Abstract
The toxicity of mistranslation of serine for alanine appears to be universal, and is prevented in part by the editing activities of alanyl-tRNA synthetases (AlaRSs), which remove serine from mischarged tRNAAla. The problem of serine mistranslation is so acute that free-standing, genome-encoded fragments of the editing domain of AlaRSs are found throughout evolution. These AlaXps are thought to provide functional redundancy of editing. Indeed, archaeal versions rescue the conditional lethality of bacterial cells harboring an editing-inactive AlaRS. In mammals, AlaXps are encoded by a gene that fuses coding sequences of a homolog of the HSP90 cochaperone p23 (p23H) to those of AlaXp, to create p23HAlaXp. Not known is whether this fusion protein, or various potential splice variants, are expressed as editing-proficient proteins in mammalian cells. Here we show that both p23HAlaXp and AlaXp alternative splice variants can be detected as proteins in mammalian cells. The variant that ablated p23H and encoded just AlaXp was active in vitro. In contrast, neither the p23HAlaXp fusion protein, nor the mixture of free p23H with AlaXp, was active. Further experiments in a mammalian cell-based system showed that RNAi-directed suppression of sequences encoding AlaXp led to a serine-sensitive increase in the accumulation of misfolded proteins. The results demonstrate the dependence of mammalian cell homeostasis on AlaXp, and implicate p23H as a cis- and trans-acting regulator of its activity.
Keywords: genetic code, protein synthesis, tRNA misacylation, amino acyl-tRNA synthetases
The potential for mistranslation caused by the confusion of serine for alanine by alanyl-tRNA synthetases (AlaRSs) is universal (1, 2). The consequences of Ala-to-Ser mistranslation include conditional lethality in bacteria (3) and severe neurodegeneration in mice (4). The Ser/Ala confusion is surprising, because serine is larger than alanine and, for other tRNA synthetases, the challenge is to eliminate similar or smaller, but not larger, amino acids from the active site. Among other examples (5–7) are misactivation of valine by IleRS (8–10), glycine by AlaRS (1), valine by LeuRS (11), and serine by ThrRS (12). The misactivated amino acids (in the form of the aminoacyl adenylate or as the mischarged tRNA, such as Val-tRNAIle) are removed by hydrolytic editing, using a second active site (13–15). The misactivation of serine by AlaRS arises from the constraints of a structural design of 3 billion years, which locks the α-amino group of the bound amino acid (such as alanine or serine or glycine) to a universally conserved acidic (carboxylate) residue. However, this carboxylate side chain can also have a serendipitous bifurcated interaction with both the serine OH and its α-amino group (2). Any mutation that removes the bifurcated interaction with serine simultaneously weakens the interaction with alanine, such that the discrimination between alanine and serine is made even worse. Thus, the problematic acidic residue is maintained by selective pressure during evolution.
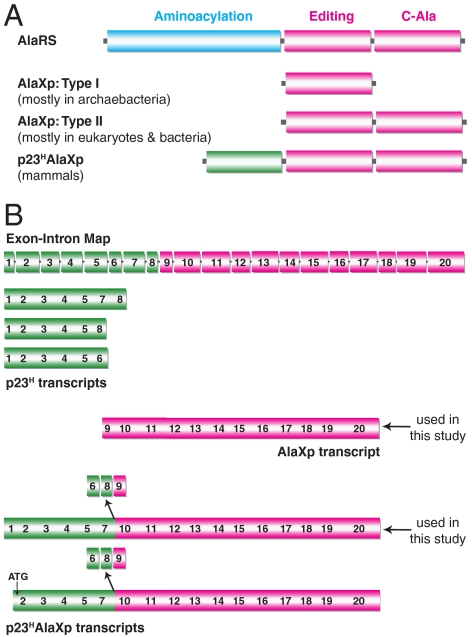
Faced with this dilemma, nature is believed to have solved the serine misactivation problem through the creation of free-standing, genome-encoded AlaXps (16). Although their phylogenetic distribution is limited, free-standing editing domains that are homologous to the editing domains of ThrRS and ProRS have also been identified and investigated (17–19). In contrast, AlaXps are found in all three kingdoms of life (20). Type I AlaXps are found mostly in archaebacteria, whereas type II AlaXps are found mostly in eukaryotes and bacteria. Type II AlaXps have an extra domain at the C-terminus that is homologous to the C-Ala domain of AlaRS (Fig. 1A). This extra domain brings together aminoacylation and editing functions to act on bound tRNAAla (21).
Fig. 1.
Architecture of AlaRS/AlaXp domains and the transcripts of the mouse Aarsd1 gene. (A) Domains of AlaRS, type I and type II AlaXps and the p23HAlaXp fusion protein. Type II AlaXps have the C-Ala like domain in addition to the editing domain. p23HAlaXp has an additional p23-like domain appended to the N-terminus of type II AlaXp. (B) The exon-intron layout of the mouse Aarsd1 gene is depicted with each rectangular box representing an exon and the intermediate lines representing introns (http://vega.sanger.ac.uk/). The alternatively spliced transcripts that are depicted are based on database information. Exons 6, 8, and 9 are absent in p23HAlaXp transcripts.
In contrast to their AlaRS counterparts and to archaeal AlaXps, all of which can catalyze hydrolysis of both Gly-tRNAAla and Ser-tRNAAla (16), eukaryotic AlaXps typically have little or no activity toward Gly-tRNAAla (2). Thus, the challenge of avoiding mistranslation of serine for alanine was so great that a redundant editing activity was needed, presumably to maintain cell viability (3). Indeed, when bacteria are exposed to excess serine, an editing-defective AlaRS cannot sustain cell growth without an AlaXp-encoding gene being expressed (22).
Previous work in the mouse showed that a severe neurodegeneration was caused by a mutation (sti) in the editing domain of AlaRS (4). This mutation caused only a small (2-fold) defect in the catalytic activity for editing in vitro. And yet, this small defect led to degeneration of Purkinje cells in the cerebellum and severe ataxia in the mouse. The phenotype was rescued by a transgene expressing wild-type AlaRS with its full editing activity. Further experiments suggested that the deleterious phenotype was caused by the toxic effects of a tiny amount of serine-for-alanine mistranslation. The extreme sensitivity of the mouse to even a slight defect in hydrolytic editing of Ser-tRNAAla provided a powerful example of the need for redundancy in the activity that corrects serine-for-alanine mistranslation.
Not investigated in those studies in the mouse was the expression of AlaXp or its activity. In mammalian cells, transcripts of AlaXp and of a fusion of AlaXp with a p23 homolog (p23H) have been annotated (http://vega.sanger.ac.uk/). Mammalian p23 is also encoded separately so that, in principle, it is produced in two formats, the actual p23 itself (23) and as p23H (encoded either as a free protein or as fused to AlaXp). The amino acid sequences encoding the two proteins—mouse p23 and p23H—have an identity of 41%. The p23 protein was first discovered as part of the Hsp90 complex. This small protein, which is conserved from yeast to humans, is believed to be a cochaperone in the Hsp90 complex that is needed to suppress denatured protein aggregation and to fold many proteins into their active forms (24).
In this work, we sought to determine whether AlaXp and the fusion designated here as p23HAlaXp can be detected, not only as transcripts that encode each, but as actual expressed proteins. For this analysis, we investigated 15 different mouse tissue samples. After establishing the expression of both AlaXp and p23HAlaXp, we then sought to demonstrate the activity of AlaXp, the role (if any) of p23H in regulating that activity, and the consequences on cell viability of blocking expression of AlaXp. The results demonstrated a significant role for AlaXp in maintaining cellular homeostasis and, in addition, revealed p23H as a strong regulator of the activity of AlaXp.
Results
Detection of AlaXp and p23HAlaXp Transcripts and Expression.
The mouse Aarsd1 gene on chromosome 11 (region 11d) has 20 exons (in an 18.64 kbp region) that collectively encode p23H, AlaXp, and p23HAlaXp (Fig. 1B). Six spliced transcripts have been reported in databases. Three of them do not encode any part of AlaXp. Instead, these transcripts exclusively encode some, but not all, of the 8 exons of p23H. These transcripts include one that skips exon 6, another that skips exons 6 and 7, and a third that terminates after exon 6. A fourth transcript encodes AlaXp (exons 9–20). Finally, the remaining two transcripts are special fusions of coding sequences for p23H with those for AlaXp. One transcript runs from exon 1 to 20, but skips exons 6, 8, and 9. The other skips the same 3 exons, but starts with a methionine within exon 2.
The significance of the aforementioned transcripts, or of the proteins that they may express, has not been investigated heretofore. We set out to determine whether AlaXp and the p23HAlaXp fusion protein were expressed in mammalian cells and, if so, whether either or both of these proteins were active in clearing Ser-tRNAAla. If activity could be detected, we then wanted to see whether this activity was needed for cellular homeostasis. Our thinking was that, if the activity of these proteins was important for cell homeostasis, it would provide support for the need in mammals for functional redundancy of an activity for correcting serine-for-alanine mistranslation.
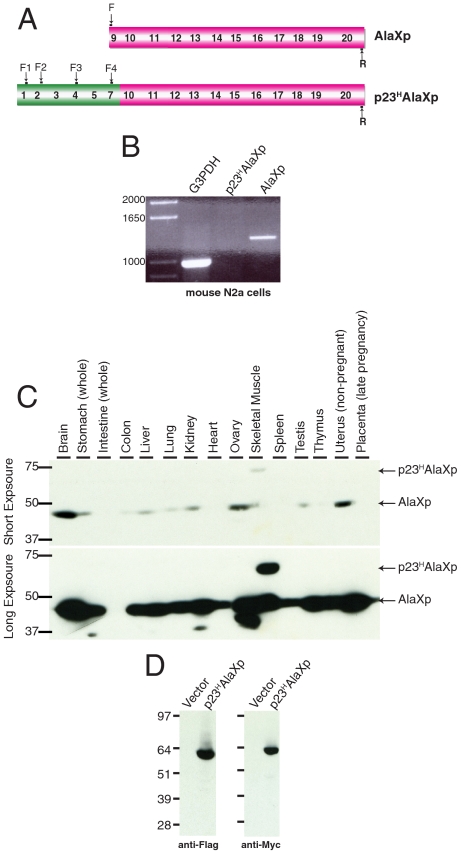
To detect endogenously expressed transcripts of AlaXp, we investigated a mouse cell line. Our goal was not to exhaustively confirm or extend the previous reports of transcripts of AlaXp and p23HAlaXp, but rather to look for representative transcripts, especially ones that encoded AlaXp and that gave evidence for a fusion between p23H and AlaXp. For this purpose, the mouse neuroblastoma N2a cell line was used for RT-PCR analysis. The generated cDNA was used as a template to carry out a standard PCR reaction, using “forward” and “reverse” primers that are displayed in Fig. 2A.
Fig. 2.
AlaXp and a variant are expressed in mouse cells. (A) Schematic representation of primers used for RT-PCR analysis. For the fusion protein, four different forward primers (F1–F4) in the p23H region were designed to ensure the detection of the p23HAlaXp protein. (B) RT-PCR analysis of mRNA obtained from mouse N2a cells confirming the presence of the AlaXp transcript. All four sets of p23HAlaXp primers failed to amplify any product, indicating the absence of this transcript in these cells. Although all 4 sets had the same negative result, only one lane representing p23HAlaXp is shown in the figure for simplicity. (C) Detection of endogenous AlaXp/p23HAlaXp expression in different mouse tissue samples. The lower panel was obtained from a longer exposure of the film. Samples were Western blotted with an anti-AlaXp antibody. (D) Overexpression of recombinant p23HAlaXp protein in mouse N2a cells. The protein was FLAG-tagged at the N-terminus and Myc-tagged at the C-terminus. No cleavage products were detected by either the anti-Flag or anti-Myc antibodies.
These primers were selected in part based on the previous reports of transcripts that were observed (Fig. 1B). Primers for transcripts expressing the G3PDH gene were used as a control. These experiments showed clearly that transcripts encoding AlaXp could be detected in N2a cells, whereas those encoding p23HAlaXp were not detected (Fig. 2B).
To understand the expression pattern of AlaXp and p23HAlaXp more broadly, we tried to detect them in 15 different cell-type (tissue) samples. For this purpose, we used premade filter-blots of proteins transferred from SDS-PAGE gels of mouse tissue lysates. Each lane in these filters contained 75 micrograms of a specific tissue lysate. The blots were probed with a custom-made antibody raised against purified mouse AlaXp protein. (The efficiency of this antibody for detecting mouse AlaXp/p23HAlaXp proteins was confirmed by Western blot analysis using purified proteins). As shown in Fig. 2C, AlaXp was detected in all but one of the 15 different mouse tissues that were tested. The levels varied from tissue to tissue, with brain having the most abundant expression. However, only skeletal muscle lysates displayed a higher molecular weight band that would correspond to the p23HAlaXp fusion.
We considered the formal possibility that all of the AlaXp seen in mouse tissues (Fig. 2C) was not produced solely by alternative splicing, but also by proteolysis in vivo of p23HAlaXp. For this purpose, we cloned a recombinant gene encoding p23HAlaXp into the p3XFlagMycCmv24 vector. This construct adds Flag and Myc tags onto the N- and C-terminus, respectively, of the protein. Therefore, in the event of a cleavage, the p23H and AlaXp portions of the protein can be independently detected, using anti-Flag and anti-Myc antibodies. Mouse N2a cells were transfected with this plasmid and lysed after 24 h. The lysates were subjected to Western blot analysis with anti-Flag and anti-Myc antibodies. As shown in Fig. 2D, only a single band corresponding to p23HAlaXp was detected by both antibodies. This result suggests that, at least in N2a cells, proteolytic cleavage is not a major mechanism for generating AlaXp and p23H.
In Vitro Editing Activity of AlaXp and p23HAlaXp.
To investigate the activity of mouse AlaXp and p23HAlaXp, we created cDNAs that corresponded to specific transcripts seen in our analysis above. These included AlaXp and one of the two transcripts for p23HAlaXp (Fig. 1B). We used the longer transcript that had an additional 18 amino acids (as compared to the one that starts with a methionine within exon 2). [All of the three p23H transcripts depicted also have these same additional 18 amino acids (see Fig. 1B)]. These constructs were then used to express the corresponding recombinant proteins in Escherichia coli. In an attempt to improve the solubility of the p23HAlaXp fusion protein, we created a gene construct that fused the maltose-binding protein (MBP) to the N-terminus of p23HAlaXp. The junction between the two coding sequences encoded a Factor Xa cleavage site. MBP-fused mouse p23HAlaXp was expressed in E. coli, purified on an amylose column, and cleaved by Factor Xa. The liberated p23HAlaXp protein was then purified on a DEAE-Sepharose column. After all of these steps that started with expression in E. coli of a MBP fusion protein, the ability to isolate p23HAlaXp suggested it retained its native form. Far UV CD analysis confirmed that the protein had ordered secondary structure.
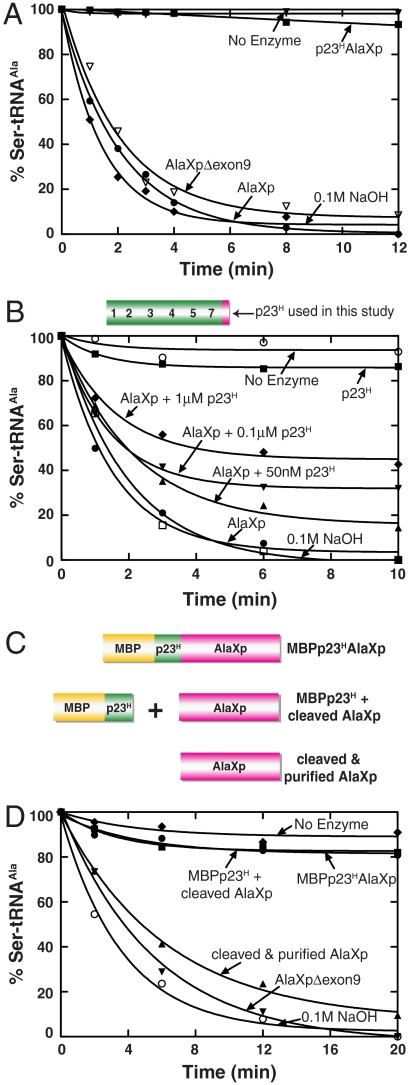
To check the deacylation activity, Ser-tRNAAla was used as a substrate. (This substrate was synthesized by in vitro transcription of the cDNA sequence for E. coli tRNAAla, which was then mischarged with serine using an editing-defective mutant of E. coli AlaRS.) The AlaXp protein displayed robust deacylation activity with mischarged Ser-tRNAAla (Fig. 3A). This editing activity was serine-specific, and did not act on Gly-tRNAAla (2).
Fig. 3.
Deacylation of AlaXp is inhibited by the presence of p23H both in cis and trans. (A) Deacylation of Ser-tRNAAla by AlaXp, p23HAlaXp and AlaXp(Δexon9). Symbols represent hydrolysis activity of the different proteins (each 100 nM, with 5 μM Ser-tRNAAla) as follows; AlaXp (●), p23HAlaXp (▪), AlaXp(Δexon9) (▿), no enzyme (▾), 0.1 M NaOH (♦). The experiment was done three times. To avoid crowding of the data, error bars are omitted. Typical variation from experiment to experiment was 5–10%. The data shown are from one representative experiment. (B) Inhibition of AlaXp editing by p23H in trans. The p23H construct includes 21 amino acids of exon 10. Increasing concentrations of p23H were added to 100 nM of AlaXp and 5 μM of Ser-tRNAAla. Symbols are as follows; AlaXp (●), p23H (▪), AlaXp + 50 nM p23H (▴), AlaXp + 100 nM p23H (▾), AlaXp + 1 μM p23H (♦), no enzyme (o), 0.1 M NaOH (□). The experiment was done three times. To avoid crowding of the data, error bars are omitted. Typical variation from experiment to experiment was 5–15%. The data shown are from one representative experiment. (C) Schematic representation of constructs used in Fig. 3D. These include MBPp23HAlaXp, MBPp23H, and AlaXp (obtained from enterokinase cleavage of MBPp23HAlaXp, but not subjected to further separation), and AlaXp (obtained from separation of the two components of cleaved MBPp23HAlaXp. (D) AlaXp released from cleavage of N-terminal MBPp23H is active for deacylation only in the absence of MBPp23H. Symbols represent hydrolysis activity (of 5 μM Ser-tRNAAla) as follows; purified 100 nM AlaXp(Δexon9) (▾), 100 nM MBPp23HAlaXp (●), 200 nM MBPp23H + AlaXp (▪), 100 nM cleaved AlaXp (▴), no enzyme (♦), 0.1 M NaOH (o). The experiment was done three times. To avoid crowding of the data, error bars are omitted. Typical variation from experiment to experiment was 5–10%. The data shown are from one representative experiment.
In contrast, the p23HAlaXp fusion protein was inactive in editing Ser-tRNAAla. The p23HAlaXp fusion lacks exon 9, which is retained in the alternatively spliced version of AlaXp, and was therefore present in the recombinant constructs of AlaXp that we made. This exon codes for 13 amino acids at the N-terminus of AlaXp. To investigate if the lack of editing of p23HAlaXp was due to the absence of exon 9, we made an additional AlaXp recombinant construct lacking this exon. However, this AlaXp(Δexon9) protein was active in deacylating mischarged Ser-tRNAAla (Fig. 3A).
These results raise the possibility that p23H is an inhibitor of the deacylation activity of AlaXp. This possibility was further explored using an engineered p23H encompassing the fusion junction, i.e. having exons 1–5, 7, and 21 of 44 amino acids of exon 10 (Fig. 3B). When increasing concentrations of this protein were added to AlaXp in trans, the editing activity of AlaXp was systematically reduced (Fig. 3B). (The apparent KI for inhibition was about 40 nM.) To further establish this point, we inserted an enterokinase cleavage site between p23H and AlaXp in the MBPp23HAlaXp construct, between exons 7 and 10 (Fig. 3C). A His tag was also inserted at the C-terminus of this construct. When cleaved with the above protease, two bands could be detected on an SDS gel, one corresponding to MBPp23H and the other corresponding to AlaXp(Δexon9). As mentioned earlier, MBPp23HAlaXp displayed no editing activity. The cleavage product mixture comprised of MBPp23H and AlaXp(Δexon9) also lacked editing. However, when AlaXp(Δexon9) was separated from this mixture using a Ni-NTA column, it displayed robust editing activity comparable to purified AlaXp(Δexon9) (Fig. 3D). Finally, to rule out the possibility of the MBP protein itself interfering with AlaXp editing, we made a MBP-AlaXp fusion construct. This protein displayed hydrolytic activity comparable to AlaXp, thus highlighting the specificity of p23H as an AlaXp inhibitor.
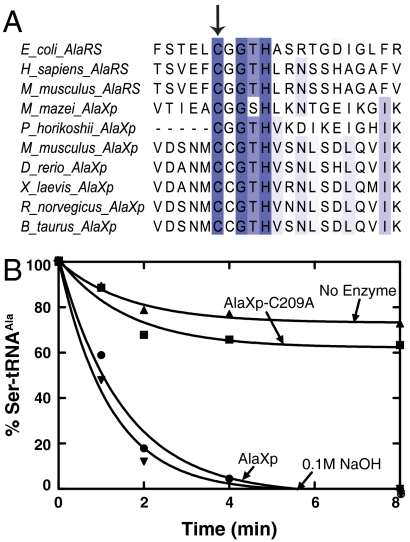
Earlier work with E. coli AlaRS enzyme has highlighted the importance of a specific cysteine residue (C666A) in the active site of the editing domain in clearing mischarged Ser-tRNAAla (3). This cysteine residue is part of a zinc binding motif CxxxH conserved in all AlaRS enzymes. To understand if this feature is common to AlaRS and AlaXp enzymes, we performed a multiple sequence alignment of these enzymes. As shown in Fig. 4A, the conservation of this cysteine is indeed a feature shared by both AlaRS and AlaXps. Mutation of this cysteine to alanine (C209A) in the mouse AlaXp enzyme resulted in a significant loss of editing activity, similar to that observed for AlaRS (Fig. 4B). Thus, the active site architecture of AlaXp and AlaRS editing domains seems well conserved.
Fig. 4.
Analysis of a predicted active-site mutation in AlaXp. (A) Sequence alignment of AlaRS and AlaXp enzymes. The conserved cysteine important for editing in AlaRS is indicated with an arrow. (B) Deacylation of AlaXp C209A mutant showing significant loss in activity. Symbols represent hydrolysis activity of the different proteins (each 100 μM, with 5 μM Ser-tRNAAla) as follows; AlaXp (●), AlaXp-C209A (▪), no enzyme (▴), and 0.1 M NaOH (▾). The experiment was done three times. To avoid crowding of the data, error bars are omitted. Typical variation from experiment to experiment was 5–10%. The data shown are from one representative experiment.
Functional Significance of AlaXp Editing in Vivo.
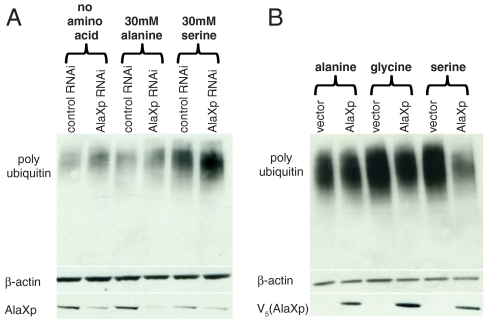
Next, we turned to the question of the functional significance of endogenous AlaXp-editing in vivo. To directly test the significance in a cell-based system, we used siRNA directed against mouse AlaXp to knockdown its expression in mouse N2a cells. At 24 h after transfection, the cells were challenged by addition of 30 mM alanine or serine to the media, with media containing no additional amino acids as a control. The cells were then allowed to grow for an additional 24 h. The lysate was subjected to Western blot analysis, and probed for the presence of misfolded proteins using the anti-polyubiquitin polyclonal antibody. As shown in Fig. 5A, knockdown of endogenous AlaXp caused a small increase in the accumulation of misfolded proteins even without the addition of more amino acids to the media. Upon addition of 30 mM serine, a large increase in the accumulation of misfolded proteins was readily observed. This effect is more obvious in samples with the knockdown of endogenous AlaXp. This result suggested that the lack of editing by endogenous AlaXp causes accumulation of misfolded proteins. In contrast, addition of equivalent amounts (30 mM) of alanine in the medium did not cause accumulation of misfolded proteins beyond what was seen in the control.
Fig. 5.
Significance of AlaXp editing in vivo. (A) Serine sensitivity and accumulation of misfolded proteins in cell lysates of mouse N2a cells transfected with control or AlaXp siRNA, followed by a challenge with high concentrations of specific amino acids. Western blotting for β-actin served as a loading control. Knockdown was determined by employing an anti-AlaXp antibody. The blots were probed with an antipolyubiquitin antibody. (B) Rescue of serine sensitivity by exogenous AlaXp. N2a cells transfected with either empty vector or AlaXp were challenged with serine (or alanine or glycine) and lysates probed for polyubiquitinated proteins after 48 h of growth. Overexpressed AlaXp protein was detected using an anti-V5 antibody.
Overexpression of exogenous AlaXp rescued a similar phenotype caused by high serine concentrations in the growth medium of mouse N2a neuroblastoma cells. Cells were transfected with either empty vector or AlaXp plasmids, and 50 mM serine was added to the medium 24 h after transfection. Geneticin (500 μg/mL) was also included in the medium to select for positive transformants. After 48 h of growth, cells were harvested and the lysate subjected to Western blot analysis. As shown in Fig. 5B, addition of serine to the medium led to the accumulation of misfolded proteins, and overexpression of exogenous AlaXp rescued this phenomenon. Because the polyubiquitination profile remained unchanged (with or without AlaXp overexpression) when similar concentrations (50 mM) of alanine or glycine were used, this rescue seemed serine-specific. Together these results demonstrate the role of AlaXp as a vital checkpoint in maintaining cellular homeostasis by protecting cells from serine toxicity.
Discussion
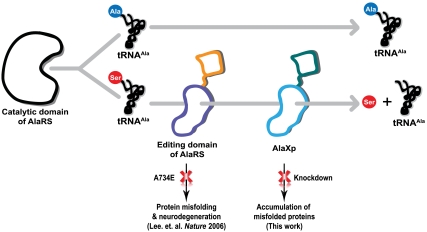
The serine paradox reflects an inherent problem faced by the historical architecture of the protein synthesis apparatus and the compromises that arose to solve this challenge (2). It was proposed earlier that multiple checkpoints are necessary to deal with this problem (25). These include having a redundant hydrolytic activity for clearance of Ser-tRNAAla encoded by the AlaRS editing domain and by free-standing AlaXp (Fig. 6). The sensitivity of organismal homeostasis to even a small defect in the editing activity of AlaRS was demonstrated by the accumulation of misfolded proteins and concomitant neurodegeneration in the mouse (4). We inferred from this result that the redundant activity of AlaXp was not sufficient to compensate for the small editing defect in murine AlaRS. Here we determined that, conversely, in mammalian cells, fully active editing activity from AlaRS was also not sufficient to maintain cell homeostasis.
Fig. 6.
Nature’s solution to the serine paradox, and the consequences of defective clearance of mischarged tRNAAla. Two checkpoints (i.e., the editing domain of AlaRS and freestanding AlaXp, are employed to ensure the hydrolysis of mischarged Ser-tRNAAla, to deal with the “serine paradox.” Both these checkpoints are important, and a defect in either could lead to mistranslation and accumulation of misfolded proteins in the cell.
Interestingly, the gene coding for AlaXp is fused to a p23 homolog. In this work we showed that the unfused AlaXp protein is expressed in almost all tissues of the mouse that were tested. However, we could detect a higher molecular weight band of AlaXp (corresponding to p23HAlaXp) in one tissue sample (skeletal muscle). Possibly, the p23HAlaXp fusion protein is expressed in a regulated fashion, and that the expression is turned off/on under specific conditions. The available information in databases implied that alternative splicing is one mechanism for the generation of AlaXp and p23HAlaXp proteins. In addition, natural proteolysis of p23HAlaXp may also liberate AlaXp under specific conditions. Possibly the p23HAlaXp fusion is designed to provide posttranscriptional temporal, “on-call” regulation of the activity of AlaXp, by using alternative splicing and proteolysis to control the supply of the active form of this free-standing editing domain. In addition to having a regulatory function, the p23HAlaXp fusion may also have a unique function unrelated to editing.
From an evolutionary perspective, it is of interest to note that p23 is a cochaperone that interacts with HSP90 to assist protein folding. At the same time, our work here showed that AlaXp is needed to suppress the appearance of unfolded proteins that, in turn, triggers the unfolded protein response. Thus, from a functional perspective, there is a connection between AlaXp and p23. Whether this functional connection was part of the selective pressure to generate the p23HAlaXp fusion protein remains to be determined.
Materials and Methods
Plasmid Construction and Protein Purification.
The plasmid for bacterial expression of mouse AlaXp (pH8GW/AlaXp) has been described previously (25). The point mutation, C209A was introduced into this vector by site directed mutagenesis. p23HAlaXp was cloned into pMALC4x vector (NEB) by PCR of the targeted sequence. For the experiment in Fig. 4D, an enterokinase site was introduced between p23H and AlaXp (exons 7 and 10), and a C-terminal His6 tag added to the above construct by site directed mutagenesis. AlaXp(Δexon9) was generated by replacing the first 13 amino acids of AlaXp with methionine, by PCR amplification and cloning into a pET28a vector (Novagen). Engineered p23H was derived from p23HAlaXp by introducing a stop codon after amino acid 21 of exon 10, by PCR amplification and cloning into a pDEST17 vector (Invitrogen). For mammalian expression AlaXp was cloned into the pcDNA3.1n/V5-DEST vector (Invitrogen) by PCR amplification. p23HAlaXp was cloned into the p3XFLAG-myc-CMV24 (Sigma) to generate a construct with N and C-terminal FLAG and Myc tags respectively.
Expression and purification of His6- tagged proteins was performed as previously described (25). MBP fusions were purified on an amylose column according to the manufacture’s protocol (NEB).
RT-PCR, Cell Culture, and Western Blot Analysis.
Culture and maintenance of mouse N2a cells was carried out as described previously (26). For RT-PCR analysis, cells were grown up to ∼90% confluence in 10 cm plates, and total RNA was isolated with the RNeasy kit (Qiagen) according to manufacturer’s protocol. This RNA was then used to make cDNA with oligo (dT)18 primers using the Advantage RT-for-PCR Kit (Clontech) according to manufacturer’s protocol. Primers used for amplification of AlaXp were Forward; 5′-GGCGTTCCTGTGTCAGCG-3′, and Reverse; 5′-CACTCCTCAGCACTCTGTGTGC-3′. For the p23HAlaXp fusion, the reverse primer was the same as above, and four different forward primers (all in the p23H region) were used, one at the junction of exon 1 and 2; 5′-GGAACGGCAGCCTGCC-3′, one in exon 2; 5′-CGTCAGTGTGCTCATTGAGGACC-3′, one in exon 4; 5′-GGCCTAGGCTCACAAAGGAGG-3′, and one in exon 7; 5′-GGCCTAGGCTCACAAAGGAGG-3′. Primers for the G3PDH gene were used as a control.
For Fig. 2C, premade mouse tissue filter blots were purchased from Zyagen, (catalog number MW-MT1). Western analysis was carried out using polyclonal anti-AlaXp antibody. For Fig. 2D, N2a cells were transfected with p3XFLAG-myc-CMV24/AlaXp plasmid using Lipofectamine 2000 (Invitrogen). Following 24 h of transfection cell lysates were extracted using cell lysis buffer (9803, Cell Signaling). Protease inhibitor cocktail was not added to this buffer.
Knockdown of AlaXp was performed by transfecting N2a cells with siRNA purchased from Dharmacon (ON-TARGET plus SMARTpool, L-053178). Nonspecific siRNAs (D-001210) were used a control. Twenty-four hours after transfection, 30 mM of glycine, or serine were added to the culture medium and incubated for an additional 24 h. Cell lysates were obtained using the above lysis buffer containing protease inhibitor cocktail. Western analysis was performed using the following antibodies; anti-polyubiquitin (PW8805, Enzo Life Sciences), anti-β-actin (ab8227, Abcam) and polyclonal anti-AlaXp.
For overexpression (Fig. 5B), pcDNA3.1n/V5-DEST plasmids (empty vector or with AlaXp construct), were transfected into N2a cells using Lipofectamine 2000. Twenty-four hours after transfection, 50 mM alanine, glycine or serine was added to the medium and cells were allowed to grow for an additional 48 h. Geneticin (500 μg/mL) was also included in the medium. Cell lysates were then extracted using the lysis buffer containing protease inhibitor cocktail. Western analysis was performed using the following antibodies; anti-polyubiquitin, anti-β-actin, and anti-V5 (R96025, Invitrogen).
Deacylation Assay.
E. coli tRNAAla(UGC) was prepared by in vitro transcription as described previously (3). Misacylated [3H]Ser-tRNAAla was produced using E. coli AlaRS C666A/Q584H as described previously (3). [3H]Ser-tRNAAla (5 μM) was incubated at room temperature with 100 nM of purified proteins in assay buffer (50 mM Hepes (pH 7.5), 20 mM KCl, 2 mM DTT and 10 mM MgCl2) in 96-well plates as described previously (27). Liberated [3H]Ser was collected in 96-well flexible plates, and radioactive counts measured on a MicroBeta plate reader. For Fig. 3B, 100 nM AlaXp was incubated with 50, 100, or 1,000 nM p23H before starting the reaction. For Fig. 3D, AlaXp released from enterokinase cleavage (before and after separation from MBPp23H) was run on an SDS gel and concentrations adjusted with 100 μM AlaXp(Δexon9) as a reference.
Acknowledgments.
We thank Professors Susan Martinis (University of Illinois) and Karin Musier-Forsyth (Ohio State University), and Dr. Mom Das (Ohio State University) for helpful comments on the manuscript. This work was supported by Grant GM23562 and a fellowship from the National Foundation for Cancer Research.
Footnotes
The authors declare no conflict of interest.
References
- 1.Tsui WC, Fersht AR. Probing the principles of amino acid selection using the alanyl-tRNA synthetase from Escherichia coli. Nucleic Acids Res. 1981;9(18):4627–4637. doi: 10.1093/nar/9.18.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo M, et al. Paradox of mistranslation of serine for alanine caused by AlaRS recognition dilemma. Nature. 2009;462(7274):808–812. doi: 10.1038/nature08612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beebe K, Ribas De Pouplana L, Schimmel P. Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 2003;22(3):668–675. doi: 10.1093/emboj/cdg065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JW, et al. Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature. 2006;443(7107):50–55. doi: 10.1038/nature05096. [DOI] [PubMed] [Google Scholar]
- 5.Jakubowski H, Goldman E. Editing of errors in selection of amino acids for protein synthesis. Microbiol Rev. 1992;56(3):412–429. doi: 10.1128/mr.56.3.412-429.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibba M, Soll D. Quality control mechanisms during translation. Science. 1999;286(5446):1893–1897. doi: 10.1126/science.286.5446.1893. [DOI] [PubMed] [Google Scholar]
- 7.Martinis SA, Boniecki MT. The balance between pre- and post-transfer editing in tRNA synthetases. FEBS Lett. 2010;584(2):455–459. doi: 10.1016/j.febslet.2009.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norris AT, Berg P. Mechanism of aminoacyl RNA synthesis: Studies with isolated aminoacyl adenylate complexes of isoleucyl RNA synthetase. Proc Natl Acad Sci USA. 1964;52:330–337. doi: 10.1073/pnas.52.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eldred EW, Schimmel PR. Rapid deacylation by isoleucyl transfer ribonucleic acid synthetase of isoleucine-specific transfer ribonucleic acid aminoacylated with valine. J Biol Chem. 1972;247(9):2961–2964. [PubMed] [Google Scholar]
- 10.Fersht AR. Editing mechanisms in protein synthesis. Rejection of valine by the isoleucyl-tRNA synthetase. Biochemistry. 1977;16(5):1025–1030. doi: 10.1021/bi00624a034. [DOI] [PubMed] [Google Scholar]
- 11.Mursinna RS, Lee KW, Briggs JM, Martinis SA. Molecular dissection of a critical specificity determinant within the amino acid editing domain of leucyl-tRNA synthetase. Biochemistry. 2004;43(1):155–165. doi: 10.1021/bi034919h. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, et al. Zinc ion mediated amino acid discrimination by threonyl-tRNA synthetase. Nat Struct Biol. 2000;7(6):461–465. doi: 10.1038/75856. [DOI] [PubMed] [Google Scholar]
- 13.Schreier AA, Schimmel PR. Transfer ribonucleic acid synthetase catalyzed deacylation of aminoacyl transfer ribonucleic acid in the absence of adenosine monophosphate and pyrophosphate. Biochemistry. 1972;11(9):1582–1589. doi: 10.1021/bi00759a006. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt E, Schimmel P. Mutational isolation of a sieve for editing in a transfer RNA synthetase. Science. 1994;264(5156):265–267. doi: 10.1126/science.8146659. [DOI] [PubMed] [Google Scholar]
- 15.Hendrickson TL, Schimmel PR. Transfer RNA-dependent amino acid discrimination by aminoacyl-tRNA synthetases. In: Lapointe J, Brakier-Gingras L, editors. Translation Mechanisms. Georgetown, Texas: Kluwer; 2003. pp. 34–64. [Google Scholar]
- 16.Ahel I, Korencic D, Ibba M, Soll D. Trans-editing of mischarged tRNAs. Proc Natl Acad Sci USA. 2003;100(26):15422–15427. doi: 10.1073/pnas.2136934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong FC, Beuning PJ, Silvers C, Musier-Forsyth K. An isolated class II aminoacyl-tRNA synthetase insertion domain is functional in amino acid editing. J Biol Chem. 2003;278(52):52857–52864. doi: 10.1074/jbc.M309627200. [DOI] [PubMed] [Google Scholar]
- 18.Korencic D, et al. A freestanding proofreading domain is required for protein synthesis quality control in Archaea. Proc Natl Acad Sci USA. 2004;101(28):10260–10265. doi: 10.1073/pnas.0403926101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An S, Musier-Forsyth K. Cys-tRNA(Pro) editing by Haemophilus influenzae YbaK via a novel synthetase.YbaK.tRNA ternary complex. J Biol Chem. 2005;280(41):34465–34472. doi: 10.1074/jbc.M507550200. [DOI] [PubMed] [Google Scholar]
- 20.Schimmel P, Ribas De Pouplana L. Footprints of aminoacyl-tRNA synthetases are everywhere. Trends Biochem Sci. 2000;25(5):207–209. doi: 10.1016/s0968-0004(00)01553-x. [DOI] [PubMed] [Google Scholar]
- 21.Guo M, et al. The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science. 2009;325(5941):744–747. doi: 10.1126/science.1174343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chong YE, Yang XL, Schimmel P. Natural homolog of tRNA synthetase editing domain rescues conditional lethality caused by mistranslation. J Biol Chem. 2008;283(44):30073–30078. doi: 10.1074/jbc.M805943200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felts SJ, Toft DO. p23, a simple protein with complex activities. Cell Stress Chaperon. 2003;8(2):108–113. doi: 10.1379/1466-1268(2003)008<0108:paspwc>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Picard D. Heat-shock protein 90, a chaperone for folding and regulation. Cell Mol Life Sci. 2002;59(10):1640–1648. doi: 10.1007/PL00012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beebe K, Mock M, Merriman E, Schimmel P. Distinct domains of tRNA synthetase recognize the same base pair. Nature. 2008;451(7174):90–93. doi: 10.1038/nature06454. [DOI] [PubMed] [Google Scholar]
- 26.Nangle LA, Zhang W, Xie W, Yang XL, Schimmel P. Charcot–Marie–Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci USA. 2007;104(27):11239–11244. doi: 10.1073/pnas.0705055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beebe K, Waas W, Druzina Z, Guo M, Schimmel P. A universal plate format for increased throughput of assays that monitor multiple aminoacyl transfer RNA synthetase activities. Anal Biochem. 2007;368(1):111–121. doi: 10.1016/j.ab.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]