Abstract
Sensory and signaling pathways are exquisitely organized in primary cilia. Bardet-Biedl syndrome (BBS) patients have compromised cilia and signaling. BBS proteins form the BBSome, which binds Rabin8, a guanine nucleotide exchange factor (GEF) activating the Rab8 GTPase, required for ciliary assembly. We now describe serum-regulated upstream vesicular transport events leading to centrosomal Rab8 activation and ciliary membrane formation. Using live microscopy imaging, we show that upon serum withdrawal Rab8 is observed to assemble the ciliary membrane in ∼100 min. Rab8-dependent ciliary assembly is initiated by the relocalization of Rabin8 to Rab11-positive vesicles that are transported to the centrosome. After ciliogenesis, Rab8 ciliary transport is strongly reduced, and this reduction appears to be associated with decreased Rabin8 centrosomal accumulation. Rab11-GTP associates with the Rabin8 COOH-terminal region and is required for Rabin8 preciliary membrane trafficking to the centrosome and for ciliogenesis. Using zebrafish as a model organism, we show that Rabin8 and Rab11 are associated with the BBS pathway. Finally, using tandem affinity purification and mass spectrometry, we determined that the transport protein particle (TRAPP) II complex associates with the Rabin8 NH2-terminal domain and show that TRAPP II subunits colocalize with centrosomal Rabin8 and are required for Rabin8 preciliary targeting and ciliogenesis.
The primary cilium is present on many cell types and functions to organize signaling (1). Primary cilia are composed of microtubule-based axonemes extending from the mother centriole, a ciliary membrane that surrounds the axoneme, and an intraflagellar transport (IFT) mechanism to traffic proteins within the cilium. Aberrant ciliary function has been linked to human genetic diseases, including Bardet-Biedl syndrome (BBS). We recently reported that a core complex of seven BBS proteins, the BBSome, associates via BBS1 with Rabin8, a guanine nucleotide exchange factor (GEF) for Rab8 (2), and that Rabin8-dependent activation of Rab8 to the GTP-bound form is required for ciliation (3).
Rab GTPases regulate multiple aspects of vesicle transport, budding, and fusion; thus Rab8 and the BBSome may cooperate with multiple unknown factors to organize ciliary membrane assembly. The BBS pathway and Rabs also may cooperate to regulate membrane-trafficking processes, including melanosome transport in zebrafish (3, 4). Despite these recent findings, we understand little about how Rab8 vesicular transport is connected to cilium formation or how membrane assembly is coupled to axoneme formation.
In 1962, Sorokin (5) published detailed electron microscopy studies suggesting a model for ciliary formation (5). During the early stages of ciliogenesis, he detected a large Golgi-like vesicle, the ciliary vesicle, localized to the distal end of the mother centriole. This vesicle flattened to form a double membrane sheath around the assembling axoneme that later would fuse with the plasma membrane. Sorokin's work suggested that the centrosome organizes vesicle trafficking during formation of the primary cilium, although the kinetics and molecular components of this process were unknown.
Rab8 ciliary localization (3, 6) and its presence in Golgi/trans-Golgi membranes (7) suggest that Rab8 could participate in ciliary membrane assembly. Previously we reported Rabin8 at the centrosome (3), a localization that probably is important for Rab8 ciliary trafficking. A candidate Rab8 GTPase activating protein (GAP) (XM_037557) has been described and prevents cilia formation when overexpressed in cells; moreover, a catalytically inactive mutant localized to the cilia (6).
An emerging mechanism for organizing vesicular transport shows the importance of Rab cascades in which an activated Rab in an upstream compartment binds to a GEF as an effector, thereby triggering activation of a downstream GDP-bound Rab (8). In yeast, a Rab cascade organizes polarized exocytosis of Sec4 vesicles for secretion and bud neck formation (9). The Rab Ypt31/32 is responsible for targeting transport of the GEF Sec2 to direct downstream activation of Sec4. Rabin8 and Rab8 are believed to be the mammalian orthologs of Sec2 and Sec4, respectively. Recently it was shown that the GTP-bound form of Rab11, the closest relative of Ypt31/32, binds Rabin8, promotes Rab8 activation, and is required for ciliogenesis and apical membrane formation (10, 11). These findings suggest a Rab11–Rab8 cascade may function in primary cilium assembly.
In the present study, we have investigated ciliary membrane biogenesis further, focusing on mechanisms regulating Rabin8 activation of Rab8. Using real-time imaging after serum starvation, we observe that GFP-tagged Rab8a (GFP-Rab8a) assembles into the ciliary membrane in human retinal pigmented epithelium (RPE) cells. Rab8 ciliary membrane formation is preceded by the vesicular trafficking of Rabin8 to the centrosome; that trafficking ceases after ciliogenesis. We find that this serum-dependent Rabin8 vesicular transport step requires Rab11. Using a localization and affinity purification-tagged Rabin8 (LAP-Rabin8), we show that Rabin8 binds via an NH2-terminal domain to the transport protein particle (TRAPP) II complex and demonstrate that TRAPPC3, -C9, and -C10 are required for Rabin8 centrosome trafficking and ciliogenesis.
Results
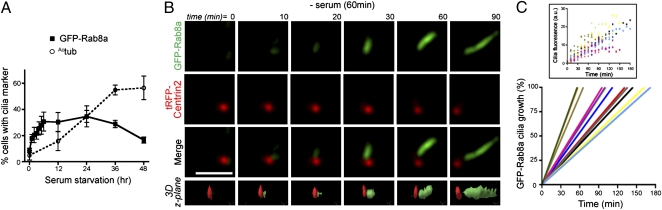
Rab8 Localizes to the Primary Cilium Membrane During Ciliogenesis.
Given the function of related Rab proteins, Rab8 might be predicted to transport membrane components or proteins important in cilia assembly. There are two isoforms of Rab8, Rab8a and Rab8b (which show 82% identity), both of which are expressed in telomere reverse transcriptase (TERT)-immortalized RPE cells (Fig. S1A). Contrary to another report (6), Rab8b colocalized with the cilia marker acetylated tubulin (Actub) in serum-starved RPE cells (Fig. S1B). RNAi-mediated inactivation of either Rab8a or Rab8b reduced ciliation, showing a stronger effect when both forms were depleted (Fig. S1A). Thus, both Rab8a and Rab8b are important for ciliation in RPE cells.
To determine where and when Rab8 functions in ciliogenesis, clonal RPE cell lines expressing GFP-Rab8a were generated. These lines showed approximately sevenfold higher expression than endogenous Rab8 (Fig. S1C) and provided sufficient signal strength for direct fluorescence microscopy studies. In some cells GFP-Rab8a localized to developing cilia within 1 h after serum withdrawal, and additional cells continued to ciliate at later time points (Fig. 1A). Developing or mature GFP-Rab8a–positive cilia were present in ∼30% of cells after 6 h (Fig. 1A). Importantly, GFP-Rab8a did not localize near the centrioles in serum-fed cells, indicating that Rab8 centrosomal targeting is induced by serum starvation (Fig. S1D). RNAi depletion of BBS1 or IFT88, a component of the IFT-B complex required for ciliogenesis (12), confirmed that Rab8a-positive structures were bona fide cilia (Fig. S1E).
Fig. 1.
Rab8 localizes to developing cilia but departs mature cilia. (A) Rab8 is an early marker of ciliogenesis. GFP-Rab8a localization in cilia was scored in live RPE GFP-Rab8a cells, and Actub-positive cilia were counted in fixed cells (n = 75 cells). (B) Rab8 accumulates in the growing cilia. One hour after serum withdrawal, RPE GFP-Rab8a cells transiently expressing tRFP-Centrin2 for 24 h were imaged every 10 min by SDC microscopy over 6 h with concurrent imaging in both red and green channels. Representative contrast-adjusted images shown are projections of 20 z-sections (step size 0.5 μm). (Bottom Row) 3D surface rendering. (Scale bar: 5 μm.) (Movie S1). (C) Kinetics of ciliogenesis. Keying on the initiation of nascent cilia, GFP-Rab8a levels in 12 developing cilia were measured from projected z-stacks collected as described in B. Regression analysis was performed on raw data (Inset) to determine the time required for GFP fluorescence to reach maximal levels (106 ± 12 min).
To understand the earliest steps in Rab8 membrane assembly leading up to ciliation, we used time-lapse video microscopy to observe Rab8a ciliary targeting after serum withdrawal (Fig. 1B and Movie S1). We coexpressed the centriolar marker Centrin2 tagged with the Tag-RFP (tRFP) cassette (tRFP-Centrin2) to highlight the centrosome. Analysis of the image series in Fig. 1B shows GFP-Rab8a nucleating a single vesicular structure near the centriole (t, 0–10 min) within 1 h after serum withdrawal. Rab8a continued to accumulate distally from the mother centriole/developing basal body, assuming the highly elongated shape characteristic of cilia. By tracking GFP fluorescence from the onset of cilia formation, we determined that Rab8a showed a period of relatively linear accumulation at the newly forming and elongating cilia for 106 ± 12 min (Fig. 1C), after which we believe that the steady-state ciliary levels of Rab8 indicate completion of ciliary membrane assembly. Fluorescence recovery after photobleaching (FRAP) studies demonstrated that the rate of Rab8a trafficking to mature cilia was ∼10-fold lower than that observed for a similar time during cilia formation (Fig. S1F). Together these results indicate that Rab8 localizes to the ciliary membrane during ciliogenesis and that Rab8 transport to the primary cilium is reduced after cilium formation.
Interestingly, after more than 24 h of serum starvation, GFP-Rab8a–positive cilia were reduced compared with Actub-positive cilia (Fig. 1A). Importantly, the loss of GFP-Rab8a from the cilia was not caused by decreased expression of Rab8a (Fig. S1C). Live imaging studies of GFP-Rab8a expressed with tRFP-tagged serotonin receptor 6 (5-HT6), a ciliary membrane protein, confirmed the loss of GFP-Rab8a from mature cilia over time (Fig. S1G). Similar results were observed with the ciliary receptor somatostatin receptor 3 (Sstr3), which localizes to the cilia more strongly after ciliogenesis, suggesting that Sstr3 entry in to the primary cilium is independent of Rab8 ciliary trafficking.
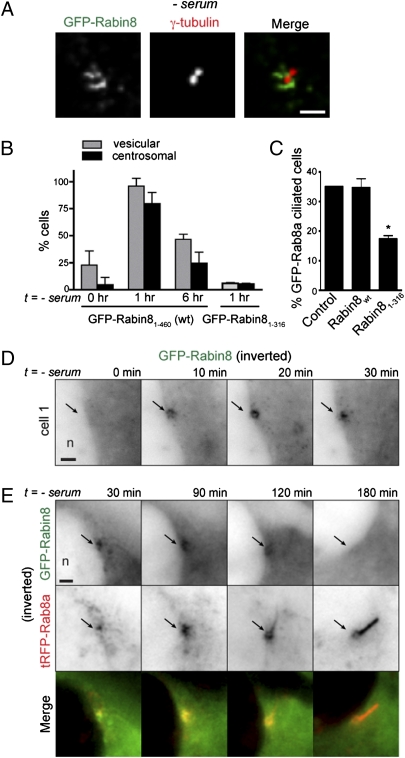
Serum Withdrawal Triggers Rabin8 Recruitment to Vesicles That Are Transported to the Centrosome Before Ciliogenesis.
Because no long-range cellular transport of GFP-Rab8a membranes to the centrosome was evident during ciliogenesis, and Rabin8 is found at the centrosome (Fig. 2A) (3), we considered whether Rabin8 trafficking is important for Rab8-dependent ciliogenesis. To study Rabin8 targeting, we used RPE cells stably expressing GFP-Rabin8. Live-cell imaging revealed that centrosomal Rabin8 was present on dynamic membrane vesicles in a small percentage (<5%) of serum-fed cells (Fig. 2B). Surprisingly, within 1 h after serum withdrawal, >90% of cells showed dramatically increased vesicle-associated Rabin8, and in most of these cells vesicular Rabin8 accumulated at the centrosome (Fig. 2 A and B, Fig. S2A, and Movie S2). Remarkably, Rabin8 was detected at the centrosome in some cells within 10 min of serum removal (Fig. 2D). Readdition of serum rapidly (<10 min) reversed Rabin8 distribution to the cytoplasm (Fig. S2A). Interestingly, 6 h after serum starvation Rabin8 centrosomal accumulation was strongly reduced in the majority of cells (Fig. 2B). This finding, together with live-imaging studies, indicates that Rabin8 centrosome targeting occurred over a span of ∼60–100 min and then diminished (Fig. 2E and Fig. S2C), distinctly paralleling the duration of Rab8 accumulation at the assembling cilium. Time-lapse imaging of GFP-Rabin8 and tRFP-tagged Rab8a (tRFP-Rab8a) after serum withdrawal demonstrated that Rabin8 centrosomal recruitment coincides with Rab8a localization on centrosomal structures, followed by the formation of Rab8-positive primary cilia and reduced Rabin8 centrosomal accumulation (Fig. 2E). Notably, RNAi depletion of Rab8, BBS1, or IFT88 did not block Rabin8 centrosomal transport (Fig. S2D). Together these results suggest that Rabin8 trafficking to the centrosome is upstream of ciliary membrane formation.
Fig. 2.
Serum withdrawal triggers Rabin8 association with vesicles that accumulate dynamically at the centrosome before ciliary membrane assembly. (A) Rabin8 localizes to dynamic centrosomal vesicles following serum withdrawal. Serum-starved (60 min) RPE GFP-Rabin8 cells were fixed and stained with the centriolar marker γ-tubulin (Movie S2). (Scale bar: 2 μm.) (B) Serum starvation causes transient association of Rabin8 with membrane vesicles and accumulation at the centrosome mediated by its COOH-terminal domain. Shown is quantification of GFP-Rabin8 and GFP-Rabin81–316 (ΔC)-positive vesicles and centrosomal accumulation in RPE cells (n = 50) (Fig. S2 A and B). (C) The Rabin8 COOH-terminal domain is required for ciliation. GFP-Rab8a–positive cilia were counted in cells transiently expressing control (tRFP), tRFP-Rabin8, and tRFP-Rabin81–316 for 72 h; serum was removed for the last 24 h. GFP-positive cilia were counted only in tRFP-positive cells (n = 50; P < 0.001). (D) Time course of RPE cells accumulating GFP-Rabin8 at the centrosome after serum removal. Fluorescence signal (black) has been inverted in D and E for better viewing of structures. (Scale bar: 2 μm.) n, nucleus. (E) Rabin8 centrosomal targeting precedes Rab8a ciliary membrane formation and is reduced after ciliogenesis. RPE GFP-Rabin8 cells transiently expressing tRFP-Rab8a for 24 h were imaged live every 30 min after serum withdrawal. GFP and tRFP images were collected separately. (Scale bar: 2 μm.)
Hattula and colleagues (2) observed that deletion of the COOH-terminal region of Rabin8 blocked association with membranes. We constructed a similar Rabin8-targeting mutant and found it failed to redistribute to vesicles or accumulate at the centrosome after serum starvation (Fig. 2B and Fig. S2B). Importantly, expression of the Rabin8 membrane-targeting defective fragment had a dominant negative effect in reducing cilia formation (Fig. 2C). Rabin8 also associates with the actin cytoskeleton (2). However, cytochalasin D-induced actin depolymerization did not affect Rabin8 centrosomal targeting (Fig. S2E). By contrast, destabilization of microtubules with nocodazole blocked Rabin8 centrosomal localization and reduced its vesicle association (Fig. S2E). Thus, we hypothesized that the dynamic microtubule-dependent vesicular transport of Rabin8 to the centrosome is required for localized activation of Rab8 near the centrosome during ciliary membrane assembly.
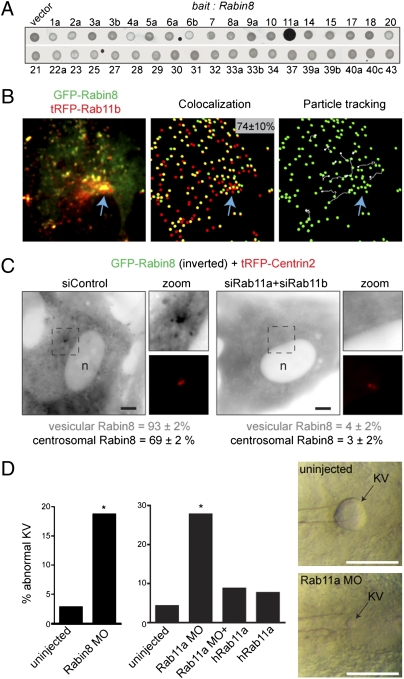
Rab11 Is Required for Rabin8 Vesicle Association and Centrosome Trafficking.
It was reported recently that Rab11-GTP binds the Rabin8 COOH-terminal domain (10). We have confirmed these observations (Fig. S3 A and B) and further show that this interaction is specific for Rab11-GTP in a yeast two-hybrid screen with 37 Rabs (Fig. 3A). Rab11 regulates endosome recycling and is a resident of the pericentriolar endosome recycling compartment (ERC), suggesting that Rab11 may direct Rabin8 to the centrosome. As predicted, Rabin8 was detected on Rab11 vesicles near the cilia base (Fig. S3C). To investigate specifically Rab11 and Rabin8 trafficking after serum withdrawal, GFP-Rabin8 and tRFP-Rab11b were imaged simultaneously in live cells. We found that 74% of GFP-Rabin8 vesicles contained tRFP-Rab11b (Fig. 3B). Vesicle-tracking analysis determined that ∼50% of these “double-positive” vesicles trafficked to the pericentriolar region of the cell. Rab11 and Rab8 have been reported to localize to the Golgi; however, GFP-Rabin8 vesicles did not colocalize with the Golgi marker GM130 (Fig. S3D). In contrast, centrosomal GFP-Rabin8 colocalized with fluorescent transferrin within 30 min of its internalization (Fig. S3E) but was not detected in transferrin-positive early endosomes (5 min uptake). Next, we tested if Rab11 was required for Rabin8 centrosomal trafficking. Simultaneous depletion of Rab11 isoforms (Fig. S3F) dramatically blocked Rabin8 vesicle association and centrosomal accumulation (Fig. 3C). Together these results suggest that vesicular GFP-Rabin8 is associated with the post-Golgi Rab11-ERC. Thus, Rabin8 is recruited by Rab11 after serum starvation for transport to the pericentriolar compartment.
Fig. 3.
Rab11 is required for Rabin8 vesicle recruitment and centrosomal trafficking and is important for ciliated KV formation in zebrafish. (A) Rabin8 specifically interacts with the GTP-locked form of Rab11a (Q70L) by yeast two-hybrid analysis. Rabin8 was screened against a library of 37 constitutively active (“GTP-locked”) Rabs. One isoform for most of the 60 known Rabs was included (exceptions were Rab24, -26, -35, -36, and -38). (B) GFP-Rabin8 and tRFP-Rab11b vesicles colocalize and are transported to the centrosome. (Left) RPE GFP-Rabin8 cells transiently expressing tRFP-Rab11b (48 h) were serum starved for 1 h and imaged by live dual-view SDC microscopy. (Center) In five cells analyzed, 74 ± 10% of GFP-Rabin8 vesicles (green spheres) colocalized with tRFP-Rab11b structures (red spheres). (Right) Particle-tracking software was used to visualize the trajectory (shown in white) of Rab11b-Rabin8 double-positive vesicles. Blue arrows mark the pericentriolar region. (C) Rab11 is required for Rabin8 trafficking. Micrographs show representative siRNA-treated RPE GFP-Rabin8 cells expressing tRFP-Centrin2 that were serum-starved for 1 h. GFP-Rabin8 vesicle recruitment and centrosomal accumulation was scored in live cells (n = 50). Representative results from three independent experiments are shown. (Scale bar: 5 μm.) (D) Rabin8 and Rab11a knockdown caused defects in KV formation in zebrafish. Embryos were untreated or injected with 5 ng Rabin8 MO and 2.5 ng Rab11a MO as previously described (4). (Right) Micrographs show KVs from 8–10 somite embryos. (Left) Rabin8 MO caused KV abnormalities in 19% of embryos (29/155; P < 0.0001), compared with >4% of control embryos (3/108). (Center) Rab11 MO caused KV abnormalities in 27% (38/137; P < 0.0001) of embryos, compared with >4% of control embryos (3/71). Coinjection of hRab11a mRNA rescued the phenotype (9%, 11/126) in Rab11a MO-treated embryos (Fig. S3 J and K).
Rab11 Is Required for Ciliogenesis and Is Associated with the BBS Pathway in Zebrafish.
Knödler et al. (10) reported that Rab11-depleted RPE cells have shorter cilia. Consistent with this proposed role in ciliogenesis, we find that Rab11a plus Rab11b siRNA depletion blocked cilia growth in RPE GFP-Rab8a cells and in parental RPE cells (Fig. S3 G and H). These results support a model wherein serum-dependent, Rab11-dependent transport of Rabin8 to the centrosome is required to activate Rab8 locally for ciliary membrane biogenesis.
Recently it was shown that Rab11 promotes Rabin8 binding to BBS1 (10). To examine further Rab11–Rabin8 associations in ciliogenesis and the connections to the BBS pathway, we tested the effects of Rabin8 and Rab11 depletion on BBS-dependent functions in zebrafish. BBS1 morpholino oligonucleotide (MO) knockdown or human Rab8a dominant negative expression causes abnormalities in ciliated Kupffer vesicle (KV) morphogenesis in zebrafish embryos and delays melanosome retraction (3, 4). Rabin8 MO injection caused an approximately sixfold increase in KV abnormalities compared with uninjected embryos (Fig. 3D) and caused delays in melanosome retraction (Fig. S3I). Expression analysis indicated that Rab11a is present during KV formation, whereas Rab11b and a Rab11a-like isoform (86% identical) were poorly expressed (Fig. S3J). KV abnormalities were six times more frequent in embryos injected with Rab11a morpholinos than in uninjected embryos, an effect rescued by coinjection of human Rab11a mRNA (Fig. 3D). Similar results were observed following dominant negative human Rab11a S25N expression (Fig. S3K). Knockdown of Rab11a also caused delays in the retraction of melanosomes (Fig. S3L), an effect that could be rescued by expression of Rab11a. Thus, Rabin8- and Rab11a-depleted zebrafish embryos display characteristic bbs phenotypes, probably linked to defects in Rabin8 activation of Rab8 membrane trafficking.
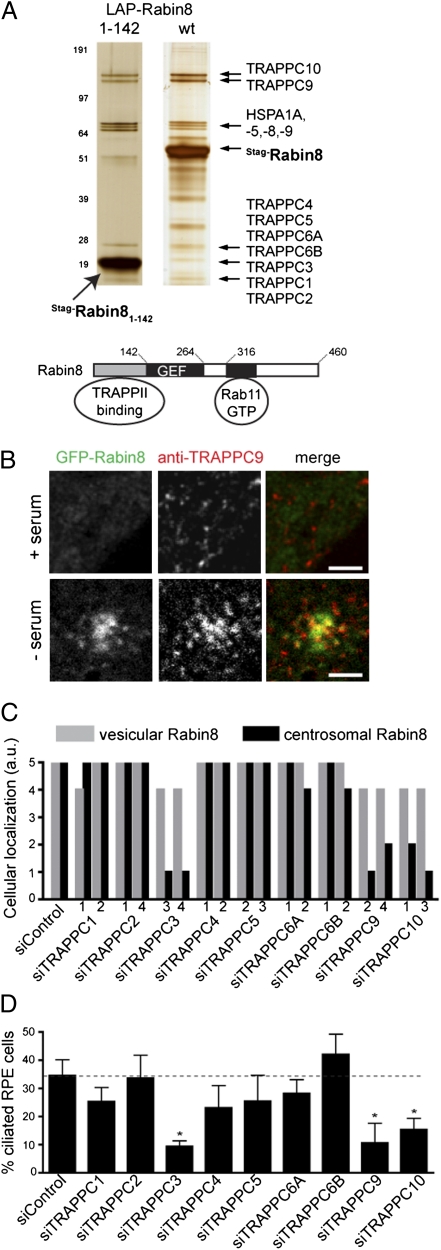
TRAPPII Complex Targets Rabin8 to the Centrosome for Primary Cilia Assembly.
Because Rabin8 vesicular transport to the centrosome precedes ciliary membrane assembly, we sought to understand better the regulation of Rabin8 trafficking. We used tandem affinity purification and mass spectrometry to identify additional Rabin8-associated proteins from 293 Trex cells and IMCD3 cells (Table S1). Silver staining of LAP-tagged Rabin8 complexes showed a distinct set of protein species. Rab11 was not detected by mass spectrometry, because we did not control for the nucleotide-bound state of the Rab (Fig. S3B). BBS1 and BBS7 were weakly detected by mass spectrometry, suggesting that BBSome interactions are transient. Most interesting was the identification of all the known components of the mammalian TRAPPII complex: TRAPPC1–5, -C6A and C6B, -C9, and -C10 (Fig. 4A and Table S1). Peptide coverage for each TRAPPC protein was generally high (46–92% in 293 Trex cells), indicating a high-confidence interaction. GFP-Rabin8 binding to TRAPPII complex subunits also was identified specifically in RPE cells (Fig. S4A). The mammalian TRAPPII complex has been shown to function as a GEF for Rab1 and to tether coat protein I (COPI)-coated vesicles to early Golgi membranes (13). We determined that TRAPPC proteins are in a high molecular weight protein complex (∼400–700 kDa) that partially cofractionates with GFP-Rabin8 (Fig. S4B). Unlike Rab8 and Rab11, the TRAPPII complex specifically bound to the NH2-terminal region of Rabin8 (Fig. 4A and Fig. S4C). Interestingly TRAPPC9 accumulated at the centrosome with GFP-Rabin8 after serum withdrawal (Fig. 4B and Fig. S4D) but was not detected at the centrosome when serum was present. Similarly, tRFP-Rabin8 colocalized with TRAPPC10-GFP on vesicular structures after serum withdrawal (Fig. S4E). The Rabin8–TRAPPII complex association is not dependent on serum starvation (Fig. S4F). Together these results suggested that the TRAPPII complex may be involved in Rabin8 vesicular trafficking.
Fig. 4.
The TRAPPII complex associates with the Rabin8 NH2-terminal domain and is required for Rabin8 centrosomal targeting and primary cilium assembly. (A) LAP-tag purification of Rabin8-associated proteins. (Upper) LAP-Rabin8 and LAP-Rabin81–142 were purified by tandem affinity from 293Trex cells, and the eluted proteins were analyzed by SDS/PAGE (4–12% gradient) and silver stained; then 14 equally spaced gel slices were cut. TRAPPC components had high-percentage peptide coverage in gel slices analyzed by LC-MS/MS corresponding to their predicted molecular weight (Table S1). (Lower) Rabin8-binding domains important for centrosomal vesicular trafficking. (B) TRAPPC9 colocalizes with GFP-Rabin8 on centrosomal vesicles after serum starvation. Micrographs show RPE GFP-Rabin8 cells grown with or without serum for 60 min and fixed and stained with anti-TRAPPC9 antibodies before confocal laser scanning microscopy. (Whole-cell images are shown in Fig. S4E.) (Scale bars: 2 μm.) (C) TRAPPC3, -C9, and -C10 are required for Rabin8 centrosome trafficking. RPE GFP-Rabin8 cells expressing tRFP-Centrin2 were treated with two different ablating TRAPPC protein siRNAs for 72 h (knockdown efficiency is shown in Table S2) followed by serum starvation for 1 h. GFP-Rabin8 subcellular localization was scored in live cells (n = 50) by comparing siRab11a+siRab11b-treated cells (score = 0; Fig. 3C) with siControl-treated cells (score = 5). Results from three independent experiments are shown (Fig. S4H). (D) TRAPPC3, -C9, and -C10 are required for ciliation. RPE cells were treated with siRNAs as in C, serum starved 24 h, and stained with Actub and pericentrin antibodies (n = 75 cells; P < 0.0001). Representative data from three independent experiments are shown.
Consequently, we tested whether TRAPPC proteins are important for Rabin8 transport and ciliation by RNAi (Table S2). We were unable to confirm that all the TRAPPII subunits were strongly depleted at the protein level; however, TRAPPC4 was eliminated almost completely, and TRAPPC2 levels correlated with mRNA levels (Fig. S4G and Table S2). In serum-starved cells, knockdown of TRAPPC proteins did not show substantial effect on Rabin8 recruitment to vesicles (Fig. 4C), compared with Rab11 ablation (Fig. 3C). However, Rabin8 centrosome localization was reduced dramatically in cells depleted of TRAPPC3, TRAPPC9, or TRAPPC10; depletion of the other TRAPPC proteins had negligible effects (Fig. 4C and Fig. S4H). We next tested whether the TRAPPII complex was important for cilia formation and found that knockdown of the three TRAPPC proteins required for Rabin8 centrosomal targeting also impaired cilia formation by more than 50%, whereas knockdown of the other TRAPPC proteins did not (Fig. 4D). Thus, these results suggest that only specific subunits of the TRAPPII complex are important for Rabin8 trafficking before ciliogenesis.
Discussion
The discovery that genes mutated in BBS are linked to Rabin8 and its small GTPase substrate Rab8 brought attention to the importance of membrane trafficking in ciliary function. Here, we establish a detailed timeline of the membrane transport steps leading to the formation of the primary cilium. We find that serum withdrawal regulates Rabin8 centrosomal trafficking to organize Rab8-dependent ciliary membrane formation. We propose that Rab11 and the TRAPPII complex provide polarized vesicular transport of Rabin8 to the centrosome for the localized activation of Rab8 (Fig. S5).
Sorokin (5) first reported the ultrastructure of the developing ciliary membrane. We show that Rab8 is localized to a focal site near the mother centriole/basal body after serum withdrawal, consistent with Sorokin's description of the ciliary vesicle. Furthermore, extensions of Rab8 structures from this site appear to parallel Sorokin's stages in ciliary membrane sheath and primary cilium membrane formation. By our estimation, this entire process takes ∼100 min. Remarkably, these events are organized by upstream recruitment of Rabin8 to Rab11 vesicles, resulting in Rabin8 targeting to the centrosome after serum withdrawal. Disruption of Rabin8 vesicle transport blocked cilia formation, with Rabin8 centrosomal trafficking occurring regardless of whether cilia formed. Interestingly, after ciliogenesis, Rabin8 vesicular accumulation at the centrosome was ablated dramatically coincident with strong reductions in Rab8 ciliary transport. Because Rab8 overexpression increases primary cilia length (3), these findings may suggest that regulation of Rabin8 centrosomal trafficking is important to control primary cilium structure and signaling. Although how the BBSome association with Rabin8 functions during ciliogenesis is still unclear, the requirement for Rabin8 and Rab11 in zebrafish KV formation and melanosome retraction suggests that Rab11 membrane trafficking of Rabin8 is associated with the BBS pathway.
We used mass spectrometry to identify Rabin8-interacting proteins associated with its ciliary function, identifying all the components of the mammalian TRAPPII complex. Our finding that TRAPPC3, -C9, and -C10 are required for Rabin8 centrosome targeting and ciliogenesis suggests that the TRAPPII complex is associated with Rab11-dependent membrane-trafficking pathways. Interestingly, the TRAPPII complex has been linked to Rab11 functioning during cytokinesis in Drosophila (14). Furthermore, the yeast TRAPPII complex has been linked previously to the ERC, because deletion of Trs130/TRAPPC10 blocked receptor recycling (15). Because Rabin8–TRAPPII complex binding is distinct from the Rab11 binding domain (Fig. 4A), is not important for Rabin8 membrane association, was not enhanced by serum starvation or blocked by Rab11a/b RNAi (Fig. S4I), our results suggest a function for the TRAPPII complex, together with Rab11, in promoting Rabin8 trafficking to the centrosome. One possibility is that the TRAPPII complex tethers Rabin8-containing Rab11 vesicles to the pericentriolar ERC, similar to its proposed function in endoplasmic reticulum –Golgi trafficking (16). Alternatively, the striking reduction in vesicular Rabin8 near the centrosome, but not cellular vesicular Rabin8, could indicate a role for TRAPPII complex subunits in the microtubule-dependent transport of Rabin8 vesicles before ciliogenesis. Unfortunately, we were not able to test this model fully because of photobleaching effects on weakly fluorescent GFP-Rabin8 structures. It is not clear if Rabin8 is associated with TRAPPII–Golgi functions or Rab1 GEF activity. However, because TRAPPC proteins were depleted only partially from cell lysates after immunoprecipitation of GFP-Rabin8 (Fig. S4A), and the Rabin8-TRAPPII complex is detected on the Rab11-ERC and not the Golgi, TRAPPII–Rabin8 binding may be distinct from TRAPPII–Golgi associations. Furthermore, the requirement for only some TRAPPII complex proteins in Rabin8 trafficking and ciliogenesis may be important in specifying functions outside the Golgi.
Rabin8 joins a growing cast of Rab11 effectors (17), and the requirement for Rab11 in cilia formation adds another role to its already known functions (receptor recycling, exocytosis, and cytokinesis). The dramatic and rapid redistribution of Rabin8 to the centrosome after serum removal suggests that Rab11 effector binding is sensitive to the serum switch that triggers assembly of the primary cilium. Although Rab11 simply may deliver Rabin8 to the centrosome to activate Rab8, it also is possible that Rab11 vesicles are converted into the Rab8 ciliary compartment (Fig. S5), similar to the Rab5–Rab7 early endosome to late endosome switch (18). Rab11 vesicles also may deliver other components important for ciliary membrane formation and signaling. The trafficking pathway we have identified to understand Rab8-dependent primary cilium assembly should help us identify factors and associated signaling networks important for the serum switch in RPE cells.
Materials and Methods
Antibodies and Reagents.
Antibodies used were TRAPPC9 (Proteintech), acetylated α-tubulin and γ-tubulin (Sigma), pericentrin (Novus), and GFP (3). HRP-conjugated antibodies used were anti-GFP (Miltenyi Biotech).
Plasmids, cDNAs and Yeast Two-Hybrid Screen.
The following plasmids were used: pEGFP-Rabin8 (2), pG-LAP1, pG-LAP3 NH2-terminal LAP-tagged, and pG-LAP5 COOH-terminal LAP-tagged (19). pDEST-tRFP was generated by removing the LAP-tag from pG-LAP3 and replacing it with the tRFP cassette from the pTag-RFP-C vector (Evrogen). Full-length cDNAs in pENTR/DONR vectors were cloned into Gateway destination vectors by LR Clonase II (Invitrogen). Yeast two-hybrid screening was performed as described (20).
Cell Culture, Transfection, and Quantitative PCR.
RPE-human TERT cell lines were transfected with pG-LAP1-Rab8a, selected (hygromycin; 1 mg/mL), and single-cell cloned. RPE cells stably expressing GFP-Rabin8 (3) were sorted by FACS for GFP. siRNA duplexes were synthesized using Dharmacon siGenome sequences; ON-TARGETplus oligonucleotides were purchased from Dharmacon. Cells were transfected with siRNA duplexes (50 nM) using RNAiMAX (Invitrogen) and were analyzed at 72 h by quantitative RT-PCR to identify effective siRNAs.
Immunofluorescence and Time-Lapse Microscopy.
RPE cells were prepared for indirect immunofluorescence as described (3). GFP-Rab8a cilia localization was observed live by epifluorescence microscopy or Marianas spinning disk confocal (SDC) microscopy (Intelligent Imaging Innovations) at 40× magnification. In fixed cells, cilia were counted after staining with Actub and pericentrin antibodies to mark cilia and the centrosome. Actub-positive cilia were counted by epifluorescence microscopy (eight fields, 40× 1.3 NA oil objective). Simultaneous time-lapse imaging of tRFP and GFP fusions was performed by an SDC microscope fitted with a dual-view device (Optical Insights). GFP-Rabin8 localization was detected by time-lapse epifluorescence microscopy (5–10 frames over 10–20 s, 63× 1.3 NA oil objective, 10 random fields) in an environmental chamber (37 °C, 5% CO2). Image acquisition and analysis used Slidebook 4.2 (Intelligent Imaging Innovations), Axiovision software (Zeiss), IMARIS (Bitplane), and Volocity (Perkin Elmer).
Analysis of Kupffer Vesicle and Melanosome Transport Assay.
Assays were performed as published (4) using an MO against the start site of Rab11a and Rabin8 (Transcript: zgc:110270 ENSDART00000059404). The sequence for Rab11a was MO-GTATTCGTCGTCTCGTGTCCCCAT; the sequence for Rabin8 was MO-TCTTCATACA GCACTCTGAGAAAAC. KVs were scored as abnormal if they were smaller than the diameter of the notochord.
Data Analysis.
Analysis of multiple groups was by one-way ANOVA with Bonferroni's post hoc test. Fisher's exact test was used for KV analysis. Error bars indicate SD unless otherwise indicated.
Acknowledgments
We thank Darryl Nishimura and Qihong Zhang for help with zebrafish bioinformatics. Jagath Junutula provided Rab reagents for the yeast two-hybrid screens. L.M.B. is supported by an Iowa Cardiovascular Center Institutional Research fellowship. This work was supported in part by National Institutes of Health Grants CA112369 (to D.C.S.) and R01 EY011298 and EY017168 (to V.C.S.).
Footnotes
Conflict of interest statement: C.J.W., K.J.W., K.E.E., L.P., C.C., D.S.K., R.H.S., and P.K.J. are employees of Genentech, Inc.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018823108/-/DCSupplemental.
References
- 1.Scholey JM, Anderson KV. Intraflagellar transport and cilium-based signaling. Cell. 2006;125:439–442. doi: 10.1016/j.cell.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Hattula K, Furuhjelm J, Arffman A, Peränen J. A Rab8-specific GDP/GTP exchange factor is involved in actin remodeling and polarized membrane transport. Mol Biol Cell. 2002;13:3268–3280. doi: 10.1091/mbc.E02-03-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nachury MV, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Yen HJ, et al. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer's vesicle cilia function. Hum Mol Genet. 2006;15:667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 5.Sorokin S. Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol. 1962;15:363–377. doi: 10.1083/jcb.15.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshimura S, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. J Cell Biol. 2007;178:363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber LA, et al. Rab8, a small GTPase involved in vesicular traffic between the TGN and the basolateral plasma membrane. J Cell Biol. 1993;123:35–45. doi: 10.1083/jcb.123.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grosshans BL, Ortiz D, Novick P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc Natl Acad Sci USA. 2006;103:11821–11827. doi: 10.1073/pnas.0601617103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz D, Medkova M, Walch-Solimena C, Novick P. Ypt32 recruits the Sec4p guanine nucleotide exchange factor, Sec2p, to secretory vesicles; evidence for a Rab cascade in yeast. J Cell Biol. 2002;157:1005–1015. doi: 10.1083/jcb.200201003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knödler A, et al. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA. 2010;107:6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bryant DM, et al. A molecular network for de novo generation of the apical surface and lumen. Nat Cell Biol. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pazour GJ, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamasaki A, et al. mTrs130 is a component of a mammalian TRAPPII complex, a Rab1 GEF that binds to COPI-coated vesicles. Mol Biol Cell. 2009;20:4205–4215. doi: 10.1091/mbc.E09-05-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robinett CC, Giansanti MG, Gatti M, Fuller MT. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J Cell Sci. 2009;122:4526–4534. doi: 10.1242/jcs.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang CJ, et al. Genetic interactions link ARF1, YPT31/32 and TRS130. Yeast. 2002;19:1075–1086. doi: 10.1002/yea.903. [DOI] [PubMed] [Google Scholar]
- 16.Sacher M, et al. TRAPP, a highly conserved novel complex on the cis-Golgi that mediates vesicle docking and fusion. EMBO J. 1998;17:2494–2503. doi: 10.1093/emboj/17.9.2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon GC, Prekeris R. Mechanisms regulating targeting of recycling endosomes to the cleavage furrow during cytokinesis. Biochem Soc Trans. 2008;36:391–394. doi: 10.1042/BST0360391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 19.Torres JZ, Miller JJ, Jackson PK. High-throughput generation of tagged stable cell lines for proteomic analysis. Proteomics. 2009;9:2888–2891. doi: 10.1002/pmic.200800873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westlake CJ, et al. Identification of Rab11 as a small GTPase binding protein for the Evi5 oncogene. Proc Natl Acad Sci USA. 2007;104:1236–1241. doi: 10.1073/pnas.0610500104. [DOI] [PMC free article] [PubMed] [Google Scholar]