Abstract
Moderate maternal nutrient restriction during pregnancy occurs in both developing and developed countries. In addition to poverty, maternal dieting, teenage pregnancy, and uterine vascular problems in older mothers are causes of decreased fetal nutrition. We evaluated the impact of global 30% maternal nutrient reduction (MNR) on early fetal baboon brain maturation. MNR induced major cerebral developmental disturbances without fetal growth restriction or marked maternal weight reduction. Mechanisms evaluated included neurotrophic factor suppression, cell proliferation and cell death imbalance, impaired glial maturation and neuronal process formation, down-regulation of gene ontological pathways and related gene products, and up-regulated transcription of cerebral catabolism. Contrary to the known benefits from this degree of dietary reduction on life span, MNR in pregnancy compromises structural fetal cerebral development, potentially having an impact on brain function throughout life.
Keywords: brain development, fetus, environment, epigenetics, malnutrition
Moderate malnutrition during pregnancy is widespread in both developing and industrialized countries. Worldwide, 852 million people experienced food insecurity in 2004 (1) and 35–40% of children experience moderate malnutrition (2). In industrialized countries, maternal lifestyle, in which dieting (including global food reduction) is used for cosmetic reasons, is a common cause of moderate malnutrition. Recently, one study has shown that most women do not improve their dietary and lifestyle patterns in pregnancy (3). Decreased nutrient delivery to the fetus also occurs in teenage pregnancy (4) and during pregnancy in women over 35 y of age (5). Severe global reduction in nutrition during pregnancy has long been known to result in fetal growth restriction and to cause permanent brain dysfunction, especially cognitive and behavior deficits (6–10). In rodents, these deficits are accompanied by alterations of neuronal excitability as well as structural changes in the developing and adult brain (11, 12). In contrast, effects of moderate maternal global reduction in nutrition on the fetus have been poorly examined. It is often assumed that moderate degrees of maternal nutrient reduction (MNR) are without unwanted consequences because it has generally been considered that the mother prioritizes fetal nutrient supply at the expense of her own needs, and brain growth is assumed to be spared from the other fetal effects of moderate decreases in fetal nutrient availability. This concept no longer appears to be tenable. Several rodent studies have demonstrated significant changes in fetal body and brain composition, often in the absence of major reduction of birth weight following poor maternal nutrition during pregnancy (11, 12). In relation to the changes in brain development that we report here in a nonhuman primate species, evidence is accumulating that birth weight at the lower end of the normal range is associated with impaired higher mental function in later life (5, 13, 14).
We addressed effects of 30% global moderate MNR on early fetal cerebral development in a well-established nonhuman primate model, the fetal baboon. This degree of dietary reduction has beneficial effects on aging and life span in nonhuman primates (15) but may have adverse effects on brain development. The gradual development of the CNS, as well as its plasticity and high energy demand, makes the developing brain vulnerable to adverse intrauterine conditions (16). Most studies have focused on the brain growth spurt (6, 9–12) that occurs between the second trimester and the second year of life in humans (6) and is mainly caused by an increase in neuropil and myelination (6, 11). We focused on the first half of pregnancy because epidemiological studies indicate that cognitive and behavior deficits are more severe the earlier that MNR occurs (7, 10, 17). During the first half of pregnancy, the mechanisms of glia- and neurogenesis, cell migration, and differentiation determine brain development. Importantly, fetal primate brain neuronal proliferation peaks at the gestational stage studied here (11, 18).
We examined gross morphology of the developing fetal brain and detected subtle changes in the subventricular proliferation zone. Therefore, we examined neural cell proliferation, migration, and maturation and found discrete alterations in neuronal and glial maturation. Examining the underlying mechanisms, we found decreased growth factors and complex alterations of major ontological pathways of biological processes and molecular function revealed by whole-genome expression profiling. To examine the relevance of these findings, we assessed protein expression by immunohistochemistry from a subset of affected genes, particularly of transcripts that encode proteins known to be widely involved in cerebral development.
Results
Maternal Age and Weight.
The age of the control and MNR baboons was 9.4 ± 0.6 y and 11.1 ± 1.3 y, respectively, and did not differ between the experimental groups. The age corresponds to approximately 25–29 y in human age based on the reproductive period in both species (Fig. S1). Control and MNR mothers weighed 13.7 ± 0.5 and 13.0 ± 0.2 kg, respectively 30 d before pregnancy and 13.7 ± 0.4 and 12.2 ± 0.3 kg at 0.5 gestation (term = 185 d) i.e., MNR caused a 9.1% drop in maternal weight (P < 0.05).
Fetal Weight and Brain Gross Morphology.
Body and brain weights for control and MNR fetuses were not different at 0.5 gestation (Table 1). The stage of cortical development studied was comparable to the six-layer stage in the human fetus at midgestation (19) (Fig. 1). MNR did not alter the gross morphology of the fetal brain, as shown by proportions of the whole hemisphere and the cortical plate (Table 1). In contrast, the thickness of the proliferative-active subventricular zone (SVZ) was reduced by 44% in MNR fetuses (P < 0.05; Fig. 2 and Table 1).
Table 1.
Effect of MNR on fetal growth and cerebral development
| Controls | MNR | |
| Fetal growth | ||
| Fetal body weight, g | 100.9 ± 3.8 | 95.4 ± 3.3 |
| Fetal brain weight, g | 32.6 ± 1.8 | 30.1 ± 0.5 |
| Ratio of the areas of the cortical plate/hemisphere | 0.17 ± 0.04 | 0.19 ± 0.03 |
| Morphometric data | ||
| Thickness, mm × 10−3 | ||
| SVZ | 0.75 ± 0.27 | 0.42 ± 0.09* |
| Cortical plate | 0.81 ± 0.06 | 0.80 ± 0.09 |
| No. of proliferating cells per mm2 | ||
| SVZ | 13,830 ± 4,568 | 19,711 ± 1,094* |
| Cortical plate | 24 ± 17 | 20 ± 18 |
| Intermediate zone | 3,612 ± 841 | 3,773 ± 619 |
| Total no. of proliferating cells in the SVZ | 106,869 ± 5,393 | 117,312 ± 1,094 |
| No. of apoptotic cells per mm2 | ||
| SVZ | 27 ± 9 | 60 ± 27* |
| Cortical plate | 9 ± 4 | 12 ± 10 |
| Intermediate zone | 978 ± 391 | 1,225 ± 473 |
| Ratio of programmed cell death/cell no. | ||
| SVZ | 0.85 ± 0.21 | 1.86 ± 0.79† |
| Cortical plate | 7.94 ± 3.02 | 9.89 ± 3.20 |
| Intermediate zone | 0.58 ± 0.21 | 0.68 ± 0.60 |
*P < 0.05, †P < 0.1 in comparison to controls.
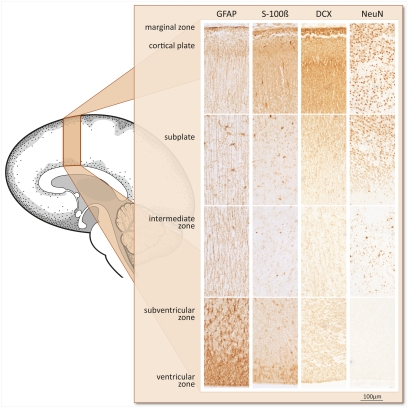
Fig. 1.
Fetal baboon brain cortical development at 0.5 gestation. Immunohistochemical distribution of glial (GFAP, S-100β) and neuronal (DCX, NeuN) marker proteins (brown precipitate) is shown. The brain at this stage of development consists of the ventricular zone and SVZ (where cells are born), the intermediate zone (through which cells migrate to target regions, such as the cortical plate), the subplate (a transient structure essential in cortical network formation), and the cortical plate and marginal zone that form the presumptive cerebral cortex. GFAP+ radial glia are highly concentrated in the SVZ. Their processes span the entire width of the cerebral wall. Sporadic GFAP+ cells with few astral branches represent mature astrocytes with growing processes. Immature S-100β+ astrocytes and DCX+ neurons are abundant in all layers. The extracellular appearance of S-100β reflects its function as a neurotrophic factor. Mature NeuN+ neurons are most prominent in the cortical plate. Their more dense occurrence in the inner than in the outer cortical plate reflects inside-out layering.
Fig. 2.
Effects of MNR in the proliferative SVZ and in the cortical plate. SVZ: MNR reduced SVZ thickness (arrowheads), increased the density of proliferative Ki-67+ cells (brown precipitate, arrowheads mark the SVZ), increased apoptotic TUNEL+ cells (dark cells marked by arrows), and reduced glial S-100β IR (brown precipitate, arrows). Cortical plate: MNR decreased local IGF-I and BDNF IR (brown precipitate), density of the neuronal network (Golgi silver impregnation, arrowheads), and O4+ preoligodendrocytes (brown precipitate, arrows) reflecting impaired cerebral myelinogenesis.
Cell Proliferation and Developmental Cell Death.
The number of Ki-67+ cells indicating proliferating cells was highest in the SVZ, followed by the intermediate zone and the cortical plate. The total number of Ki-67+ cells was similar in both experimental groups, but the cell density of Ki-67+ cells was increased by 42.5% in the SVZ of MNR fetuses (P < 0.05; Fig. 2 and Table 1). The number of TUNEL+ cells indicating developmental cell death was highest in the intermediate zone, followed by the SVZ and the cortical plate (Table 1). MNR increased the number of TUNEL+ cells in the SVZ (P < 0.05; Fig. 2 and Table 1), resulting in a raised ratio of cell death by 118.9 ± 97.6% (P < 0.1; Fig. 2 and Table 1).
Glial and Neuronal Maturation.
MNR did not affect the GFAP immunoreactivity (IR) as a marker of radial glia or vimentin IR as a marker of immature astrocytes (Table 2), but reduced SVZ S-100β IR, a marker of immature astrocytes and glial neurotrophic factor (P < 0.05; Fig. 2 and Table 2). No differences were found in the immunohistochemical distribution of immature doublecortin (DCX)-positive and mature neuron-specific nuclear antigen (NeuN)-positive neurons between fetuses of control and MNR mothers (Table 2). In contrast, MNR decreased the density of neuronal processes and suppressed O4 IR, a marker of pre-oligodendrocytes, in the cortical plate (P < 0.05; Fig. 2 and Table 2).
Table 2.
Effect of MNR on glial and neuronal maturation
| Protein expression (area of IR, mm2 × 10−2) | Controls | MNR |
| GFAP | ||
| SVZ | 2.47 ± 0.97 | 1.79 ± 0.43 |
| Intermediate zone | 3.20 ± 0.84 | 3.22 ± 0.53 |
| Cortical plate | 0.84 ± 0.41 | 0.77 ± 0.20 |
| Vimentin | ||
| SVZ | 0.32 ± 0.08 | 0.33 ± 0.17 |
| Intermediate zone | 0.15 ± 0.07 | 0.13 ± 0.05 |
| Cortical plate | 0.24 ± 0.08 | 0.25 ± 0.05 |
| S-100β | ||
| SVZ | 1.97 ± 0.28 | 0.68 ± 0.39* |
| Intermediate zone | 0.73 ± 0.17 | 0.91 ± 0.14 |
| Cortical plate | 1.04 ± 0.24 | 1.16 ± 0.54 |
| O4 | ||
| SVZ | 0.29 ± 0.19 | 0.14 ± 0.09 |
| Intermediate zone | 0.19 ± 0.02 | 0.16 ± 0.02 |
| Subplate/cortical plate | 0.30 ± 0.04 | 0.21 ± 0.04* |
| NeuN | ||
| SVZ | 0.01 ± 0.006 | 0.02 ± 0.014 |
| Intermediate zone | 0.39 ± 0.21 | 0.50 ± 0.34 |
| Cortical plate | 1.69 ± 1.22 | 1.69 ± 0.94 |
| DCX | ||
| SVZ | 2.32 ± 0.47 | 2.68 ± 0.52 |
| Intermediate zone | 0.66 ± 0.27 | 0.43 ± 0.08 |
| Cortical plate | 0.54 ± 0.31 | 0.99 ± 0.34 |
| Golgi | ||
| Subplate | 1.90 ± 0.33 | 1.23 ± 0.50* |
*P < 0.05 in comparison to controls.
Trophic Factors.
MNR reduced local brain-derived neurotrophic factor (BDNF) mRNA but did not affect cortical plate insulin-like growth factor-I (IGF-I), IGF-I receptor, or IGF binding protein 3 (IGFBP3) mRNA expression (Table 3). In contrast, IGF-I protein was reduced by 24.0 ± 18.0% (P < 0.05; Fig. 2 and Tables 3). MNR lowered BDNF IR by 36.6 ± 13.4% and 33.5 ± 26.3% in the intermediate zone and the cortical plate, respectively (P < 0.05; Fig. 2 and Table 3).
Table 3.
Effect of MNR on fetal growth factors
| Controls | MNR | |
| Gene expression, ΔRQ | ||
| IGF-I | 1.35 ± 0.34 | 1.56 ± 0.19 |
| IGF-I receptor | −0.26 ± 0.05 | −0.29 ± 0.23 |
| IGFBP3 | 1.36 ± 0.27 | 1.25 ± 0.36 |
| BDNF | 10.21 ± 0.62 | 5.33 ± 2.26* |
| Protein expression (area of IR, mm2 × 10−3) | ||
| IGF-I | 11.40 ± 5.95 | 5.68 ± 1.30* |
| BDNF | 9.25 ± 1.89 | 6.15 ± 2.44* |
*P < 0.05 in comparison to controls. ΔRQ, relative quantity.
Differentially Expressed Genes in the Fetal Brain by MNR.
Whole-genome expression profiling on cortical plate RNA from control fetuses and MNR fetuses at 0.5 gestation resulted in 318 differently expressed genes: 157 genes were up-regulated and 161 genes were down-regulated in RNA samples of MNR fetuses vs. controls (Table S1).
Ontological Pathway Analysis of Differentially Expressed Genes.
Differentially expressed genes were analyzed by ontological groups. Ontological pathways involved in biological processes, such as anti-cell cycle progression, RNA splicing, protein phosphorylation, biopolymer metabolism, and catabolism, were all up-regulated in the cortical plate RNA of MNR fetuses vs. control fetuses (Table S2). Consistent with these changes, ontological pathways involved in molecular function describing activities that occur at the molecular level were up-regulated for enzyme activator activity, GTPase activator activity, oxidoreductase activity, protein binding, protein methyltransferase activity, small conjugating protein-specific protease activity, thiolester hydrolase activity, ubiquitin thiolesterase activity, and ubiquitin-specific protease activity (Table S2). In contrast, biological ontological pathways related to nervous system development, neurogenesis, cell division and migration, cell motility, cell–cell adhesion, extracellular matrix (ECM) organization and biogenesis, establishment of cellular localization, neurite morphogenesis and axonal guidance, DNA-dependent transcription, and antiapoptosis were down-regulated, as were carboxylic acid biosynthesis, fatty acid biosynthesis, and protein folding (Table S2). Similarly, molecular ontological pathways, such as general RNA polymerase II transcription factor activity, phosphatase regulator activity, phosphoprotein binding, GTP binding and GTPase activity, microtubule binding, and dynein binding, were down-regulated (Table S2).
Expression of Key Proteins for Cerebral Development.
Genes prominently involved in the altered ontological pathways are BCL2-associated athanogene 3, ephrin (EPH) receptor B2, acetylcholinesterase (AChE; Yt blood group), neuronal cell adhesion molecule (NCAM), nonmuscle myosin heavy chain 10, and β-actin, which were all down-regulated, and zinc finger protein 259 was found to be up-regulated (P < 0.05; Table 4). Consistent with gene expression changes, the expression of encoded proteins was decreased for β-actin and ephrin-B2 (EphB2) in the cortical plate, for nonmuscle-specific myosin II heavy chain B (MHC-B) in the SVZ, and for polysialic acid associated (PSA-) NCAM in the intermediate zone (P < 0.05; Fig. 3 and Table 5). In contrast, Bcl-2 expression was increased in all three regions (P < 0.05; Fig. 3 and Table 5). AChE and zinc finger protein 1 (ZPR1) remained unchanged (Table 5).
Table 4.
Effects of MNR on gene expression of key regulatory proteins of cerebral development
| Gene name | Gene ID | Protein encoded | Biological function | Controls | MNR | Ratio | Direction | P value | GenBank accession no. | Affymetrix probe ID |
| AChE | ACHE | AChE | ECM organization | 0.64 ± 0.13 | −0.42 ± 0.33 | 2.1 | Down | 0.01 | NM_015831 | 205378_s_at |
| Actin, β | ACTB | β-Actin | Cell motility | 6.74 ± 0.04 | 6.58 ± 0.03 | 1.1 | Down | 0.01 | X00351 | 00351_m_at |
| BCL2-associated athanogene 3 | BAG3 | Bcl-2 | Antiapoptosis Cellular macromolecule and protein metabolism | −0.41 ± 0.09 | −1.00 ± 0.22 | 1.5 | Down | 0.03 | NM_004281 | 217911_s_at |
| EPH receptor B2 | EPHB2 | EphB2 | Axonogenesis and guidance | 0.93 ± 0.06 | 0.52 ± 0.14 | 1.3 | Down | 0.02 | L41939 | 210651_s_at |
| Cell migration and motility | ||||||||||
| Cellular macromolecule and protein metabolism | ||||||||||
| Neurogenesis and neuronal differentiation | ||||||||||
| Myosin, heavy chain 10, nonmuscle | MYH10 | MHC-B | Cell division Cell migration and motility Cellular macromolecule and protein metabolism | 4.26 ± 0.05 | 4.04 ± 0.05 | 1.2 | Down | 0.02 | AK026977 | 212372_at |
| NCAM | NRCAM | PSA-NCAM | Cell-cell adhesion Cell migration and motility | 1.90 ± 0.14 | 1.16 ± 0.29 | 1.7 | Down | 0.04 | NM_005010 | 204105_s_at |
| ECM organization | ||||||||||
| Axo-, neurogenesis, and neuronal differentiation | ||||||||||
| Zinc finger protein 259 | ZNF259 | ZPR1 | DNA-dependent transcription | −0.47 ± 0.10 | 0.01 ± 0.14 | 1.4 | Up | 0.02 | NM_003904 | 200054_at |
Fig. 3.
Effects of MNR on proteins that participate in regulation of brain development. MNR decreased MHC-B IR in the SVZ, PSA-NCAM IR in the intermediate zone, and EphB2 and β-actin IR in the cortical plate (brown precipitate). In contrast, antiapoptotic Bcl-2 increased (brown precipitate), probably in response to increased proliferative cell death.
Table 5.
Effect of MNR on regulatory key proteins
| Protein expression (area of IR, mm2 × 10−2) | Controls | MNR |
| AChE | ||
| SVZ | 6.52 ± 2.04 | 5.07 ± 3.00 |
| Intermediate zone | 2.76 ± 0.69 | 2.45 ± 0.79 |
| Cortical plate | 6.16 ± 2.20 | 6.83 ± 2.44 |
| β-Actin | ||
| SVZ | 9.95 ± 4.06 | 9.94 ± 2.50 |
| Intermediate zone | 1.89 ± 0.87 | 1.61 ± 0.45 |
| Cortical plate | 13.89 ± 4.09 | 8.29 ± 2.59* |
| EphB2 | ||
| SVZ | 0.60 ± 0.17 | 0.85 ± 0.32 |
| Intermediate zone | 1.15 ± 0.58 | 0.88 ± 0.57 |
| Cortical plate | 5.13 ± 1.67 | 2.84 ± 0.66* |
| MHC-B | ||
| SVZ | 13.32 ± 2.80 | 9.18 ± 2.32* |
| Intermediate zone | 6.76 ± 2.53 | 7.89 ± 1.47 |
| Cortical plate | 8.42 ± 1.88 | 7.60 ± 1.63 |
| PSA-NCAM | ||
| SVZ | 6.28 ± 2.75 | 5.85 ± 1.53 |
| Intermediate zone | 4.38 ± 0.84 | 2.61 ± 0.70* |
| Cortical plate | 6.14 ± 1.88 | 4.59 ± 1.51 |
| ZPR1 | ||
| SVZ | 9.26 ± 5.74 | 6.78 ± 1.35 |
| Intermediate zone | 6.01 ± 1.79 | 6.11 ± 1.40 |
| Cortical plate | 13.28 ± 2.06 | 13.01 ± 2.31 |
| Bcl-2 (optical density, arbitrary units) | ||
| SVZ | 53.92 ± 14.27 | 85.48 ± 13.97** |
| Intermediate zone | 41.50 ± 11.44 | 62.28 ± 10.84* |
| Cortical plate | 65.08 ± 19.19 | 93.24 ± 12.10* |
*P < 0.05; **P < 0.01 in comparison to controls.
Discussion
There is currently much interest in the level of nutrition required for optimal health and longevity. In contrast to the beneficial effects of this 30% dietary restriction on aging and life span in several species, including nonhuman primates (15), the present study shows that the same degree of nutrient restriction during pregnancy results in major impairment of fetal brain development.
MNR at the level studied here occurs in both developing and industrialized countries as a consequence of low income or self-imposed maternal dieting. The developing brain is susceptible to adverse intrauterine conditions, particularly because of its high-energy demand [during neuroglial growth and process formation, the fetal brain requires half of the fetal energy consumed (16)]. Therefore, moderate MNR probably contributes to the increasing number of children with persisting intellectual and attention deficits (6, 13, 14).
We examined the effects of a moderate degree of global MNR on early fetal cerebral development in a nonhuman primate model. We observed impaired cell proliferation as indicated by a smaller expansion of the SVZ, increased proliferative cell death, impaired neuronal maturation as indicated by reduced formation of neuronal processes and suppressed myelinogenesis in the prospective cerebral cortex. These changes were accompanied by a decrease of growth factors, such as circulating IGF-I bioavailability (20), and decreased cerebral IGF-I, BDNF, and glial neurotrophic factor S-100β. These changes are attributable to complex alterations of major biological ontological pathways and gene products that regulate cerebral development and cerebral metabolism. Most importantly, these marked effects on neurodevelopment occurred at a moderate level of malnutrition that did not affect overall fetal body and brain weight and affected maternal weight only marginally. By comparing the reproductive life spans of female baboons and women, we approximated the age of pregnant baboons to an age of life that is without risk for age-related fetal compromise (4, 5) supported by the finding that the age-dependent increase of fetal loss in baboons starts at the age of 14 y (21).
Fetal primate brain neuronal proliferation peaks at 0.5 gestation (22), the gestational stage studied here. Because overall cell number was unchanged by MNR, the presence of the smaller SVZ in MNR fetuses with more densely packed proliferating cells indicates altered ECM properties. This finding is strongly supported by the down-regulation of biological ontological pathways related to ECM organization and biosynthesis, cell-cell adhesion, and cell motility. The reduced transcriptional activity and protein expression of MHC-B known to be involved in cell motility and migration may affect cell motility, resulting in higher packaging of proliferating cells in the SVZ in MNR fetuses. Further, the diminished gene and protein expression PSA-NCAM shown here very likely interferes with ECM organization (23). Reduction of ECM may have adverse effects on CNS development (24, 25). The smaller SVZ in fetuses of nutrient-restricted mothers may also reflect less cell proliferation during early development before brain evaluation. This is supported by down-regulation of the ontological pathways related to cell proliferation, DNA-dependent transcription, anti-cell cycle progression, and neurogenesis consistent with the gene and protein expression of MHC-B and PSA-NCAM. If diminished proliferation was present at an early stage, proliferation had caught up in the MNR fetuses by 90 d because the number of proliferating cells in the smaller SVZ did not differ between control and MNR fetuses. This explanation of the thinner SVZ would suggest that modest MNR delays proliferation rates during the first half of gestation. Normalization of proliferation at the time of examination is reflected in the normal protein expression of ZPR1, a regulatory protein and transcription factor of DNA-dependent transcription, in MNR fetuses. The widespread changes of biological and molecular ontological pathways at the gene level suggest ongoing impairment of cerebral development, however.
The enhanced proliferative cell death in the MNR fetuses was accompanied by reduced bioavailability of IGF-I (20), reduced cerebral expression of IGF-I and BDNF, and up-regulation of antiapoptotic pathways. The increased expression of the antiapoptotic Bcl-2 may indicate compensatory regulation of increased apoptosis. During early stages of development, IGF-I reduces proliferative cell death (26). The increase of programmed cell death relative to cell proliferation in the SVZ may reflect higher rates of superfluous cells produced because programmed cell death in the proliferation zones contributes to the elimination of defective cells (27). The high rate of programmed cell death in the intermediate zone, however, suggests that most of the newborn cells are not eliminated in the proliferative zone. In keeping with our results, Rakic and Zecevic (18) have shown in 13 human embryos and fetuses from 4.5 to 27 wk of gestation that in relation to total cell number, the number of apoptotic cells peaks at 17 wk of gestation in the SVZ and is sustained at high levels in the intermediate zone and cortical plate from 17 to 21 wk of gestation.
Neuronal precursors are the main cell population affected by developmental cell death in the first trimester of human pregnancy (18). In agreement with the sequential generation of precursor cells for neuronal and glial lineages, it has been suggested that glial cells probably undergo programmed cell death from midgestation (18). We did not discriminate between apoptotic cells of neuronal and glial lineages. Nevertheless, MNR led selectively to a reduction of glial S-100β IR, a marker of immature astrocytes and glial neurotrophic factor. MNR also suppressed O4+ preoligodendrocytes in the cortical plate, a finding compatible with impaired myelinogenesis and cerebral myelination.
The reduced bioavailability of IGF-I and the prominent reduction of S-100β after MNR may also have contributed to the diminished formation of neuronal processes that probably will contribute to delayed maturation of the neuronal network. Reduced neuronal process formation may have contributed to the tendency to a lower brain weight that we observed in MNR fetuses. IGF-I promotes proliferation, survival, and differentiation of neurons and oligodendrocytes as well as neurite outgrowth and branching, synaptogenesis, and myelin formation (28, 29). The reduced bioavailability of circulating IGF-I and the decrease of local IGF-I in the brain following MNR are compatible with the enhanced proliferative cell death, reduced neuritogenesis, and suppressed oligodendrocyte maturation shown here. The prominent reduction of BDNF and glial neurotrophic factor S-100β may also have contributed to the diminished formation of neuronal processes. BDNF and S-100β enhance neuronal survival, stimulate neurite outgrowth, and modulate synaptic function (30, 31). The MNR-related drop in β-actin, EphB2, and NCAM gene and protein expression in the migration and target zones may cause suppression of neuritogenesis and axonal guidance (32). During early stages of development, IGF-I increases branching and total extent of dendrites of pyramidal cells (29). The greater than fivefold increase in circulating fetal IGFBP3 and 57% decrease in IGF-I:IGFBP3 that we have shown previously (20) may have contributed to the reduced bioavailability of IGF-I and differs from the reduction in IGFBP3 shown in fetal cord serum of preterm growth-retarded human babies (33). This difference may be attributable to the different stages of gestation studied as well as the heterogeneous challenges resulting in human intrauterine growth restriction compared with the controlled and specific nutritional challenge used in this study. Regulation of IGFBPs is complex and depends on the stage of development (34). The observed reduction of cerebral cortical IGF-I expression by MNR is supported by the reported decreased cerebral cortical IGF-I binding in neonatal rats during protein-calorie malnutrition (35).
Our data, obtained at various levels between gene expression and structural development, indicate subtle but widespread disturbances of early organizational processes in cerebral development. Gross structural and biochemical markers, such as overall cerebral protein, DNA, RNA, cholesterol, phospholipid, or water content, do not seem to be sufficient to reflect developmental changes induced by MNR. In a study in the pregnant rhesus monkey, these parameters did not change in the presence of even more severe decreases in nutrient availability (50% caloric combined with 70% protein reduction) (36). Our results substantially extend the data in rodents showing that moderate MNR results in a variety of minimal brain dysfunctions responsible for various developmental disturbances (9). In contrast to these altricial species, in which studies of nutritional effects are generally conducted postnatally at a very different pO2 and in a different hormonal milieu, the nonhuman primate model provides the closest possible relationship to humans from which such data cannot be obtained.
We hypothesize that the disturbed brain development following moderate dietary reduction during early pregnancy may have long-term effects on offspring cerebral function and contribute to the impaired higher mental function in offspring at the lower end of normal birth weight in human pregnancy (5, 13, 14). In contrast to the beneficial effects of dietary reduction on aging and life span (15), nutrient reduction during pregnancy at the same moderate level is an important epigenetic factor that provides suboptimal conditions for appropriate fetal brain development with potential life-long consequences.
Materials and Methods
All procedures were approved by the Southwest Foundation for Biomedical Research and University of Texas Health Science Center Institutional Animal Care and Use Committees and conducted in American Association for the Accreditation of Laboratory Animal Care International-approved facilities.
In brief, pregnant baboons (Papio hamadryas) were individually fed ad libitum with a standard monkey diet (n = 8) or 70% of the recorded ad libitum food intake starting from 30 d of gestation (n = 6). Harvested at 90 d of gestation, fetal brain tissue samples were fixed with 4% (wt/vol) paraformaldehyde solution in saline for immunohistochemistry or flash-frozen in liquid nitrogen for gene expression analysis. Histological examination focused on the cerebral hemispheres and included gross morphology, cell proliferation and developmental cell death, and neuronal and glial cell maturation using well-accepted marker proteins (Table S3). Morphometric measures and semiquantification of immunohistochemical staining were performed using image analysis software (Scion Image 6.21; National Institutes of Health). A whole-genome expression profile of the cortical plate was determined for each RNA sample using the Affymetrix Human Genome U133 Plus 2.0 Array and GCOS software (Affymetrix). Data were filtered to account for baboon-human gene sequence differences, and data analysis was performed using GeneSifter software (GeneSifter.Net; VizX Labs). Gene expression of IGF-I, IGFBP3, and IGF receptors in the fetal cortical plate was estimated using a two-step quantitative real-time PCR protocol (Applied Biosystems). To confirm the results of the genome analysis, we assessed the protein expression in a subset of genes by immunohistochemistry.
Differences between the values of the experimental groups were tested for significance using the two-tailed Student's t test (P < 0.05). Data are presented as mean ± SEM. Pathway significance was determined by z score (≤2 or >2). Details are given in SI Materials and Methods.
Acknowledgments
We thank Natalia E. Schlabritz-Loutsevitch for supervising the breeding and care of the animals, Sue L. Jenkins for statistical support, and Michael Brodhun for helpful discussions of staining procedures. We are grateful to Marie Silva, Claudia Sommer, Ina Ingrisch, and Antonio Perez for excellent technical assistance. This work was supported by Grants HD 21350-17, Bundesministerium für Bildung und Forschung (BMBF) 01ZZ0105, and BMBF 0315581 (JenAge).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 2641.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009838108/-/DCSupplemental.
References
- 1.The State of Food Insecurity in the World. Rome: Food and Agriculture Organization of the United Nations; Food and Agriculture Organization of the United Nations (2004) [Google Scholar]
- 2.United Nations International Children's Emergency Fund. State of the World's Children. New York: Oxford Univ Press; 2001. [Google Scholar]
- 3.Crozier SR, Robinson SM, Godfrey KM, Cooper C, Inskip HM. Women's dietary patterns change little from before to during pregnancy. J Nutr. 2009;139:1956–1963. doi: 10.3945/jn.109.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker PN, et al. A prospective study of micronutrient status in adolescent pregnancy. Am J Clin Nutr. 2009;89:1114–1124. doi: 10.3945/ajcn.2008.27097. [DOI] [PubMed] [Google Scholar]
- 5.Beard JR, et al. Socioeconomic and maternal determinants of small-for-gestational age births: Patterns of increasing disparity. Acta Obstet Gynecol Scand. 2009;88:575–583. doi: 10.1080/00016340902818170. [DOI] [PubMed] [Google Scholar]
- 6.Olness K. Effects on brain development leading to cognitive impairment: A worldwide epidemic. J Dev Behav Pediatr. 2003;24:120–130. doi: 10.1097/00004703-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Benton D ILSI Europe a.i.s.b.l. The influence of children's diet on their cognition and behavior. Eur J Nutr. 2008;47(Suppl 3):25–37. doi: 10.1007/s00394-008-3003-x. [DOI] [PubMed] [Google Scholar]
- 8.Walker SP, et al. International Child Development Steering Group. Child development: Risk factors for adverse outcomes in developing countries. Lancet. 2007;369:145–157. doi: 10.1016/S0140-6736(07)60076-2. [DOI] [PubMed] [Google Scholar]
- 9.Grantham-McGregor S, Baker-Henningham H. Review of the evidence linking protein and energy to mental development. Public Health Nutr. 2005;8(7A):1191–1201. doi: 10.1079/phn2005805. [DOI] [PubMed] [Google Scholar]
- 10.Morley R, Lucas A. Nutrition and cognitive development. Br Med Bull. 1997;53:123–134. doi: 10.1093/oxfordjournals.bmb.a011595. [DOI] [PubMed] [Google Scholar]
- 11.Morgane PJ, et al. Prenatal malnutrition and development of the brain. Neurosci Biobehav Rev. 1993;17:91–128. doi: 10.1016/s0149-7634(05)80234-9. [DOI] [PubMed] [Google Scholar]
- 12.Levitsky DA, Strupp BJ. Malnutrition and the brain: Changing concepts, changing concerns. J Nutr. 1995;125(Suppl):2212S–2220S. doi: 10.1093/jn/125.suppl_8.2212S. [DOI] [PubMed] [Google Scholar]
- 13.Matte TD, Bresnahan M, Begg MD, Susser E. Influence of variation in birth weight within normal range and within sibships on IQ at age 7 years: Cohort study. BMJ. 2001;323:310–314. doi: 10.1136/bmj.323.7308.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richards M, Hardy R, Kuh D, Wadsworth ME. Birth weight and cognitive function in the British 1946 birth cohort: Longitudinal population based study. BMJ. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Colman RJ, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibbons A. Solving the brain's energy crisis. Science. 1998;280:1345–1347. doi: 10.1126/science.280.5368.1345. [DOI] [PubMed] [Google Scholar]
- 17.Neugebauer R, Hoek HW, Susser E. Prenatal exposure to wartime famine and development of antisocial personality disorder in early adulthood. JAMA. 1999;282:455–462. doi: 10.1001/jama.282.5.455. [DOI] [PubMed] [Google Scholar]
- 18.Rakic S, Zecevic N. Programmed cell death in the developing human telencephalon. Eur J Neurosci. 2000;12:2721–2734. doi: 10.1046/j.1460-9568.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- 19.Bystron I, Blakemore C, Rakic P. Development of the human cerebral cortex: Boulder Committee revisited. Nat Rev Neurosci. 2008;9:110–122. doi: 10.1038/nrn2252. [DOI] [PubMed] [Google Scholar]
- 20.Li C, et al. Effects of maternal global nutrient restriction on fetal baboon hepatic insulin-like growth factor system genes and gene products. Endocrinology. 2009;150:4634–4642. doi: 10.1210/en.2008-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schlabritz-Loutsevitch NE, et al. The baboon model (Papio hamadryas) of fetal loss: Maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol. 2008;37:337–345. doi: 10.1111/j.1600-0684.2008.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex. 2003;13:541–549. doi: 10.1093/cercor/13.6.541. [DOI] [PubMed] [Google Scholar]
- 23.Nielsen J, Kulahin N, Walmod PS. Extracellular protein interactions mediated by the neural cell adhesion molecule, NCAM: Heterophilic interactions between NCAM and cell adhesion molecules, extracellular matrix proteins, and viruses. Adv Exp Med Biol. 2010;663:23–53. doi: 10.1007/978-1-4419-1170-4_2. [DOI] [PubMed] [Google Scholar]
- 24.Jones FS, Jones PL. The tenascin family of ECM glycoproteins: Structure, function, and regulation during embryonic development and tissue remodeling. Dev Dyn. 2000;218:235–259. doi: 10.1002/(SICI)1097-0177(200006)218:2<235::AID-DVDY2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 25.Garcion E, Faissner A, ffrench-Constant C. Knockout mice reveal a contribution of the extracellular matrix molecule tenascin-C to neural precursor proliferation and migration. Development. 2001;128:2485–2496. doi: 10.1242/dev.128.13.2485. [DOI] [PubMed] [Google Scholar]
- 26.O'Kusky JR, Ye P, D'Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435–8442. doi: 10.1523/JNEUROSCI.20-22-08435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oppenheim RW. Cell death during development of the nervous system. Annu Rev Neurosci. 1991;14:453–501. doi: 10.1146/annurev.ne.14.030191.002321. [DOI] [PubMed] [Google Scholar]
- 28.D'Ercole AJ, Ye P. Expanding the mind: Insulin-like growth factor I and brain development. Endocrinology. 2008;149:5958–5962. doi: 10.1210/en.2008-0920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niblock MM, Brunso-Bechtold JK, Riddle DR. Insulin-like growth factor I stimulates dendritic growth in primary somatosensory cortex. J Neurosci. 2000;20:4165–4176. doi: 10.1523/JNEUROSCI.20-11-04165.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Eldik LJ, Wainwright MS. The Janus face of glial-derived S100B: Beneficial and detrimental functions in the brain. Restor Neurol Neurosci. 2003;21:97–108. [PubMed] [Google Scholar]
- 31.Cohen S, Greenberg ME. Communication between the synapse and the nucleus in neuronal development, plasticity, and disease. Annu Rev Cell Dev Biol. 2008;24:183–209. doi: 10.1146/annurev.cellbio.24.110707.175235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rønn LC, Hartz BP, Bock E. The neural cell adhesion molecule (NCAM) in development and plasticity of the nervous system. Exp Gerontol. 1998;33:853–864. doi: 10.1016/s0531-5565(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 33.Giudice LC, et al. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab. 1995;80:1548–1555. doi: 10.1210/jcem.80.5.7538146. [DOI] [PubMed] [Google Scholar]
- 34.Fowden AL. The insulin-like growth factors and feto-placental growth. Placenta. 2003;24:803–812. doi: 10.1016/s0143-4004(03)00080-8. [DOI] [PubMed] [Google Scholar]
- 35.Maheshwari HG, Mermelstein S, vonSchlegell AS, Shambaugh GE., III Alteration in IGF-I binding in the cerebral cortex and cerebellum of neonatal rats during protein-calorie malnutrition. Neurochem Res. 1997;22:313–319. doi: 10.1023/a:1022447007154. [DOI] [PubMed] [Google Scholar]
- 36.Cheek DB, et al. Nutritional studies in the pregnant rhesus monkey—The effect of protein-calorie or protein deprivation on growth of the fetal brain. Am J Clin Nutr. 1976;29:1149–1157. doi: 10.1093/ajcn/29.10.1149. [DOI] [PubMed] [Google Scholar]