryos at E10.5 (yellow arrowheads in A and B). Similarly, vessel sprouting into the spinal cord (yellow arrowheads in F and G) from the PNVP (blue arrowheads in F and G) was reduced in the dorsal spinal cord and absent in the ventral spinal cord of KO embryos at E10.5. Vessels were grossly aberrant in the ventral forebrain in KO embryos at E12.5, featuring glomeruloid tuft–like endings (yellow arrowheads in D); large regions in the periventricular portion of the ganglionic eminences, and in the lateral pallium, remained avascular. A thickening of the PNVP often occurred along the lateral pallium (blue arrowheads in C and D). Abnormal vessel formations also were present in the ventral spinal cord of KO embryos at E12.5 (yellow arrowheads in I). Histochemical staining for endogenous peroxidase contained in red blood cells (E and J) clearly demonstrated accumulation of red blood cells in neural tissue surrounding abnormal vessels in both brain and ventral spinal cord as well as in the central canal (arrowheads in E and J). Sections in E and J were counterstained with eosin. VFB, ventral forebrain; MGE, medial ganglionic eminence; LGE, lateral ganglionic eminence.
Between E10.5 and E12.5, the ventral forebrain expands, forming the medial and lateral ganglionic eminences, and acquires an increasingly complex vascular network (Fig. 3C). In Gpr124Lz/Lz embryos at E12.5, vessels that sprouted into the forebrain ended in disorganized vascular structures or “glomeruloid tufts” (Fig. 3D). Similarly, the few vessels that sprouted into the ventral spinal cord had a tufted appearance (Fig. 3 H and I). Blood cells were present within the neural parenchyma surrounding the vascular tufts (Fig. 3 E and J). Gpr124Lz/Lz embryos often had buildup in the density of the PNVP, particularly in the dorsal forebrain (Fig. 3D).
At E15.5, the dorsal forebrain of Gpr124Lz/Lz embryos remained sparsely vascularized with abnormal, sometimes tufted vessels (SI Appendix, Fig. S6 C and D). The hypothalamus contained enlarged vessels at reduced density (SI Appendix, Fig. S6 A and B). Vessel development in all other parts of the brain, and in all other parts of the embryo, including the lungs, appeared grossly normal (SI Appendix, Figs. S6 E and F and S7), and the development of these organs, with the exception of the lungs, was indistinguishable between null mutants and control littermates.
Gpr124 Expression Is Specifically Associated with Vessels in Brain and Spinal Cord.
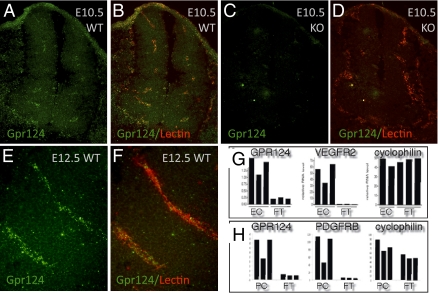
We examined embryo sections containing both normal and abnormal vessels labeled for Gpr124 by in situ hybridization or stained for the LacZ reporter for Gpr124 (Fig. 4 A–F and SI Appendix, Fig. S8). Gpr124 expression occurred within CNS vessels and in the PNVP, as well as in abnormal vascular tufts of null embryos, but was not detected in the neuroepithelium at E10.5 (Fig. 4 A–D) or at E12.5 (Fig. 4 E and F and SI Appendix, Fig. S8 A and B). By using flow cytometry, to specifically sort endothelial cells and pericytes from brains at E15.5, and quantitative PCR analysis, we showed that Gpr124 mRNA was expressed in both these cell types (Fig. 4 G and H).
Fig. 4.
Gpr124 is expressed in normal developing CNS vessels of E10.5 and E12.5 embryos. Fluorescent RNA in situ hybridization with a probe for Gpr124 (green) coupled with fluorescent histochemical staining of endothelial cells by GS lectin I (red) in cross-sections through the neural tube of E10.5 WT embryos (A and B) showed Gpr124 expression within the developing CNS vasculature. Gpr124 expression was not detected in Gp124 KO embryos (C and D). Gpr124 (green) continued to be expressed within the vasculature of the CNS in WT E12.5 embryos (E and F). RT-PCR measurements on platelet endothelial cell adhesion molecule–positive endothelial cells and PDGF receptor β–positive pericytes isolated by FACS from the brains of WT embryos at E15.5 demonstrated that both endothelial cells (EC) and pericytes (PC) have enriched expression of Gpr124 relative to nonendothelial cells [“flow through” (FT)] and nonpericyte cells (FT), respectively (G and H). Control probes for VEGF receptor 2, PDGF receptor β, and cyclophilin confirmed the endothelial identity of isolated cells, the pericyte identity of isolated cells, and the integrity of mRNA samples, respectively. The y axis scale is proportional to RNA copy number.
Interestingly, embryonic Gpr124 reporter expression was also associated with vasculature in parts of the brain and spinal cord that develop normal vasculature after deletion of Gpr124 and was associated with normally developing arteries located outside the CNS (SI Appendix, Fig. S8C). In the lung primordia at E12.5, Gpr124 reporter expression was detected in vessels and in lung mesenchyme (SI Appendix, Fig. S8D). In the palatal shelf at E12.5, at the onset of hard-palate formation, Gpr124 reporter appeared to be expressed only in mesenchyme (SI Appendix, Fig. S8E).
Vessels of the Abnormal Vascular Tufts Recruit Pericytes and Express Molecules Important for Vascular Development.
During angiogenic sprouting into the brain, endothelial cells recruit pericytes that migrate along the sprouting vessels (24), and a failure in recruitment and/or coverage results in increased capillary diameter, increased permeability, and formation of microaneurysms (25–27). By histological staining for pericytes, we found that they were present along vessels and in association with glomeruloid tufts in Gpr124Lz/Lz mutants at E12.5, indicating successful recruitment of pericytes in the absence of Gpr124 (SI Appendix, Fig. S9 Part 1 A–D).
To determine whether the expression of vascular receptors that are essential for VEGF or TGF-β signaling depend on Gpr124 expression, we performed immunostaining for VEGF receptors and the nonsignaling TGF-β coreceptor endoglin. These receptors were normally distributed on vessels of the brain and spinal cord of Gpr124Lz/Lz mutants at E12.5 (SI Appendix, Fig. S9 Part 2 A–L). However, we did discover that vessels of Gpr124Lz/Lz embryos at E12.5 lack expression of GLUT-1, a marker of blood–brain barrier formation (2) normally seen in the brain vasculature at this stage (SI Appendix, Fig. S10 A and B). In contrast, the neuroepithelium displayed intense staining for GLUT-1, suggesting that these regions are hypoxic (28).
The Neural Tube of Gpr124 Null Mutants Has Normal Patterning of Radial Glial Cell Processes.
Angiogenic sprouts are guided centripetally from the PNVP by a radial glial cell scaffold (29), and defects in radial glial cell patterning may cause secondary defects in CNS angiogenesis. In Gpr124Lz/Lz embryos, the patterning of the radial glia appeared normal when evaluated by immunostaining for the glial marker RC2 (SI Appendix, Fig. S11 A–D), suggesting that the defect in angiogenesis in the Gpr124Lz/Lz embryos was not secondary to defective patterning of the radial glia scaffold.
Abnormal Basement Membrane Deposition and Smooth Muscle Cell Association with Gpr124-Deficient Vessels.
Failure to properly form a vascular basal lamina can cause abnormal vascular development (30). Evaluation of basal lamina by immunostaining for collagen IV showed that the distribution of collagen IV staining was sparse in some parts of vascular tufts and abnormally dense in others, suggesting that a defect in basal lamina formation in Gpr124-deficient vessels may be associated with the formation of abnormal vessels (SI Appendix, Fig. S9 Part 1 E–H).
Mural cells expressing the smooth muscle marker smooth muscle actin (SMA) are rarely detected within the developing brain (SI Appendix, Fig. S9 Part 1 I and J). However, in null embryos, we observed a marked expression of SMA in cells associated with the glomeruloid tufts, indicating that these aberrant vascular formations contain mural cells with an abnormal phenotype (SI Appendix, Fig. S9 Part 1 K and L).
Abnormal Vascular Tufts Display Changes in Gene Expression That Indicate a Disruption of TGF-β Signaling in Response to Gpr124 Deletion.
We used laser microdissection to isolate samples from vessels of the ganglionic eminence and from the surrounding neuroepithelium at E12.5 (SI Appendix, Fig. S12A), and we analyzed these samples by RNA microarray to identify signaling pathways that might be altered and thus contribute to the vascular defects in Gpr124-deficient mice. Because of the similarities in the phenotype of Gpr124 mutants and that of various mutants of the TGF-β or Wnt pathways (SI Appendix, Table S1), we looked for changes in target genes of TGF-β (phospho-Smad) or Wnt (β-catenin) pathways. To establish the validity of results obtained by this method, we determined whether differences in gene expression existed that were consistent with our histological observations. As expected, the level of Gpr124 expression in glomeruloid tufts of Gpr124Lz/Lz embryos at E12.5 relative to normal ganglionic eminence vessels of WT control embryos was dramatically reduced, whereas robust expression of the lacZ reporter gene was detected (SI Appendix, Fig. S12B). Enhanced expression of smooth muscle cell markers α-SMA (Acta2) and SM22a (Tagln), and of the transcription factor myocardin (Myocd), in glomeruloid tufts reflected the increased abundance of α-SMA seen by immunostaining (SI Appendix, Fig. S12C). There was a pronounced expression of numerous marker genes for red blood cells in the brain parenchyma of Gpr124Lz/Lz embryos relative to normal control (SI Appendix, Fig. S12D). Increased expression of Glut1 (Slc2a1) in the brain parenchyma of Gpr124 null mutants (SI Appendix, Fig. S12E) matched our GLUT-1 immunostaining results. Along with Glut1, expression of several additional genes indicative of hypoxia were elevated (SI Appendix, Fig. S12E). We found that several endothelial cell markers were expressed more abundantly in samples from Gpr124Lz/Lz mutants than in samples of normal vessels, by about threefold, suggesting that mutant vessel samples might be enriched in endothelial cells (SI Appendix, Fig. S12F). Therefore, when evaluating all changes in gene expression in our vessel samples, we gave greater emphasis to measurements showing changes of threefold or greater.
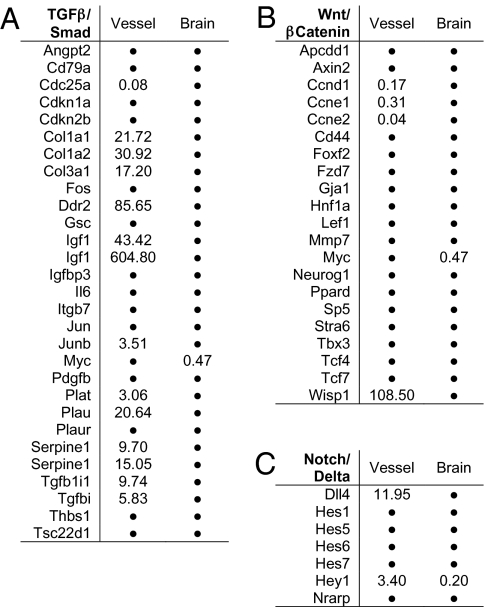
To search for a possible effect of Gpr124 deletion on the TGF-β or Wnt7a/7b pathways, we compiled lists of TGF-β (or pSmad) and Wnt7a/7b (or β-catenin) target genes based on PCR array lists (SABioscience), public databases (Ingenuity), and literature searches, and we determined whether these genes were significantly regulated (≥1.5-fold change, P < 0.05) in vessel samples or neuroepithelium samples. We found that the expression of a striking number of TGF-β target genes (12 of 27, 44%) was altered in the glomeruloid tuft vessel samples of Gpr124Lz/Lz embryos relative to normal. All but one of these genes was up-regulated, including plasminogen activators (Plat and Plau), plasminogen activator inhibitor (Serpine1), and TGF-β–inducible genes (Tgfb1i1 and Tgfbi) (Fig. 5A). Our vessel samples also showed an 8.4-fold increase in Id3, a target gene of both TGF-β and bone morphogenetic proteins (BMPs). We did not see increased expression of BMP receptor genes in vessel samples but did detect a 35-fold increase in BMP7. Whether enhanced BMP signaling might also occur in glomeruloid tufts warrants further investigation. Expression was altered for far fewer target genes of β-catenin (4 of 21, 19%) or Delta/Notch (2 of 7, 29%) (Fig. 5 B and C).
Fig. 5.
Microarray analysis of alterations in embryonic brain and vascular gene expression resulting from global deletion of Gpr124. The effect of deleting Gpr124 on gene expression is shown for sets of genes representing TGF-β target genes (A), β-catenin target genes (B), and Notch target genes (C). The analysis was performed on mRNA extracted from isolated samples of vessels from the ventral forebrain and samples of ventral forebrain neuroepithelium (excluding vessels) at E12.5 collected by laser microdissection. Values are mean fold change in Gpr124Lz/Lz (KO) samples versus Gpr124WT/WT (WT) samples (n = 3–4 KO embryos; n = 3 WT embryos). Only values with a mean fold change >1.5, or <0.66, and a KO vs. WT t test P value ≤ 0.05 are shown. Values not meeting both of these criteria were considered to be unchanged and are represented by dots. Genes represented more than once showed significant changes in expression by multiple probes.
Although the microarray data did not reveal a significant increase in VEGF itself in the neuroepithelium (SI Appendix, Fig. S12G), we found that a large number of genes indicative of VEGF stimulation of endothelium were up-regulated in the vessels of Gpr124Lz/Lz mutants, including Ang2, Dll4, and Kcne3 (31–33). Therefore, the abnormal vessel development in Gpr124 null mutants is not likely because of a deficiency in VEGF or VEGF signaling.
We also found numerous genes expressing components of the basal lamina that were up-regulated in vessels (SI Appendix, Fig. S12H). Many genes (including Ddr2, Lama2, Lamb1-1, and Nid2) showed large changes, and matrix metallopeptidase 2, which degrades collagen IV present in basal lamina during vessel growth, was up-regulated 30-fold, suggesting that the abnormal vessels of Gpr124 null mutants are in an activated state of basal lamina modeling.
Gpr124 mRNA Expression Is Induced by TGF-β1 in Endothelial Cells in Vitro.
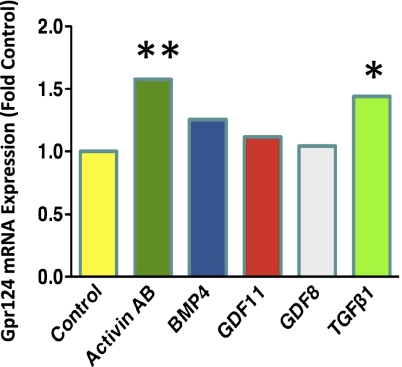
To determine whether expression of Gpr124 might be regulated by TGF-β1, we measured Gpr124 mRNA expression by microarray in human umbilical vein endothelial cells (HUVECs) after a 6-h stimulation by TGF-β1 or by the related growth factors activin AB, growth differentiation factor 11, growth differentiation factor 8, or BMP4. Both TGF-β1 and activin AB induced a significant increase in Gpr124 mRNA (Fig. 6 and SI Appendix, Fig. S13).
Fig. 6.
Gpr124 mRNA expression is induced by TGF-β1 in HUVECs. Treatment of HUVECs for 6 h with 50 pM TGF-β1 induced a 1.44-fold increase in expression of Gpr124 mRNA, as measured by microarray. Treatment with 600 pM activin AB induced a 1.58-fold increase. Values are the mean of three replicates. *P < 0.05; **P < 0.01.
Discussion
Our data demonstrate that the orphan LNB GPCR Gpr124 is required for normal angiogenic sprouting from the PNVP into the developing forebrain and spinal cord but, remarkably, not for vascular development in other regions of the CNS or in other embryonic tissues. Intriguingly, genetic ablation of genes from two signaling pathways, the TGF-β pathway and the Wnt7a/7b pathway, results in abnormally formed vessels specifically in embryonic forebrain and spinal cord that are strikingly similar to the vascular phenotype we see in Gpr124 null mutants (SI Appendix, Table S1 and refs. therein). We found that Gpr124 is expressed specifically in vessels of the CNS, both in endothelial cells and pericytes, consistent with a recent report that also showed that endothelial-specific deletion of Gpr124 produces an abnormal vascular phenotype similar to that seen upon global deletion (22). The presence of Gpr124 in vascular cells raises the question of whether and how this receptor interacts with the TGF-β and/or Wnt signaling pathways to modulate angiogenic sprouting into the developing CNS.
In addition to the abnormal CNS vessel phenotype, Gpr124 null mutants exhibited a cleft palate and reduced lung size, and these were the only other developmental abnormalities that we observed through birth. Lending further support to the hypothesis that Gpr124 may interact with the TGF-β and/or Wnt pathways, a cleft palate is also seen after deletion of TGF-β3, integrin αv, or integrin β8, and hypoplastic lung development occurs after deletion of TGF-β3 or Wnt7b (SI Appendix, Table S1 and refs. therein). TGF-β3 promotes endothelial–mesenchymal transition during fusion of the palatal shelves (7, 34), and thus Gpr124 is implicated in this process as well.
The signaling mechanisms used by the large family of LNB GPCRs have largely remained an enigma. Although we have not elucidated the direct mechanisms of signal transduction by Gpr124, our detailed phenotype analysis has suggested an interesting connection between this receptor and other signaling pathways. Our microarray analysis on samples of vessels from the ganglionic eminence of Gpr124 null mutants showed that the expression of numerous TGF-β target genes was altered. The number of genes affected, together with the relatively large magnitude of the changes, offers compelling evidence that Gpr124 normally modulates signaling through the TGF-β pathway. TGF-β signaling occurs via a heteromeric receptor composed of TGF-βR2 in combination with Alk5 (TGF-βR1) or the endothelial-specific receptor Alk1 (Acvrl1). The endothelial-specific receptor endoglin serves as a coreceptor for Alk1. Deletion of Gpr124 may modestly increase the expression of endoglin (microarray results showed a sixfold increase), but we did not see evidence for substantial changes in expression of other TGF-β receptor subunits. Although deletion of Gpr124 altered the expression of several target genes of the Wnt and the Notch pathways, the effect on these pathways was not as pervasive as it was for the TGF-β signaling pathway, suggesting that Gpr124 preferentially interacts with the TGF-β pathway. This conclusion is consistent with a recent report that ablation of Gpr124 does not disrupt β-catenin signaling in developing CNS endothelial cells (22).
Both our immunohistochemistry and microarray analyses revealed the presence of abnormal expression of SMA in cells associated with the glomeruloid tufts in Gpr124 null mutants. In addition, we observed abnormal distribution and deposition of vascular basal lamina collagen in the glomeruloid tufts. Formation of basal lamina, and recruitment or differentiation of smooth muscle cells, are hallmark responses to TGF-β stimulation (24, 35, 36), lending further credence to an elevated TGF-β signaling in the vessels of Gpr124 null mutants.
We demonstrated that TGF-β1 induces an up-regulation of Gpr124 expression in endothelial cells in vitro, as does activin AB, which, like TGF-β1, exerts transcriptional effects through Alk receptor–mediated phosphorylation of Smad2/3. Together, our data suggest a potential model for the relationship of Gpr124 to the TGF-β signaling pathway and its known functions (SI Appendix, Fig. S14). Interaction of TGF-β family members with their receptors leads to phosphorylation of Smad proteins, which participate in the transcriptional activation of specific target genes. These target genes can include Gpr124 and genes related to basal lamina and extracellular matrix production. We propose that Gpr124 may be a downstream effecter for some of the cellular responses to TGF-β. Potential functional roles for Gpr124 in endothelial cells, suggested by in vitro studies, include directed endothelial cell migration and contact inhibition of endothelial cell proliferation (21, 22). Our data also suggest that Gpr124 normally acts as a negative-feedback regulator to dampen signal transduction by the pathway (SI Appendix, Fig. S14).
The vascular defects observed in mice lacking the TGF-β, Wnt7a/7b, or Gpr124 pathways appear to be remarkably similar to those described in human premature infants. Cerebral vascular defects and hemorrhages (e.g., germinal matrix hemorrhage, intraventricular hemorrhage) are seen in one-fifth of all premature births in developed countries and are a major cause of prenatal/perinatal morbidity and mortality (37). A better understanding of CNS vessel development may offer insights into not only the cause but also the prevention of these serious vascular abnormalities.
Materials and Methods
Animals and Treatments.
All work was performed by using protocols approved by the Institutional Animal Care and Use Committee. At least three specimens were analyzed for each genotype and stage. The morning of vaginal plug was designated E0.
Engineering of a Gpr124 Null Allele.
Targeted ES cells harboring a null allele of Gpr124 were generated by using VelociGene technology (23). Further details are described in SI Appendix.
TaqMan Analysis of Endothelial Cells and Pericytes Isolated from E15.5 Mouse Brains.
Brains from E15.5 WT (Gpr124WT/WT) C57BL/6 embryos were dissected and pooled into three samples of ∼10 brains each for analysis. Further details of the protocol are described in SI Appendix.
Microarray Analysis of Laser-Microdissected Vessels and Brains.
Laser microdissection was performed on brain sections of E12.5 embryos with a Leica LMD6000 system to isolate samples of blood vessels and samples of brain parenchyma absent blood vessels (SI Appendix, Fig. S12). RNA isolated from these samples was amplified and hybridized to a custom Agilent array comprising 43,538 60-mer oligonucleotides covering the entire mouse transcriptome. Fold change and Student's t test P values were calculated with GeneSpring (Agilent) from ratios normalized by nonlinear LOWESS normalization. Further details of the protocol are described in SI Appendix.
Histochemistry, Immunohistochemistry, and in Situ Hybridization.
Sections cut on the cryostat were immunostained following standard procedures as described in SI Appendix, Materials and Methods. β-Gal histochemistry was performed overnight at 37 °C, as previously described (38). In situ hybridization for Gpr124 was performed on fresh-frozen tissue sections with a riboprobe specific for Gpr124 mRNA (39), as described further in SI Appendix.
Microarray Analysis of HUVECs Treated with TGF-β and Related Growth Factors.
Pooled HUVECs were treated with growth factors for 6 h, and mRNA expression was determined by microarray analysis, as described further in SI Appendix.
Acknowledgments
We thank the following individuals for technical assistance and/or helpful discussions: Jennifer Schmahl, Ron Deckelbaum, Gabor Halasz, Richard Corpina, Ourania Yancopoulos, Vincent Idone, Thomas DeChiara, David Frendewey, Jose Rojas, Nick Papadopoulos, John Rudge, Doug MacDonald, Wojtek Auerbach, William Poueymirou, Russell Chernomorsky, Joyce McClain, Ivan Lobov, Charles Pan, Sam Davis, Wen Fury, and Ying Huang.
Footnotes
Conflict of interest statement: The authors are employees of Regeneron Pharmaceuticals, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019761108/-/DCSupplemental.
References
- 1.Hogan KA, Ambler CA, Chapman DL, Bautch VL. The neural tube patterns vessels developmentally using the VEGF signaling pathway. Development. 2004;131:1503–1513. doi: 10.1242/dev.01039. [DOI] [PubMed] [Google Scholar]
- 2.Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–129. doi: 10.1007/s00441-003-0751-z. [DOI] [PubMed] [Google Scholar]
- 3.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 4.Daneman R, et al. Wnt/β-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCarty JH. Integrin-mediated regulation of neurovascular development, physiology and disease. Cell Adh Migr. 2009;3:211–215. doi: 10.4161/cam.3.2.7767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stenman JM, et al. Canonical Wnt signaling regulates organ-specific assembly and differentiation of CNS vasculature. Science. 2008;322:1247–1250. doi: 10.1126/science.1164594. [DOI] [PubMed] [Google Scholar]
- 7.Kaartinen V, et al. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial–mesenchymal interaction. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 8.Mu Z, Yang Z, Yu D, Zhao Z, Munger JS. TGF-β1 and TGF-β3 are partially redundant effectors in brain vascular morphogenesis. Mech Dev. 2008;125:508–516. doi: 10.1016/j.mod.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Yang Z, et al. Absence of integrin-mediated TGF-β1 activation in vivo recapitulates the phenotype of TGF-β1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang LT, Li WY, Kaartinen V. Tissue-specific expression of Cre recombinase from the Tgfb3 locus. Genesis. 2008;46:112–118. doi: 10.1002/dvg.20372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all αv integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- 12.McCarty JH, et al. Selective ablation of αv integrins in the central nervous system leads to cerebral hemorrhage, seizures, axonal degeneration and premature death. Development. 2005;132:165–176. doi: 10.1242/dev.01551. [DOI] [PubMed] [Google Scholar]
- 13.Proctor JM, Zang K, Wang D, Wang R, Reichardt LF. Vascular development of the brain requires β8 integrin expression in the neuroepithelium. J Neurosci. 2005;25:9940–9948. doi: 10.1523/JNEUROSCI.3467-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, et al. β8 integrins are required for vascular morphogenesis in mouse embryos. Development. 2002;129:2891–2903. doi: 10.1242/dev.129.12.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yona S, Lin HH, Siu WO, Gordon S, Stacey M. Adhesion-GPCRs: Emerging roles for novel receptors. Trends Biochem Sci. 2008;33:491–500. doi: 10.1016/j.tibs.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Bjarnadóttir TK, Fredriksson R, Schiöth HB. The adhesion GPCRs: A unique family of G protein-coupled receptors with important roles in both central and peripheral tissues. Cell Mol Life Sci. 2007;64:2104–2119. doi: 10.1007/s00018-007-7067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasnoperov V, et al. Post-translational proteolytic processing of the calcium-independent receptor of α-latrotoxin (CIRL), a natural chimera of the cell adhesion protein and the G protein-coupled receptor. Role of the G protein-coupled receptor proteolysis site (GPS) motif. J Biol Chem. 2002;277:46518–46526. doi: 10.1074/jbc.M206415200. [DOI] [PubMed] [Google Scholar]
- 18.Lagerström MC, Schiöth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- 19.St Croix B, et al. Genes expressed in human tumor endothelium. Science. 2000;289:1197–1202. doi: 10.1126/science.289.5482.1197. [DOI] [PubMed] [Google Scholar]
- 20.Vallon M, Essler M. Proteolytically processed soluble tumor endothelial marker (TEM) 5 mediates endothelial cell survival during angiogenesis by linking integrin αvβ3 to glycosaminoglycans. J Biol Chem. 2006;281:34179–34188. doi: 10.1074/jbc.M605291200. [DOI] [PubMed] [Google Scholar]
- 21.Vallon M, Rohde F, Janssen K-P, Essler M. Tumor endothelial marker 5 expression in endothelial cells during capillary morphogenesis is induced by the small GTPase Rac and mediates contact inhibition of cell proliferation. Exp Cell Res. 2010;316:412–421. doi: 10.1016/j.yexcr.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Kuhnert F, et al. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenzuela DM, et al. High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat Biotechnol. 2003;21:652–659. doi: 10.1038/nbt822. [DOI] [PubMed] [Google Scholar]
- 24.Stenzel D, et al. Peripheral mural cell recruitment requires cell-autonomous heparan sulfate. Blood. 2009;114:915–924. doi: 10.1182/blood-2008-10-186239. [DOI] [PubMed] [Google Scholar]
- 25.Hellström M, et al. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J Cell Biol. 2001;153:543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Armulik A, et al. Pericytes regulate the blood–brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 27.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood–brain barrier integrity during embryogenesis. Nature. 2010;468:562–566. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vannucci SJ, Seaman LB, Vannucci RC. Effects of hypoxia-ischemia on GLUT1 and GLUT3 glucose transporters in immature rat brain. J Cereb Blood Flow Metab. 1996;16:77–81. doi: 10.1097/00004647-199601000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Gerhardt H, et al. Neuropilin-1 is required for endothelial tip cell guidance in the developing central nervous system. Dev Dyn. 2004;231:503–509. doi: 10.1002/dvdy.20148. [DOI] [PubMed] [Google Scholar]
- 30.Gould DB, et al. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308:1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- 31.Del Toro R, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holash J, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 33.Lobov IB, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Proetzel G, et al. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blobe GC, Schiemann WP, Lodish HF. Role of transforming growth factor β in human disease. N Engl J Med. 2000;342:1350–1358. doi: 10.1056/NEJM200005043421807. [DOI] [PubMed] [Google Scholar]
- 36.Goumans M-J, Liu Z, ten Dijke P. TGF-β signaling in vascular biology and dysfunction. Cell Res. 2009;19:116–127. doi: 10.1038/cr.2008.326. [DOI] [PubMed] [Google Scholar]
- 37.Leung A, Islam O. Germinal matrix hemorrhage imaging. eMedicine Specialties. 2008. Available at http://emedicine.medscape.com/article/408862-imaging. Accessed January 18, 2011.
- 38.Adams NC, Gale N. High resolution gene expression analysis in mice using genetically inserted reporter genes. In: Pease S, Lois C, editors. Mammalian and Avian Transgenesis—New Approaches, Principles and Practice Series. Berlin: Springer; 2006. pp. 131–172. [Google Scholar]
- 39.Homma S, Shimada T, Hikake T, Yaginuma H. Expression pattern of LRR and Ig domain-containing protein (LRRIG protein) in the early mouse embryo. Gene Expr Patterns. 2009;9:1–26. doi: 10.1016/j.gep.2008.09.004. [DOI] [PubMed] [Google Scholar]