Abstract
Increased intraabdominal (visceral) fat is associated with a high risk of diabetes and metabolic syndrome. We have previously shown that the mesodermal developmental transcription factor Tbx15 is highly differentially expressed between visceral and subcutaneous (s.c.) fat in both humans and rodents, and in humans visceral fat Tbx15 expression is decreased in obesity. Here we show that, in mice, Tbx15 is 260-fold more highly expressed in s.c. preadipocytes than in epididymal preadipocytes. Overexpression of Tbx15 in 3T3-L1 preadipocytes impairs adipocyte differentiation and decreases triglyceride content. This defect in differentiation can be corrected by stimulating cells with the PPARγ agonist rosiglitazone (Rosi). However, triglyceride accumulation remains decreased by ∼50%, due to a decrease in basal lipogenic rate and increase in basal lipolytic rate. 3T3-L1 preadipocytes overexpressing Tbx15 also have a 15% reduction in mitochondrial mass and a 28% reduction in basal mitochondrial respiration (P = 0.004) and ATP turnover (P = 0.02), and a 45% (P = 0.003) reduction in mitochondrial respiratory capacity. Thus, differential expression of Tbx15 between fat depots plays an important role in the interdepot differences in adipocyte differentiation, triglyceride accumulation, and mitochondrial function that may contribute to the risk of diabetes and metabolic disease.
Keywords: thiazolidinedione, bioenergetics profile, Oil Red O
Most organisms store energy in the form of fat (1). In higher organisms, fat accumulates as triglycerides in white adipose tissue (WAT) depots, and this depends on the balance between triglyceride synthesis (fatty acid esterification and lipogenesis) and breakdown (lipolysis and fatty acid oxidation). During energy excess, triglyceride synthesis and storage increases, resulting in excessive fat accumulation in WAT, overweight, and obesity and increased risk for diseases, including type 2 diabetes mellitus and metabolic syndrome (2).
In addition to the degree of obesity (as measured by body mass index, BMI), fat distribution (clinically measured as waist–hip ratio, WHR) is a strong predictor for disease development (3). Increased intraabdominal or visceral obesity is associated with a high risk of developing metabolic disease, whereas s.c. obesity presents little or no risk (4), and may even be protective (5). This phenomenon is related to the differential function of adipose tissue depots, including differences in adipokine secretion, lipolytic rate, and free fatty acid release (6, 7). These properties are intrinsic to the cells in each depot and can be observed even after adipose tissue transplantation (8).
Recently, we and others have shown that several developmental genes, including several Hox genes, Shox2, Engrailed-1, and Tbx15, are differentially expressed between intraabdominal and s.c. adipocytes and preadipocytes of rodents and humans, in many cases by several orders of magnitude (9–12). We hypothesized that these genes may play a role in adipose depot development and ultimately in differential adipose depot function. In humans, three of these developmental genes exhibit relationships with BMI and WHR (11). One of the genes that exhibits a large interdepot difference in expression, and whose expression closely correlates with the level of obesity and pattern of fat distribution, is the developmental transcription factor T-box 15 (Tbx15) (11).
Tbx15 is a member of a phylogenetically conserved family of 18 genes, which share a characteristic sequence similarity within the DNA-binding domain (T domain) and are involved in the control of a variety of developmental processes, including mesoderm specification (13, 14). In rodents, Tbx15 shows a dorsoventral pattern that mirrors the distribution of the agouti protein (15). Inactivation of the Tbx15 gene in mice and mutations of Tbx15 in humans result in severe skeletal malformation (16, 17). Tbx15, acting in conjunction with corepressors of the Groucho family, decreases transcription of a number of genes, suggesting that Tbx15 mainly acts as a repressor (18). A recent metaanalysis of genomewide association studies revealed that genetic variation in the Tbx15 locus is strongly associated with WHR in both men and women who are obese (19).
Here, we investigated the potential role of Tbx15 in regulating adipocyte differentiation and function. We find that increasing expression of Tbx15 in 3T3-L1 preadipocytes delays adipocyte differentiation and reduces triglyceride accumulation, and this is partially rescued by the PPARγ agonist rosiglitazone (Rosi). The decreased triglyceride content is due to decreased lipogenesis and increased lipolysis. In addition, Tbx15 overexpression decreases mitochondrial mass, reduces expression of genes encoding members of the mitochondrial electron transport chain, and decreases basal and maximal mitochondrial respiration. Together, these results indicate an important role of Tbx15 in controlling depot-specific adipocyte development and function.
Results
Expression Pattern of Tbx15 in Preadipocytes.
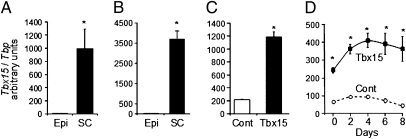
We previously reported differential expression of developmental genes in intraabdominal versus s.c. adipose depots in both humans and rodents and postulated that they may play a role in the control of differential adipocyte development and function between depots (1, 11). Striking was the 36-fold higher expression of the mesodermal developmental gene Tbx15 in visceral versus s.c. fat of lean humans. In visceral fat, Tbx15 expression was tightly inversely correlated with BMI and central fat distribution, as measured by WHR (11). In rodents, Tbx15 was also differentially distributed among depots, with levels in s.c. interscapular WAT > brown adipose tissue ∼ (equivalent) s.c. flank WAT ∼ perirenal WAT > intraabdominal epididymal WAT ∼ intraabdominal mesenteric WAT (20). This differential appeared to be present even at the preadipocyte stage. Thus, Tbx15 mRNA levels were 200-fold higher in the stromovascular fraction (SVF) of s.c. flank compared with intraabdominal epididymal WAT (Fig. 1A), and when preadipocytes were purified from SVF by fluorescence-activated cell sorting using negative selection for CD45, CD31, and Ter119 and positive selection for Sca1 and CD34 (21), this was confirmed with Tbx15 expression being 261-fold higher in preadipocytes from s.c. versus epididymal adipose tissue (Fig. 1B).
Fig. 1.
Depot-specific expression of Tbx15 and overexpression in 3T3-L1. (A) Tbx15 mRNA expression in SVF of epididymal (Epi) and s.c. (SC) adipose tissues of 6- to 8-wk-old C57BL/6 male mice, using qPCR. (B) Tbx15 expression in preadipocytes isolated by FACS from SVF of epididymal (Pre-Ad Epi) and s.c. (Pre-Ad SC) adipose tissue using qPCR. (C) Tbx15 mRNA expression in 3T3-L1 preadipocytes infected with empty (Cont) or Tbx15-expressing (Tbx15) retroviruses and selected with puromycin. (D) Tbx15 mRNA expression in 3T3-L1 preadipocytes overexpressing Tbx15 (black squares, solid line) or an empty vector (open circles, dotted line) during adipocyte differentiation. Tbx15 mRNA level was measured using qPCR and normalized to Tbp expression. Data are mean ± SEM of n = 5 (A–C) or three separate experiments (D). Statistics were analyzed by Student's t test (two-way, unequal variance). *P < 0.05.
Adipocyte Differentiation and Maturation Is Delayed in Cells Overexpressing Tbx15.
To investigate the role of Tbx15 in adipose tissue development, we stably overexpressed Tbx15 in 3T3-L1 preadipocytes (3T3-L1-Tbx15) by retroviral gene transduction. Endogenous Tbx15 mRNA was easily detected in 3T3-L1 preadipocytes reflecting the mesodermal lineage of these cells. 3T3-L1-Tbx15 cells exhibited a moderate (approximately fivefold) and relatively constant overexpression of Tbx15 during the differentiation time course (Fig. 1 C and D), a level comparable to that observed in SVF of s.c. WAT (Fig. 1 A and C).
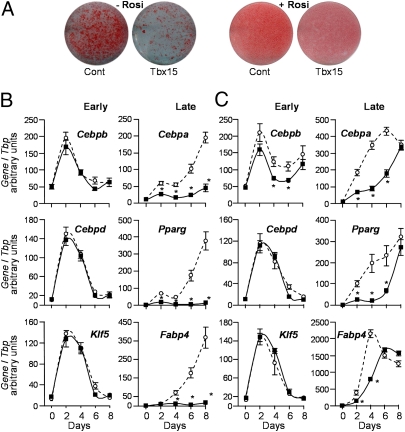
Adipocyte differentiation is tightly controlled by a transcriptional cascade. As previously described (22), induction of differentiation in control cells transiently increases expression of the transcription factors C/EBPβ (Cebpb), C/EBPδ (Cebpd), and KLF5 (Klf5), which peaks at day 2 then declines, followed by increases in expression of C/EBPα (Cebpa), PPARγ (Pparg), and aP2 (Fabp4) (Fig. 2B). Whereas no difference in induction of Cebpb, Cebpd, and Klf5 was observed in 3T3-L1-Tbx15 cells compared with control, the induction of Cebpa, Pparg, and Fabp4 was dramatically impaired (Fig. 2B). Thus, in 3T3-L1-Tbx15 cells Cebpa and Pparg expression was reduced by 56 ± 3% and 73 ± 2%, respectively, after 2 d of differentiation and levels remained low until day 8 (∼70% reduction for Cebpa and 95% reduction for Pparg), by which time, control cells had achieved full maturation and Cebpa and Pparg expression was at a maximum. Consequently, Fabp4 expression, which is regulated by Cebpa and Pparg, was reduced by 80–95% between days 4 and 8 in 3T3-L1-Tbx15 cells, and there was a marked reduction in lipid accumulation in these cells (Fig. 2A). Thus, Tbx15 overexpression in 3T3-L1 preadipocytes impaired adipocyte differentiation, as measured by gene expression and triglyceride accumulation.
Fig. 2.
Overexpression of Tbx15 in 3T3-L1 preadipocytes impairs their capacity to differentiate. 3T3-L1-Tbx15 (Tbx15, black squares, solid line) or control cells (Cont, open circles, dotted line) were induced to differentiate into adipocytes for 8 d without (−Rosi) or with (+Rosi) 1 μM rosiglitazone. Triglyceride was visualized using Oil Red O at day 8 (A). mRNA was extracted every 2 d during induction of adipocyte differentiation in the absence (B) or in the presence (C) of rosiglitazone. Expression of early adipogenic genes (Cebpb, Cebpd, and Klf5) and late adipogenic genes (Cebpa, Pparg, and Fabp4) was determined using qPCR normalized to Tbp. Values are means ± SEM of at least six experiments. For each time point, differences between control and 3T3-L1-Tbx15 were statistically analyzed by Student's t test (two-way, unequal variance). *P < 0.05.
Rosiglitazone Restores Differentiation of 3T3-L1-Tbx15 Cells, but Not Triglyceride Content.
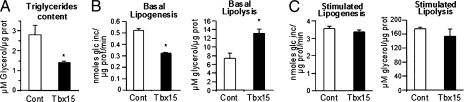
Consistent with previous studies (23), rosiglitazone (1 μM) enhanced adipocyte differentiation and increased triglyceride accumulation in both control and 3T3-L1-Tbx15 cells (Fig. 2A). Induction of expression of early transcription factors, Cebpd, Cebpb, and Klf5, during the first days of differentiation was identical in all cells, although Cebpb levels were slightly lower in the Rosi-treated 3T3-L1-Tbx15 cells. In control cells, induction of the late adipogenic transcription factors, Cebpa and Pparg, was more rapid in the presence of rosiglitazone, reaching 80–85% of their maximal expression by day 4 (Fig. 2C). In Tbx15 overexpressing cells, expression of these transcription factors was delayed, although similar levels were reached by the end of day 8. Consequently, in control cells, the expression of Fabp4 reached its maximum by day 4, whereas equivalent levels of Fabp4 in 3T3-L1-Tbx15 cells were not observed until day 6 and day 8. Thus, enhancing activation of PPARγ by rosiglitazone was sufficient to overcome the block in adipocyte differentiation in 3T3-L1-Tbx15 cells, although the time course of differentiation was delayed. Despite reaching the same level of differentiation by gene expression, triglyceride accumulation estimated both by Oil Red O staining (Fig. 2A) and enzymatic assay (Fig. 3A) was reduced by 49 ± 2% in 3T3-L1-Tbx15 cells. Hence, whereas rosiglitazone restored differentiation of 3T3-L1-Tbx15 cells at the level of gene expression, normal steady state levels of triglyceride accumulation were not attained, suggesting that Tbx15 regulates both the ability of adipocytes to differentiate and their function once differentiated.
Fig. 3.
Overexpression of Tbx15 impairs triglyceride accumulation by decreasing basal lipogenesis and increasing basal lipolysis. (A) Triglyceride content of 3T3-L1-Tbx15 (black bar) and control cells (white bar) after 8 d of adipocyte differentiation induced in the presence of 1 μM rosiglitazone. (B) Basal lipogenic activity (Left), reflected by 14C-d-glucose incorporation into lipid and basal lipolytic activity (Right), measured as glycerol released into the medium was determined in control cells (white bar) and 3T3-L1-Tbx15 (black bar). (C) Maximally stimulated lipogenic activity (100 mM insulin) and lipolytic activity (1 μM isoproterenol) were measured in control (white bar) and 3T3-L1-Tbx15 cells (black bar). Values are means ± SEM of three separate experiments, each performed in duplicate. Differences were statistically analyzed by Student's t test. *P < 0.05.
Tbx15 Overexpression Increases Basal Lipolysis and Decreases Basal Lipogenesis.
In adipocytes, triglyceride storage at steady state represents a balance between triglyceride synthesis and triglyceride breakdown. In 3T3-L1 cells, triglyceride accumulation is derived primarily from de novo synthesis of fatty acids from glucose, followed by esterification with glycerol. Conversely, triglyceride breakdown is due to hydrolysis into fatty acids and glycerol. Direct assessment of lipogenic and lipolytic activities revealed a 40 ± 6% decrease in basal lipogenic activity accompanied by an 80 ± 13% increase in basal lipolytic activity (Fig. 3B) of differentiated 3T3-L1-Tbx15 adipocytes compared with controls. However, maximal lipogenic activity stimulated by 100 nM of insulin and maximal lipolytic response stimulated by 1 μM of the β-adrenergic agonist isoproterenol did not differ from control (Fig. 3C). Thus, the reduced triglyceride levels observed in 3T3-L1-Tbx15 cells was due to the combination of decreased basal lipogenic activity and increased basal lipolytic activity, in addition to the delay in terminal adipocyte differentiation.
Overexpression of Tbx15 Decreases Mitochondrial Mass in 3T3-L1 Preadipocytes.
Lipogenesis and lipolysis are complex biochemical pathways involving the sequential function of several enzymes and proteins. Among these proteins, the glucose transporter GLUT4 (Slc2a4), the fatty acid synthase (Fasn), and the 1-acylglycerol-3-phosphate O-acyltransferase 2 (Agpat2) play critical roles in the de novo synthesis of fatty acids and esterification to form triglycerides, whereas the β3-adrenergic receptor (Adrb3), hormone-sensitive lipase (Lipe), and adipose triglyceride lipase (Pnpla2) are involved in limiting steps regulating lipolysis. Consistent with the equal level of differentiation markers in cells treated with rosiglitazone, there were no significant differences in gene expression of each of these proteins (Fig. S1).
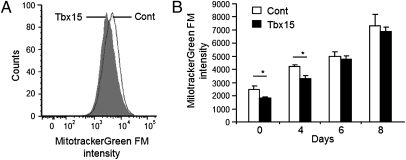
Mitochondria play an important role in controlling adipocyte fatty acid storage by regulating fatty acid degradation through oxidation, as well as their synthesis (24, 25). Mitochondrial mass, measured by Mitotracker Green FM dye accumulation and fluorescence-activated cell sorting analysis, was reduced by 25 ± 2% (P < 0.05) in 3T3-L1-Tbx15 preadipocytes (day 0) compared with controls (Fig. 4 A and B). During differentiation in the presence of rosiglitazone, mitochondrial mass increased in all cells. However, at day 4, the magnitude of increase in mass was lower in 3T3-L1-Tbx15 cells, indicating delayed adipocyte differentiation, but by days 6 and 8, mitochondrial mass was identical in both cell types (Fig. 4B).
Fig. 4.
Tbx15 overexpression decreases mitochondrial mass of 3T3-L1 preadipocytes. Total mitochondrial mass was measured by fluorescent staining, using 50 nM MitoTracker Green FM, of control (white bar) or 3T3-L1-Tbx15 (black bar) preadipocytes (A) and adipocytes differentiated for 8 d in the presence of 1 μM rosiglitazone (B). Fluorescence was analyzed by FACS on a population of 200,000 cells. Values are means ± SEM of triplicates of three separate experiments. Differences were statistically analyzed by Student's t test (two-way, unequal variance). *P < 0.05.
Increased Expression of Tbx15 in 3T3-L1 Preadipocytes Impairs Mitochondrial Respiration.
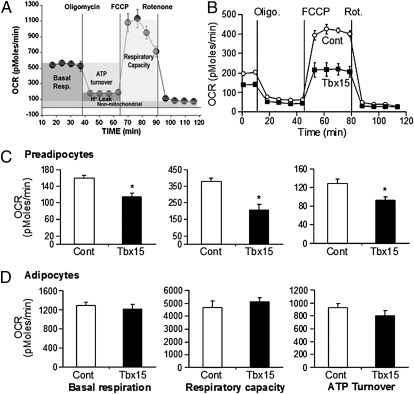
To determine the effects of Tbx15 on mitochondrial respiratory function, we measured oxygen consumption rates (OCR) using an extracellular flux analyzer. A typical bioenergetics profile is shown in Fig. 5A and involves a four-step analysis: (i) basal OCR is measured in medium containing 4.5 g/L (25 mM) glucose; (ii) ATP synthesis turnover and respiration driving proton leak are determined by measuring OCR after inhibition of ATP synthase by oligomycin (10 μM); (iii) maximal mitochondrial respiratory capacity is assessed by measuring OCR after stimulation with the uncoupling agent carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) (1 μM); and (iv) finally, nonmitochondrial respiration assessed by measuring OCR after addition of complex I inhibitor: rotenone (1 μM).
Fig. 5.
Bioenergetics profiles of 3T3-L1-Tbx15 and control preadipocytes and adipocytes. Oxygen consumption rate (OCR) was used to determine the bioenergetics profile of 3T3-L1 preadipocytes and adipocytes differentiated in presence of 1 μM rosiglitazone for 8 d (as in A) using a Seahorse Bioscience 24XF extracellular flux analyzer (see text and SI Materials and Methods). The bioenergetics profile of confluent 3T3-L1 preadipocytes expressing Tbx15 (black square) or an empty vector (white circle) was compared (B). Basal respiration, ATP turnover, and respiratory capacity were determined by calculating average value for each phase in preadipocytes (C) and adipocytes differentiated in presence of 1 μM rosiglitazone (D). Values are means ± SEM of five replicates of three (C) to five (D) separate experiments. Statistics were analyzed by Student's t test (two-way, unequal variance). *P < 0.05.
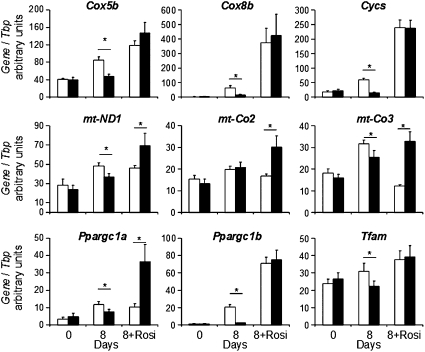
The bioenergetics profiles of control and 3T3-L1-Tbx15 preadipocytes (Fig. 5B) revealed no significant differences in nonmitochondrial respiration or proton leak; however, three important differences between Tbx15-overexpressing and control cells were observed. First, basal mitochondrial respiration of 3T3-L1-Tbx15 cells was reduced by 28 ± 5.5% compared with controls (P < 0.05) (Fig. 5 B and C). This difference resulted in a 27.7 ± 5.8% decrease in the respiration used for the generation of ATP (P < 0.05), reflecting lower ATP turnover in 3T3-L1-Tbx15 cells than in control cells (Fig. 5 B and C). Finally, maximal mitochondrial respiratory capacity of the 3T3-L1-Tbx15 preadipocytes, in response to the uncoupling agent FCCP, was reduced by 45 ± 8% (P < 0.05) (Fig. 5 B and C). Thus, higher Tbx15 expression in 3T3-L1 preadipocytes impaired both basal and maximal mitochondrial respiratory capacity. These defects in mitochondrial oxidative metabolism occurred without changes in expression of representative members of the electron transport chain (ETC) genes, including the mitochondrial-encoded genes, cytochrome c oxidase polypeptide II (mt-Co2), cytochrome c oxidase polypeptide III (mt-Co3), and NADH dehydrogenase subunit 1 (mt-Nd1) and the nuclear-encoded genes, cytochrome C oxidase subunit Vb (Cox5b), cytochrome C oxidase subunit VIIIb (Cox8b), and cytochrome C somatic (Cycs). Likewise, there were no differences in expression levels of regulators of mitochondrial biogenesis, e.g. peroxisome proliferator-activated receptor-γ coactivator 1-α (Ppargc1a) and -β (Ppargc1b), or the mitochondrial transcription factor A (Tfam) (Fig. 6). However, during differentiation without rosiglitazone, expression of all of these genes, with the exception of mt-Co2, was significantly reduced in 3T3-L1-Tbx15 compared with control cells (mt-Co3, −20%; mt-ND1, −25%; Cox8b, −97%; Cox5b, −45%; Cycs, −75%; Ppargc1a, −37%; Ppargc1b, −89%; and Tfam, −28%).
Fig. 6.
Expression level of electron transport chain genes and their regulators in 3T3-L1-Tbx15 preadipocytes and adipocytes. mRNA expression of electron transport chain genes, mt-Co2, mt-Co3, mt-ND1, Cycs, Cox5b, and Cox8b as well as regulators of mitochondrial number and activity, Ppargc1a, Ppargc1b, and Tfam was measured in 3T3-L1-Tbx15 (black bar) and control (white bar) preadipocytes (“0”) and adipocytes differentiated for 8 d in the absence (“8”) or presence of 1 μM of rosiglitazone (“8+Rosi”). mRNA levels were measured using qPCR and normalized to Tbp expression. Values are means ± SEM of at least six experiments. Differences were analyzed by Student's t test (two-way, unequal variance). *P < 0.05.
When cells were differentiated into adipocytes in the presence of rosiglitazone, all components of mitochondrial respiration were markedly up-regulated (compare scales in Fig. 5 C and D). After differentiation, there were no significant differences in basal respiration, ATP turnover, and maximal mitochondrial respiratory capacity between 3T3-L1-Tbx15 and control adipocytes (Fig. 5D). Interestingly, whereas expression of the nuclear-encoded ETC genes Cox5b, Cox8b, and Cycs were similar, those encoded by the mitochondrial genome, mt-ND1, mt-Co2, and mt-Co3, were more highly expressed in 3T3-L1-Tbx15 adipocytes (Fig. 6). This was associated with higher Ppargc1a expression, whereas Ppargc1b and Tfam were not affected. Taken together, these results indicate that preadipocytes with higher levels of Tbx15 exhibit a lower respiratory capacity. This defect can be restored during adipocyte differentiation with rosiglitazone, and this is associated with increased expression of Ppargc1a.
Discussion
Both obesity and fat distribution influence the risk of developing diabetes and metabolic syndrome. Indeed, intraabdominal/visceral fat accumulation is associated with a higher risk of diabetes and metabolic syndrome, whereas increased s.c. fat in the thighs and hips may even have a protective effect (4, 8, 26). A growing body of evidence suggests that these depot-specific associations are due to intrinsic differences in the properties of adipocytes in each depot (27, 28) and the consequence of a divergence in their developmental origin (1, 9–12, 29). Consistent with this hypothesis, we and others observed high differential expression of developmental genes between intraabdominal and s.c. adipose depots in both rodents and humans (11, 12). Of these genes, Tbx15 expression in human intraabdominal adipose depots was the most tightly correlated with obesity and the pattern of fat distribution, suggesting a role for Tbx15 in the depot-specific control of adipose tissue development and function. Recently, a metaanalysis of genomewide association studies identified a SNP in the first intron of the Tbx15 gene associated with WHR, corrected for BMI, in both men and women at a P < 10−24 level (19).
In the present study, we demonstrate differential Tbx15 expression in adipocytes and SVF of s.c. versus intraabdominal mouse adipose tissue, and this difference is even more striking in adipocyte progenitors isolated from the SVF of these depots. To better understand the potential role played by Tbx15 in preadipocytes and adipocyte development and function, we overexpressed Tbx15 in the murine preadipocyte model cell line 3T3-L1. This resulted in impaired differentiation capacity, reflected by decreased adipogenic gene expression and decreased triglyceride accumulation. Although the expression of early drivers of adipocyte differentiation, Cebpb, Cebpd, and Klf5 was not affected by Tbx15 overexpression, the expression of late drivers, Cebpa and Pparg, was impaired. Thus, it appears that Tbx15 regulates differentiation by acting at this critical checkpoint between the early and late phases of adipocyte differentiation or may alter the ability of Cebpa and Pparg to interact with one another and enhance each other's expression (30). Although addition of the Pparg agonist rosiglitazone overcame this block, allowing 3T3-L1-Tbx15 to fully differentiate, development of the normal adipogenic gene expression was delayed, and triglyceride content of the resultant adipocytes was reduced. This decrease in stored triglyceride is the result of two factors. First, the delayed differentiation rate of 3T3-L1-Tbx15 cells and, more importantly, a decrease in the basal lipogenic rate and increase in the basal lipolytic rate, which results in a decrease in the steady-state level of triglyceride accumulation. These differences occur despite normal levels of expression of lipogenic and lipolytic genes. The finding that 3T3-L1 cells with higher Tbx15 expression have less accumulated triglycerides is consistent with our previous observation that in humans, high Tbx15 levels in visceral fat are associated with less adiposity and lower WHR (11), indicating fewer and/or smaller adipocytes, especially in the visceral depot. Our results in culture mirror this phenomenon, with high Tbx15 expression resulting in less lipid accumulation and fewer mature adipocytes. Thus, Tbx15 may regulate intraabdominal adipose tissue development and fat accumulation and thus play a role in the development of central obesity and metabolic syndrome.
In a recent study, Heid et al. confirmed differential expression of Tbx15 between s.c. (gluteal) and visceral (omental) fat depots (19). However, in contrast to our previous study, in this report the Tbx15 risk allele associated with WHR was positively correlated with Tbx15 mRNA expression in omental fat. Further studies will be required to explore the reason for these differences, but it likely reflects differences in the specific depots sampled. Thus, we have found a negative correlation between Tbx15 expression and BMI/WHR in mesenteric fat but a positive association between Tbx15 expression and BMI/WHR in abdominal s.c. adipose tissue of humans, suggesting that alterations in Tbx15 expression with obesity and/or fat distribution are depot specific. Indeed, in mice, there are dramatic differences in expression of Tbx15 when one compares different intraabdominal depots (20). Potential functional differences in different intraabdominal fat depots has also been demonstrated in the dog, where removal of only a specific area of omental fat, but not other areas of intraabdominal fat, improves insulin sensitivity (31).
In adipose tissue, mitochondrial respiration appears to be required for fatty acid synthesis preceding triglyceride synthesis (24, 32). Indeed, during the early stages of adipocyte differentiation, mitochondrial mass increases rapidly (32), and agents that inhibit mitochondrial respiration during this early stage impair differentiated functions (24). Overexpression of Tbx15 significantly reduces preadipocyte mitochondrial mass, mitochondrial basal respiration, and mitochondrial respiratory capacity, which leads to decreased adipocyte differentiation and reduced triglyceride accumulation. The role of Tbx15 in regulating mitochondrial function in adipocytes is consistent with the lower mitochondrial respiration observed in s.c. preadipocytes compared with intraabdominal perigonadal preadipocytes. This role of mitochondrial respiration in adipocyte function appears to be biphasic—complete inhibition of mitochondrial respiration by rotenone in preadipocytes can increase triglyceride accumulation without inducing differentiation (33), whereas partial inhibition of mitochondrial respiration during preadipocyte differentiation decreases the ability of these cells to differentiate and store triglycerides (34). By comparison, overexpression of Tbx15 in 3T3-L1 preadipocytes causes a moderate decrease in mitochondrial respiration and mitochondrial mass, which results in impaired adipocyte differentiation capacity and reduced triglyceride accumulation. Increasing mitochondrial biogenesis using rosiglitazone restores adipocyte differentiation but not normal triglyceride accumulation.
During differentiation, 3T3-L1 preadipocytes undergo mitochondrial remodeling controlled by factors regulating the fusion and the fission of the mitochondria, which when inhibited, impairs triglyceride accumulation (25). Such reorganization of the mitochondrial network during differentiation of 3T3-L1-Tbx15 cells could account for the impaired adipocyte differentiation and triglyceride accumulation. However, mRNA expression of the fusion factor Opa1 and fission factor Fis1 is not altered in 3T3-L1-Tbx15 preadipocytes (Fig. S2).
Regulation of mitochondrial respiration in adipose tissue may therefore have a different outcome depending on the developmental stage of the cells. In mature adipocytes, fat accumulation can be regulated by modulation of mitochondrial respiration in vitro and in vivo. Mild mitochondrial uncoupling by FCCP in 3T3-L1 cells decreases triglyceride content, without affecting PPARγ and C/EBPα expression (35). In mice, low doses of the mitochondrial uncoupling agent 2,4-dinitrophenol enhances tissue respiratory rates and decreases body weight (36), and ectopic expression of the uncoupling protein 1 (UCP1) in WAT protects against obesity (37), suggesting that increased mitochondrial respiration in WAT decreases adipocyte fat content. However, mitochondrial dysfunction in preadipocytes inhibits adipocyte differentiation and subsequently triglyceride accumulation in vitro (34). Interestingly, the nucleoside reverse transcriptase inhibitors, which induce lipodystrophies in some patients being treated for HIV-1, decrease mitochondrial activity and lipid content in cell culture (38). This inhibition of adipocyte differentiation can be averted in cell culture (39) and in humans (40) with uridine treatment, which prevents mitochondrial dysfunction.
In summary, the mesodermal developmental gene Tbx15 plays an important role in adipocyte differentiation and function. High levels of Tbx15 expression in preadipocytes, such as occurs in visceral fat of lean individuals, reduces adipocyte differentiation capacity and impairs adipocyte triglyceride accumulation. These effects appear to be due to decreased mitochondrial mass and reduced respiratory capacity of preadipocytes. Conversely, low levels of Tbx15 expression, as occurs in visceral fat of obese subjects, enables normal mitochondrial function and permits an expansion of adipose tissue and increased fat accumulation. Targeting the mechanism by which Tbx15 controls mitochondrial mass and activity in preadipocytes, as well as their capacity to differentiate and accumulate triglycerides in a depot-specific manner, could provide new strategies for the prevention of visceral fat accumulation and potentially lead to therapies for obesity-linked insulin-resistant states.
Materials and Methods
Details are presented in SI Materials and Methods. For the 3T3-L1 studies, Tbx15 was stably introduced by retroviral transduction. Cells were differentiated into adipocytes using a standard induction protocol (41) in absence or in presence of 1μM of rosiglitazone. Gene expression was measured by qPCR. Triglyceride content was visualized by Oil Red O and measured using an enzymatic assay. Lipogenesis was measured by incorporation of 14C-d-glucose into lipids, and lipolytic activity measured by glycerol release (42). Mitochondrial mass was measured using MitoTracker Green FM (32), and mitochondrial function was determined by measuring the OCR using the Seahorse Bioscience XF24 extracellular flux analyzer (43).
For animal studies, C57BL6 male mice were fed a standard low-fat diet (LFD) containing 22% calories from fat and 55% from carbohydrates (Diet 9F 5020; PharmaServ), and were allowed ad libitum access to water and food. Adipocyte precursors were obtained from the stromovascular fraction of perigonadal and flank s.c. adipose tissue of 8-wk-old C57BL/6 male mice by FACS on the basis of negative selection of cells using anti-CD45, anti-Ter119, and anti-CD31 followed by positive selection for CD34 and SCA1 (21).
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK82659 and DK60837, Diabetes and Endocrinology Research Center Grant DK36836, the Mary K. Iacocca Professorship, and grants from the Eli Lilly and Loveman foundations.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019704108/-/DCSupplemental.
References
- 1.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Romao I, Roth J. Genetic and environmental interactions in obesity and type 2 diabetes. J Am Diet Assoc. 2008;108(4, Suppl 1):S24–S28. doi: 10.1016/j.jada.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Pischon T, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med. 2008;359:2105–2120. doi: 10.1056/NEJMoa0801891. [DOI] [PubMed] [Google Scholar]
- 4.Kissebah AH, Krakower GR. Regional adiposity and morbidity. Physiol Rev. 1994;74:761–811. doi: 10.1152/physrev.1994.74.4.761. [DOI] [PubMed] [Google Scholar]
- 5.Tankó LB, Bagger YZ, Alexandersen P, Larsen PJ, Christiansen C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107:1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68. [DOI] [PubMed] [Google Scholar]
- 6.Lafontan M, Berlan M. Do regional differences in adipocyte biology provide new pathophysiological insights? Trends Pharmacol Sci. 2003;24:276–283. doi: 10.1016/S0165-6147(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 7.Klöting N, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vohl MC, et al. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 10.Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol. 2003;194:225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 11.Gesta S, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci USA. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tchkonia T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 13.Showell C, Binder O, Conlon FL. T-box genes in early embryogenesis. Dev Dyn. 2004;229:201–218. doi: 10.1002/dvdy.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wardle FC, Papaioannou VE. Teasing out T-box targets in early mesoderm. Curr Opin Genet Dev. 2008;18:418–425. doi: 10.1016/j.gde.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Candille SI, et al. Dorsoventral patterning of the mouse coat by Tbx15. PLoS Biol. 2004;2:E3. doi: 10.1371/journal.pbio.0020003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh MK, et al. The T-box transcription factor Tbx15 is required for skeletal development. Mech Dev. 2005;122:131–144. doi: 10.1016/j.mod.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Lausch E, et al. TBX15 mutations cause craniofacial dysmorphism, hypoplasia of scapula and pelvis, and short stature in Cousin syndrome. Am J Hum Genet. 2008;83:649–655. doi: 10.1016/j.ajhg.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farin HF, et al. Transcriptional repression by the T-box proteins Tbx18 and Tbx15 depends on Groucho corepressors. J Biol Chem. 2007;282:25748–25759. doi: 10.1074/jbc.M703724200. [DOI] [PubMed] [Google Scholar]
- 19.Heid IM, et al. MAGIC. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42:949–960. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamamoto Y, et al. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 2010;18:872–878. doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 22.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 23.Forman BM, et al. 15-Deoxy-delta 12, 14-prostaglandin J2 is a ligand for the adipocyte determination factor PPAR γ. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 24.Shi X, et al. Paradoxical effect of mitochondrial respiratory chain impairment on insulin signaling and glucose transport in adipose cells. J Biol Chem. 2008;283:30658–30667. doi: 10.1074/jbc.M800510200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kita T, et al. Possible role of mitochondrial remodelling on cellular triacylglycerol accumulation. J Biochem. 2009;146:787–796. doi: 10.1093/jb/mvp124. [DOI] [PubMed] [Google Scholar]
- 26.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11, Suppl 1):S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lafontan M, Girard J. Impact of visceral adipose tissue on liver metabolism. Part I: Heterogeneity of adipose tissue and functional properties of visceral adipose tissue. Diabetes Metab. 2008;34:317–327. doi: 10.1016/j.diabet.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Tchkonia T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 29.Billon N, Monteiro MC, Dani C. Developmental origin of adipocytes: New insights into a pending question. Biol Cell. 2008;100:563–575. doi: 10.1042/BC20080011. [DOI] [PubMed] [Google Scholar]
- 30.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lottati M, Kolka CM, Stefanovski D, Kirkman EL, Bergman RN. Greater omentectomy improves insulin sensitivity in nonobese dogs. Obesity (Silver Spring) 2009;17:674–680. doi: 10.1038/oby.2008.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilson-Fritch L, et al. Mitochondrial biogenesis and remodeling during adipogenesis and in response to the insulin sensitizer rosiglitazone. Mol Cell Biol. 2003;23:1085–1094. doi: 10.1128/MCB.23.3.1085-1094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vankoningsloo S, et al. Mitochondrial dysfunction induces triglyceride accumulation in 3T3-L1 cells: Role of fatty acid beta-oxidation and glucose. J Lipid Res. 2005;46:1133–1149. doi: 10.1194/jlr.M400464-JLR200. [DOI] [PubMed] [Google Scholar]
- 34.Lu RH, Ji H, Chang ZG, Su SS, Yang GS. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Mol Biol Rep. 2010;37:2173–2182. doi: 10.1007/s11033-009-9695-z. [DOI] [PubMed] [Google Scholar]
- 35.Tejerina S, et al. Mild mitochondrial uncoupling induces 3T3-L1 adipocyte de-differentiation by a PPARgamma-independent mechanism, whereas TNFalpha-induced de-differentiation is PPARgamma dependent. J Cell Sci. 2009;122:145–155. doi: 10.1242/jcs.027508. [DOI] [PubMed] [Google Scholar]
- 36.Caldeira da Silva CC, Cerqueira FM, Barbosa LF, Medeiros MH, Kowaltowski AJ. Mild mitochondrial uncoupling in mice affects energy metabolism, redox balance and longevity. Aging Cell. 2008;7:552–560. doi: 10.1111/j.1474-9726.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 37.Kopecky J, Clarke G, Enerbäck S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caron M, et al. The HIV-1 nucleoside reverse transcriptase inhibitors stavudine and zidovudine alter adipocyte functions in vitro. AIDS. 2004;18:2127–2136. doi: 10.1097/00002030-200411050-00004. [DOI] [PubMed] [Google Scholar]
- 39.Walker UA, et al. Uridine abrogates the adverse effects of antiretroviral pyrimidine analogues on adipose cell functions. Antivir Ther. 2006;11:25–34. [PubMed] [Google Scholar]
- 40.Sutinen J, et al. Uridine supplementation for the treatment of antiretroviral therapy-associated lipoatrophy: A randomized, double-blind, placebo-controlled trial. Antivir Ther. 2007;12:97–105. [PubMed] [Google Scholar]
- 41.Bezy O, Vernochet C, Gesta S, Farmer SR, Kahn CR. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity. Mol Cell Biol. 2007;27:6818–6831. doi: 10.1128/MCB.00375-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blüher M, et al. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3:25–38. doi: 10.1016/s1534-5807(02)00199-5. [DOI] [PubMed] [Google Scholar]
- 43.Abe Y, et al. Bioenergetic characterization of mouse podocytes. Am J Physiol Cell Physiol. 2010;299:C464–C476. doi: 10.1152/ajpcell.00563.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]