Abstract
Conditional gene repair mutations in the mouse can assist in cell lineage analyses and provide a valuable complement to conditional gene inactivation strategies. We present a method for the generation of conditional gene repair mutations that employs a loxP-flanked (floxed) selectable marker and transcriptional/translational stop cassette (neostop) located within the first intron of a target gene. In the absence of Cre recombinase, expression of the targeted allele is suppressed generating a null allele, while in the presence of Cre, excision of neostop restores expression to wild-type levels. To test this strategy, we have generated a conditional gene repair allele of the mouse Huntington’s disease gene homolog (Hdh). Insertion of neostop within the Hdh intron 1 generated a null allele and mice homozygous for this allele resembled nullizygous Hdh mutants and died after embryonic day 8.5. In the presence of a cre transgene expressed ubiquitously early in development, excision of neostop restored Hdh expression and rescued the early embryonic lethality. A simple modification of this strategy that permits the generation of conventional gene knockout, conditional gene knockout and conditional gene repair alleles using one targeting construct is discussed.
INTRODUCTION
The introduction of site-specific modifications into the mouse genome via homologous recombination in embryonic stem (ES) cells has revolutionized mouse genetics and enabled the study of gene function in the context of an entire organism (1). The targeted disruption of genes in mice, for example, provides a causal relationship between loss-of-function and phenotype. However, many genes have critical functions throughout development and gene ablation can result in embryonic or early postnatal lethality, precluding the analysis of gene function at more advanced developmental stages or in the adult (2). Conditional gene inactivation, usually employing the Cre/loxP site-specific recombination system, provides a means to control the developmental and tissue-specificity of gene disruption, thus circumventing early lethality in knockouts of developmentally critical genes (3).
In the Cre/loxP conditional knockout strategy, a site-specific Cre recombinase encoded by the bacteriophage P1 cre gene catalyzes excision of DNA sequences between pairs of 34 bp sequences (loxP sites), provided that they are in the same orientation (4). In practice, Cre/loxP conditional mutagenesis requires crosses between Cre-producing and Cre-responding strains of mice (3). The temporal and tissue-specificity of recombination is controlled by both the expression of the cre transgene and the ability of the loxP-modified (floxed) allele to respond to Cre (5).
Recently, a strategy complementary to conditional gene inactivation was proposed that would be useful in studies aiming to characterize the function of gene products by rescuing lineage or developmental stage-specific knockout phenotypes by conditional gene repair (6,7). This approach relies on the targeted insertion of a positive selection gene cassette (typically the neomycin phosphotransferase gene, neo) flanked by loxP sites into an intron. Positive selection cassettes have the potential to interfere with normal expression of the targeted allele by promoter interference, disruption of normal splicing patterns or premature transcript termination (8–12). A potential drawback to this approach is the unpredictable effect of neo on the expression level of the target gene. Insertion of neo within an intron may result in unaltered expression, a reduction in targeted gene expression (generating a hypomorphic allele), or complete inactivation (5,8,10,13).
The Cre/loxP system has also been used to activate conditionally transgene expression by employing a floxed synthetic transcriptional/translational ‘stop’ cassette (STOP) (14). The STOP cassette consists of the 3′ portion of the yeast His3 gene, an SV40 polyadenylation sequence and a false translation initiation codon followed by a 5′ splice donor site. The floxed STOP cassette is inserted between the promoter and coding sequences of a transgene, ensuring that few, if any, transcripts containing the coding region are generated. In the presence of Cre, recombination at the loxP sites excises the STOP cassette, thus activating expression of the transgene. A similar strategy employing a floxed neo cassette was used to conditionally activate expression of a hepatitis C viral cDNA transgene in liver cells following infection with an adenovirus vector expressing Cre (15). In situations where leaky expression of the transgene cannot be tolerated, combining the use of both a neo and strong STOP cassette should effectively block transcription through downstream coding sequences. This strategy was employed successfully recently to control expression of an attenuated diphtheria toxin gene targeted into the endogenous mouse D1 dopamine receptor gene (16). A floxed cassette combining both neo and STOP elements (neostop) was placed between the dopamine receptor promoter and the toxin coding sequences. In the absence of Cre expression, toxin gene expression was not detected. Conditional activation of toxin expression was achieved following expression of a cre transgene under the control of the adenovirus EIIA promoter.
In this study, we describe a method for conditional gene repair of targeted loci that is based on the use of a neostop cassette as a more reliable substitute for the unpredictable effect of floxed neo insertions on targeted gene expression. In this strategy, the neostop cassette is inserted into the first intron of a target gene via homologous recombination in ES cells. Targeted insertion of the neostop cassette within the intron generates a null allele that can be activated by Cre-mediated recombination. To test this approach in a model system, we have generated a conditional gene repair allele of the mouse homolog of the Huntington’s disease gene (HdhSTOP). Mice that are heterozygous for the HdhSTOP allele and a Hdh null allele (HdhSTOP/–) generate no detectable Hdh mRNA transcripts, are indistinguishable from Hdh–/– mutants and die by embryonic day 9.5 (E9.5) of gestation. Cre-mediated excision of the neostop cassette at the two-cell stage of embryonic development results in normal expression from the recombined allele (HdhΔSTOP). Early embryonic lethality is bypassed and adult mutant mice are indistinguishable from their wild-type littermates. Advantages and applications of this approach as a complement to existing conditional gene modification strategies are discussed.
MATERIALS AND METHODS
Construction of the HdhSTOP targeting vector
The neostop cassette was assembled by combining a pgk-neo gene cassette with the His3-SV40 pA sequences from a Cre-mediated recombination reporter plasmid (pEF1a-lox-STOP-lox-LacZ). Two loxP sequences in the same orientation were then inserted flanking the neo and STOP sequences (Fig. 1A). The cassette was cloned into pBluescript SK+ (pBSK+) and can be excised with XbaI and KpnI. The neostop cassette was blunted with Klenow DNA polymerase, and then cloned into a unique KpnI site (blunted) located within the first intron of a 4.7 kb genomic HindIII Hdh fragment containing the promoter, first exon and a portion of the first intron cloned into pBSK+. To enrich for targeted clones by negative selection with ganciclovir, a thymidine kinase (tk) gene cassette was cloned adjacent to the 3′ flanking homology (17). To extend the 5′ flanking homology, a 7.1 kb NotI–HindIII (partial) genomic fragment was inserted between the NotI site within the pBSK+ polylinker and the 5′ HindIII site of the 4.7 kb Hdh fragment. The final construct contained 9.1 and 2.75 kb of 5′ and 3′ flanking homology, respectively (Fig. 1B).
Figure 1.
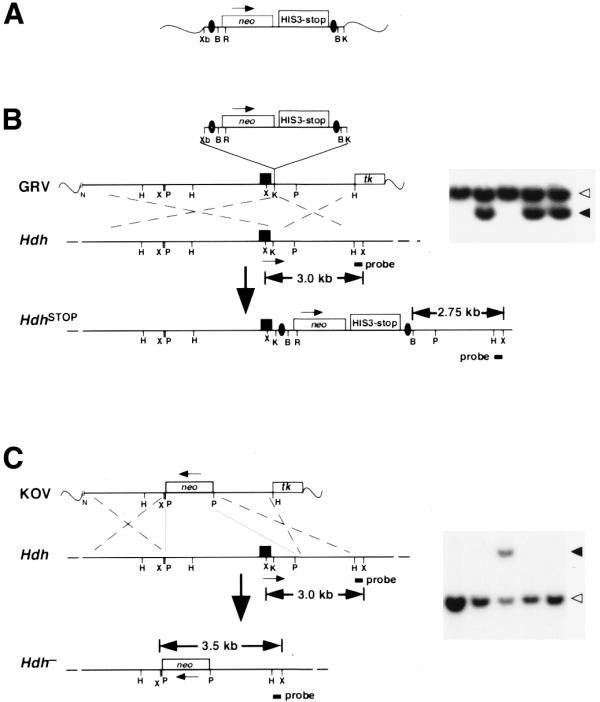
Generation of a conditional gene repair allele of Hdh. (A) Schematic of the neostop cassette. The positions of the neo cassette (neo) and the transcriptional/translational stop sequences (HIS3-stop) are indicated along with the positions of the loxP sites (black ovals). (B) Schematic of the HdhSTOP gene repair targeting vector (GRV), showing the location of the neostop cassette within intron 1 near Hdh exon 1 (black rectangle, containing the translation initiation site), together with the wild-type Hdh allele (Hdh) and the product of homologous recombination, HdhSTOP. The position of a thymidine kinase gene cassette (tk) for negative selection of the transfected ES cells is indicated. (C) Schematic of the Hdh– knockout targeting vector (KOV), wild-type Hdh allele (Hdh) and the targeted Hdh null allele (Hdh–). The 5′ and 3′ portions of the flanking homology of the Hdh– targeting vector are identical to the sequences used in the HdhSTOP targeting construct. The overall homology, however, is smaller in the Hdh– construct because of the promoter and exon 1 deletion. The position of a Hdh 3′ flanking probe along with the sizes of diagnostic restriction fragments identifying the wild-type, HdhSTOP and Hdh– alleles are indicated in (B) and (C). The transcriptional orientations of the Hdh gene and the neo cassette are indicated with arrows, and wavy lines indicate plasmid vector sequence. Southern blots showing DNA prepared from ES cell clones digested with either BamHI–XmnI or XmnI and hybridized with the Hdh 3′ flanking probe are shown to the right of the schematics in (B) and (C), respectively. Hybridizing DNA fragments representing the wild-type and targeted alleles are indicated with white and black arrowheads, respectively. The restriction sites are: XbaI (Xb), XmnI (X), BamHI (B), EcoRI (R), KpnI (K), NotI (N), HindIII (H), PstI (P).
Construction of the Hdh– knockout vector
The same flanking genomic sequences employed in the HdhSTOP targeting vector were used in the knockout vector with the exception that 4.4 kb of sequence including the promoter, exon 1 and part of intron 1 were deleted and replaced with a pgk-neo cassette as described (18). The final construct contained 5.2 and 2.2 kb of 5′ and 3′ homology, respectively, as well as a tk cassette for the enrichment of targeted clones by negative selection with ganciclovir (Fig. 1C).
Gene targeting
Targeting vector DNA was linearized with NotI and introduced by electroporation into W9.5 ES cells grown on mitomycin-C-treated G418-resistant primary mouse embryonic fibroblasts as described (19). ES cell DNA purified from G418 and ganciclovir-resistant clones was analyzed by Southern blotting using a probe that distinguishes between the targeted and wild-type Hdh alleles. Targeted ES cells were injected into the blastocoel cavity of E3.5 C57BL/6 embryos using standard procedures (20). Germline chimeras were produced with three independent HdhSTOP/+ ES clones.
Genotyping
For routine genotyping of progeny, the HdhSTOP allele was detected by PCR (3 min denaturation at 94°C, followed by 30 cycles consisting of 45 s denaturation at 94°C, 45 s annealing at 61°C and 1 min extension at 72°C) using the primers HDINT1(forward) 5′-GGCCTGCGTGCTGGGCATG-3′ and PGK (reverse) 5′-AGCGCATGCTCCAGACTGCC-3′ to generate a 200 bp product. Employing the same PCR cycle conditions that were used to amplify a portion of the HdhSTOP allele, the Hdh– allele was detected using the primers NEO-6 (forward) 5′-AACACCGAGCGACCCTGCAG-3′ and NEO-3 (reverse) 5′-AGAGCAGCCGATTGTCTGTTGT-3′ to generate a 130 bp product. The Hs-cre1 transgene was detected using the primers CRE-1 (forward) 5′-CTGCCACGACCAAGTGACAGC-3′ and CRE-2 (reverse) 5′-CTTCTCTACACCTGCGGTGCT-3′ to generate a 324 bp product corresponding to a portion of the cre coding region. To discriminate between the recombined HdhΔSTOP allele and the wild-type allele, PCR amplification across the single loxP site remaining following Cre-mediated recombination was performed using the primers HDINT1 (forward) and HDINT0 (reverse) 5′-CTGACCCGGCTCTGTCTCCT-3′ to generate 140 and 240 bp products corresponding to the wild-type allele and recombined allele, respectively.
RT–PCR
RNA was purified from embryos using Trizol reagent (Gibco BRL) and treated with RNase-free DNase I (Boehringer Mannheim) to eliminate contaminating genomic DNA. cDNA was synthesized in a 20 µl reaction containing 2 µg total RNA template using a mixture of random hexamer and oligo(dT) primers in the presence and absence (control) of Superscript II RNase H– reverse transcriptase (Gibco BRL) and then treated briefly with RNase H (15 min at 37°C). Aliquots of the cDNA product (2 µl) were then amplified in PCR reactions for β-actin (1 min denaturation at 94°C and 2 min extension at 72°C for 27 cycles) using the primers actin 1 (forward) 5′-GACAACGGCTCCGGCATGTGCAAAG-3′ and actin 2 (reverse) 5′-TTCACGGTTGGCCTTAGGGTTCAGGG-3′ to generate a 320 bp product. Hdh exon 1 sequence was amplified (30 cycles consisting of 45 s denaturation at 94°C, 45 s annealing at 57°C and 1 min extension at 72°C) using the primers Hdh1 (forward) 5′-CATTCATTGCCTTGCTGCTAAG-3′ and Hdh2 (reverse) 5′-CTGAAACGACTTGAGCGACTC-3′ to generate a 140 bp product. Hdh cDNA sequences within exons 3–5 and positions 5069–5367 (GenBank accession no. L23312) were amplified (30 cycles consisting of 45 s denaturation at 94°C, 45 s annealing at 61°C and 1 min extension at 72°C) using the primers Hdhex3 (forward) 5′-TCAGAAACTCTTGGGCATCGCT-3′and Hdhex5 (reverse) 5′-TCTGAGGTCGAACCAGGTGAG-3′ to generate a 240 bp product and Hdh11 (forward) 5′-TGTGGATATCTGGAATCCTCGC-3′and Hdh12 (reverse) 5′-GAACGTATGCTGCTGTTCACTC-3′ to generate a 300 bp product, respectively.
Northern analysis
Total RNA was isolated from adult brain tissue using Trizol reagent according to the protocol provided by the manufacturer. RNA (15 µg) was fractionated on 1% agarose MOPS/sodium acetate/EDTA gels containing formaldehyde. RNA was then transferred to nylon membranes in 10× SSC and the blots hybridized with a 32P-labeled random primed DNA probe corresponding to Hdh exon 1 or to a DNA fragment corresponding to Hdh cDNA positions 281–1425.
Western analyses
Western analyses were performed using antibodies recognizing either the N-terminal or C-terminal regions of huntingtin [HP-1 and HF-1, respectively (21)]. Briefly, tissues were homogenized by douncing in hypotonic lysis buffer (10 mM HEPES pH 8.3, 1.5 mM MgCl2, 1 mM DTT, 1 mM PMSF, 5 µg/ml leupeptin, 5 µg/ml pepstatin), crude cellular debris was pelleted by centrifugation and the protein concentration in the supernatant fraction was determined using a dye-binding assay (Bio-Rad). An aliquot of 50 µg protein was fractionated on 4–20% gradient polyacrylamide gels using a 3% stacking gel containing 4.5% N,N′-diallyltartardiamide (DATD) crosslinker. Protein was transferred electrophoretically to a nitrocellulose membrane and probed overnight at 4°C with a 1:1000 or 1:2500 dilution of HP-1 or HF-1, respectively. Signal was visualized using enhanced chemiluminescence reagents (ECL, Amersham).
RESULTS
Generation of a conditional gene repair allele of Hdh
To generate a conditional gene repair Hdh allele, we assembled a targeting vector containing a neostop cassette inserted into the Hdh intron 1 near the 5′ splice donor site (Fig. 1A and B). The neo gene within the neostop cassette provides for the positive selection of transfected ES cells and may interfere with the expression of the target gene. The addition of the STOP sequence within the cassette enhances any potential interference conferred by neo. To compare directly targeting efficiencies obtained with the conditional gene repair vector and a standard deletion/replacement vector, we assembled in parallel a targeting construct that replaces the Hdh promoter, exon 1 and a part of intron 1 with a pgk-neo cassette (Fig. 1C). Both constructs were derived using the same Hdh genomic sequences, although the deletion/replacement vector contains 4.4 kb less flanking homology in comparison with the conditional gene repair vector because of the promoter/exon/intron deletion (Materials and Methods). The deletion/replacement vector is similar to a construct described previously that was used to generate a Hdh null allele [Hdhtm1szi (18)] except for the substitution of a pgk-neo positive selection cassette for an MC1-neo cassette. In parallel experiments using the same ES cell preparation, the frequency of gene targeting was higher using the gene repair insertion vector in comparison with the frequency obtained using the standard deletion vector (30 targeted out of 96 and 12 targeted out of 96 analyzed ES cell clones, respectively; Fig. 1B and C).
HdhSTOP/STOP and HdhSTOP/– mice resemble Hdh–/– homozygous mutants
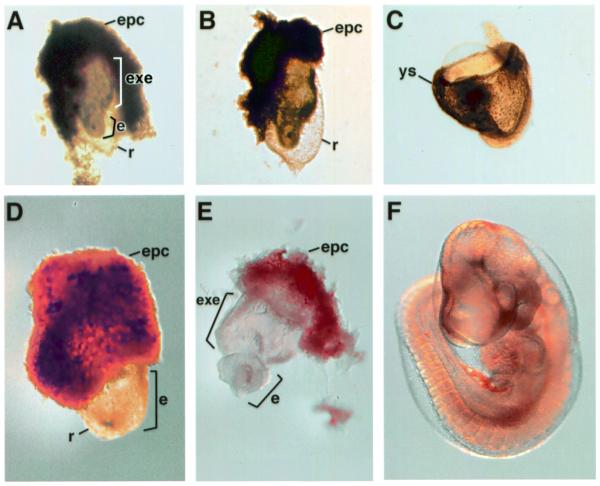
Mice heterozygous for the HdhSTOP allele were indistinguishable from wild-type littermates (data not shown). To determine if the HdhSTOP allele was equivalent functionally to a null allele, HdhSTOP heterozygotes were either intercrossed or crossed with mice heterozygous for a null allele (Hdh+/–). Genotyping of progeny at postnatal day 10 (P10) showed that while the heterozygous and wild-type progeny were obtained at the expected Mendelian ratio of approximately 2:1, HdhSTOP/STOP and HdhSTOP/– offspring (χ2 P = 0.0003 and <0.0001, respectively) were absent (Table 1). Thus, mice either homozygous for the HdhSTOP allele or carrying both the HdhSTOP and null alleles were dying during embryonic development. To determine the time of embryonic lethality, pregnant females were killed at 8.5 or 9.5 days postcoitum (E8.5 or E9.5). Embryos were dissected, scored for morphological abnormalities and genotyped by PCR (Table 1). At E8.5 and E9.5, HdhSTOP/STOP and HdhSTOP/– embryos were now obtained with the expected Mendelian frequencies (χ2 P = 0.92 and 0.98, respectively) and resembled morphologically Hdh–/– embryos at E8.5 and E9.5 (Fig. 2). Although wild-type and heterozygous E8.5 embryos had developed neural head folds and up to seven somites, the E8.5 HdhSTOP/STOP and HdhSTOP/– embryos were developmentally retarded in appearance (Fig. 2 and Table 1). The embryonic portion of the conceptus was small and stubby with no obvious neural folds and somites were absent. At E9.5, although the HdhSTOP/STOP and HdhSTOP/– embryos had increased slightly in size, the embryonic portion had begun to resorb (Fig. 2).
Table 1. Genotyped progeny.
| Genetic cross | Age | No. of litters | Number genotyped (anumber expected) | ||||
| |
|
|
Hdh: +/+ |
STOP/+ |
STOP/STOP |
STOP/– |
+/– |
| HdhSTOP/+ × HdhSTOP/+ | E8.5 | 3 | 6 (6) | 15 (12) | 5b (6) | NAc | NA |
| E9.5 | 3 | 7 (8) | 15 (16) | 8b (8) | NA | NA | |
| P10 | 8 | 20 (20) | 36 (40) | 0 (20) | NA | NA | |
| Hdh+/– × HdhSTOP/+ | E8.5 | 4 | 11 (10) | 12 (10) | NA | 9b (10) | 10 (10) |
| E9.5 | 5 | 12 (13) | 14 (13) | NA | 13b (13) | 14 (13) | |
| P10 | 17 | 42 (40) | 40 (40) | NA | 0 (40) | 38 (40) | |
aCalculated using the predicted Mendelian ratio.
bEmbryos with abnormal morphology resembling Hdh(–/–) mutants. The ratio of normal to abnormal embryos was 3:1, as expected. Embryonic resorptions were not observed at the ages examined with the exception of two empty decidua.
cNA, not applicable.
Figure 2.
HdhSTOP/– embryos resemble Hdh–/– embryos. Examples of Hdh–/– (A and D), HdhSTOP/– (B and E), wild-type (C) and Hdh+/– (F) embryos at E8.5 (A–C) and E9.5 (D–F) are shown. The embryonic portions (e) of the Hdh–/– and HdhSTOP/– mutants are stubby and retarded in development in comparison to their wild-type counterparts. The extraembryonic (exe) portion of the mutant embryos and the yolk sac (ys) of the E8.5 wild-type embryo are indicated. The ectoplacental cone (epc) of the embryos in (A), (B), (D) and (E) was not removed during dissection, while the yolk sac was removed from the embryo in (F) for better visibility. Reichert’s membrane (r) is visible as a thin transparent sac surrounding the mutant embryos in (A), (B) and (D) but was removed during dissection from the embryos in (C) and (E).
HdhSTOP is a null allele
To determine if insertion of neostop within the Hdh intron 1 resulted in loss of Hdh expression, northern analysis was performed on total RNA isolated from the brains of wild-type, Hdh+/–, and HdhSTOP/+ mice using as a probe a Hdh cDNA fragment corresponding to cDNA positions 281–1425 (Fig. 3A). Predominant RNA transcripts of 13 and 10 kb corresponding to the two major Hdh mRNA transcripts differing at the 3′ end by alternative polyadenylation were detected in all RNA samples. Half the amount of Hdh mRNA was detected in the HdhSTOP/+ lane relative to the wild-type lane and equivalent amounts of mRNA were detected in the Hdh+/– and HdhSTOP/+ lanes indicating that little, if any, full-length RNA transcripts were produced from the HdhSTOP allele. Long exposure of the same blot that was stripped of the original probe and hybridized with a Hdh exon 1-specific probe failed to detect any truncated RNA transcripts that may have originated from the HdhSTOP allele (data not shown).
Figure 3.
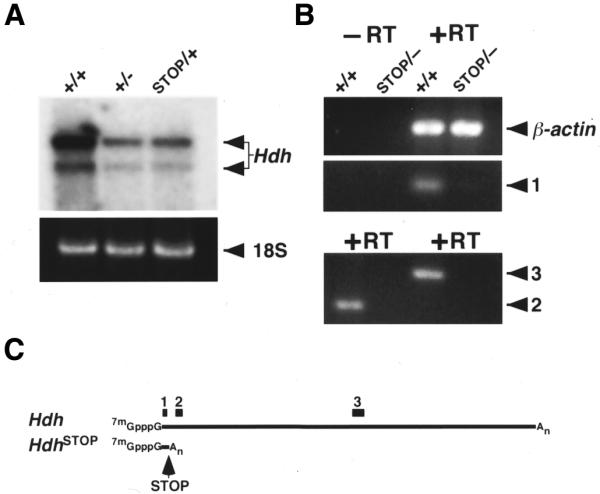
neostop suppresses Hdh expression. (A) Northern analysis of total RNA from adult brain obtained from a wild-type (+/+), Hdh heterozygote (+/–) and HdhSTOP/+ (STOP/+) mice is shown in the top panel. Hdh expression in the HdhSTOP/+ brain is equivalent to the expression in a Hdh+/– heterozygote. The two major Hdh mRNA transcripts (differing at their 3′ ends by the use of alternative polyadenylation sites) are indicated with arrows. A region of the RNA gel containing the 18S rRNA species (stained with ethidium bromide to control for sample loading and indicated with an arrow on the right) is shown in the bottom panel. (B) To determine if any transcripts were produced from the HdhSTOP allele, RT–PCR analyses were performed with total RNA isolated from wild-type (+/+) or HdhSTOP/– (STOP/–) E8.5 embryos using oligonucleotide primer pairs amplifying a region within exon 1 [1], exons 3–5 [2] and in the middle of the Hdh mRNA [3]. Reverse transcriptase was omitted from the cDNA synthesis reaction to control for DNA contamination (–RT), while amplification of β-actin cDNA was used as a control for RNA integrity and cDNA synthesis (β-actin). Note that PCR products corresponding to primer pairs 2 and 3 were not detected by ethidium bromide staining using the HdhSTOP/– cDNA template but were detected when using wild-type cDNA template. In contrast, a PCR product corresponding to β-actin was detected in equivalent amounts using the wild-type and HdhSTOP/– RNA samples. A PCR product corresponding to a putative truncated HdhSTOP RNA [1] was barely visible by ethidium bromide staining. (C) Schematics of the Hdh wild-type mRNA transcript and the putative truncated RNA transcript produced by the HdhSTOP allele (HdhSTOP). The positions of the PCR amplification products corresponding to the primer pairs 1, 2 and 3 are indicated by black rectangles.
To determine if any HdhSTOP RNA transcripts were produced that may have been below the northern blotting detection limits, we performed RT–PCR assays on total RNA prepared from E8.5 HdhSTOP/– and normal littermate embryos. Amplification of a region within exon 1 (Hdh cDNA nucleotides 31–171), and two regions downstream of intron 1 (nucleotides 417–655 spanning Hdh exons 3–5, and nucleotides 5069–5367) was performed on random hexamer and oligo(dT)-primed cDNA (Fig. 3B and C). To control for the quality of the RNA substrate and the cDNA synthesis reaction, amplification with β-actin primers was performed in parallel. Amplification with each pair of primers was also performed in the absence of reverse transcriptase to control for genomic DNA contamination of the cDNA template (Fig. 3B and data not shown). RT–PCR products of the predicted size corresponding to sequences within exon 1, exons 3–5 and a region in the middle of the Hdh mRNA transcript were obtained using cDNA prepared from wild-type littermate controls (Fig. 3B). In contrast, Hdh RT–PCR products corresponding to sequence within exon 3–5 and nucleotides 5069–5367 were not detected using the HdhSTOP/– cDNA as template. Interestingly, an RT–PCR product containing Hdh exon 1 sequence was barely visible following 30 amplification cycles in reactions using HdhSTOP/– cDNA as template, suggesting that a truncated RNA transcript was produced that was either unstable or present in very low amounts.
Rescue of HdhSTOP expression and HdhSTOP/– early embryonic lethality by Cre-mediated recombination
To demonstrate the feasibility of conditional gene rescue using our strategy, we used a ‘deleter’ cre transgenic line (Hs-cre1) that expresses Cre at the two-cell stage of development (5). Cre-mediated recombination occurs throughout the embryo prior to the blastocyst stage of development resulting in progeny that are 100% recombined at a floxed locus. For our purposes, we crossed mice heterozygous for the null Hdh allele and either hemizygous or homozygous for the Hs-cre1 transgene with mice heterozygous for the HdhSTOP allele. Cre-mediated recombination at the loxP sites results in removal of neostop and retention of a single loxP site within Hdh intron 1 (HdhΔSTOP). The remaining loxP site within the intron does not interfere with either the expression or normal processing of the HdhΔSTOP RNA transcript (Fig. 4A and C). Progeny carrying the Hs-cre1 transgene, a Hdh– allele and a recombined HdhSTOP allele (HdhΔSTOP/–; Hs-cre1/+) were obtained at the expected Mendelian frequency (χ2 P = 0.93 and 0.99 for the crosses using Hdh+/– mice hemi- or homozygous for the cre transgene, respectively; see Table 2). Southern analysis of tail DNA obtained from HdhΔSTOP/+ and HdhΔSTOP/– progeny demonstrated that 100% recombination had occurred (Fig. 4B). The HdhΔSTOP/– mice are fertile, have a normal life span and are indistinguishable from their wild-type littermates.
Figure 4.
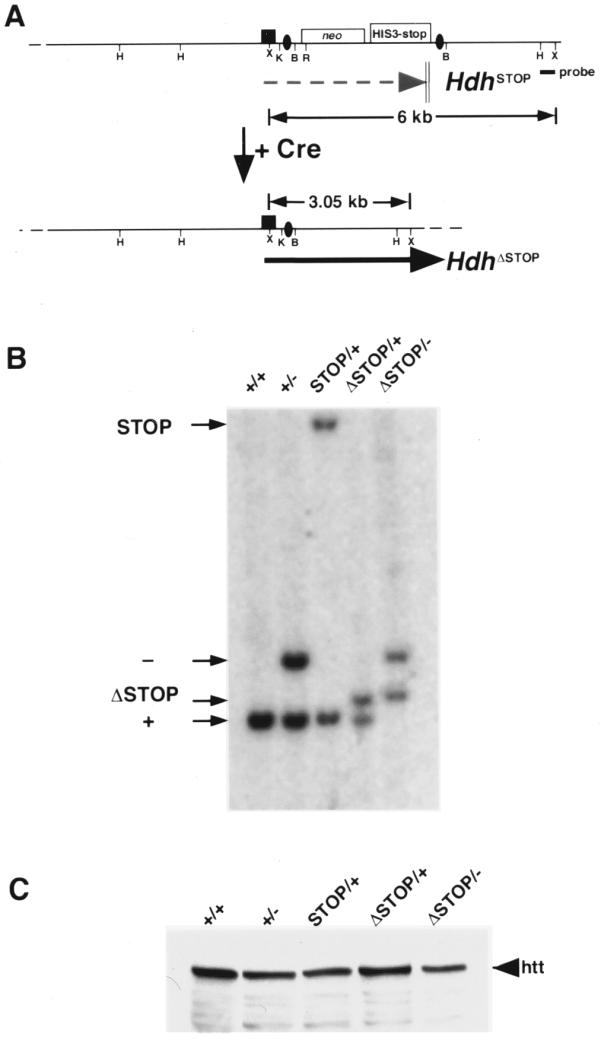
Cre-mediated excision of the neostop cassette restores Hdh expression. (A) Schematic of the HdhSTOP allele before and after (HdhΔSTOP) Cre-mediated recombination. Termination of the HdhSTOP transcript within the neostop cassette is indicated with a dashed gray arrow. Following Cre-mediated recombination, the neostop cassette is excised leaving a single loxP site (black oval) within the HdhSTOP intron 1 that does not interfere with expression [black arrow, see (C) below]. The position of a 3′ flanking probe that is able to distinguish between the HdhSTOP and HdhΔSTOP alleles is indicated with a black rectangle along with the sizes of diagnostic XmnI restriction fragments. Restriction sites are: HindIII (H), XmnI (X), KpnI (K), BamHI (B), EcoRI (R). (B) Southern analysis of tail biopsy DNA from wild-type (+/+), Hdh+/– (+/–), HdhSTOP/+ (STOP/+), HdhΔSTOP/+ (ΔSTOP/+) and HdhΔSTOP/– (ΔSTOP/–) mice. DNA digested with XmnI was blotted and hybridized with the 3′ flanking Hdh probe that recognizes the 6 kb STOP, 3.5 kb null (see Fig. 1C for a map of the Hdh– allele and diagnostic restriction fragments), 3.05 kb ΔSTOP and 3.0 kb wild-type genomic fragments. HdhΔSTOP/– mice were obtained in the predicted Mendelian ratio and were indistinguishable from their wild-type littermates. (C) Western analysis of cytoplasmic extracts prepared from brain tissue dissected from mice with the same genotypes analyzed in (B) using an anti-huntingtin antibody. The amount of huntingtin (htt) in brain extracts prepared from HdhΔSTOP/– mice was equivalent to the amount of huntingtin in Hdh+/– or HdhSTOP/+ extracts.
Table 2. Cre-mediated gene repair.
| Genetic cross | No. of litters | Cre | aNumber genotyped (bnumber expected) | |||||
| |
|
|
Hdh: +/+ |
+/– |
STOP/+ |
ΔSTOP/+ |
STOP/– |
ΔSTOP/– |
| Hdh+/–; Cre/+ × HdhSTOP/+ | 8 | – | 9 (9) | 9 (9) | 8 (9) | NAc | 0 (9) | NA |
| + | 9 (9) | 11 (9) | NA | 9 (9) | NA | 7 (9) | ||
| Hdh+/–; Cre/Cre × HdhSTOP/+ | 4 | + | 10 (10) | 9 (10) | NA | 11 (10) | NA | 9 (10) |
aAll progeny genotyped at P10.
bCalculated using the predicted Mendelian ratio.
cNA, not applicable.
To confirm that Cre-mediated recombination restored Hdh expression, we performed western analyses on protein extracts prepared from the brains of adult wild-type, Hdh+/–, HdhSTOP/+, HdhΔSTOP/+ and HdhΔSTOP/– littermates (Fig. 4C). Following Cre-mediated recombination, expression of huntingtin in the HdhΔSTOP/+ and HdhΔSTOP/– brains was restored to wild-type and heterozygote-equivalent levels, respectively (Fig. 4C). We note that a truncated huntingtin polypeptide corresponding to a translation product of a putative truncated HdhSTOP RNA transcript was not detected using an N-terminal-specific anti-huntingtin antibody (data not shown).
DISCUSSION
The targeted insertion of a floxed neo cassette within a transcription unit was proposed recently as a means to generate conditional gene repair mutations in order to study lineage-specific gene rescue or cell type-specific gene repair in the mouse (6,7). This strategy has been used successfully to rescue expression of an N-myc hypomorphic allele created by insertion of a floxed neo cassette within the second N-myc intron (10). Although it is difficult to predict the effect of the neo cassette on a target gene’s expression, hypomorphic mutations are generated frequently when the neo cassette is placed within an intron. Using this targeting strategy, the magnitude of the interference generated by the neo insertion within an intron can be influenced somewhat by the orientation of the neo cassette with respect to the targeted gene (22).
To generate a conditionally repairable null mutation, we have combined the promoter interference effects of the neo cassette with the transcriptional/translational termination properties of a strong STOP cassette (14). A stopneo cassette (the orientation of neo and the order of the neo and STOP sequences is reversed in comparison to neostop) was used recently to introduce a point mutation within exon 23 of the murine α1 collagen gene (col1α1) in order to generate a mouse model for osteogenesis imperfecta (23). This was the first report to describe insertion of a floxed transcription/translation stop cassette within an intron in an attempt to suppress gene expression from a targeted allele. Unfortunately, the activation of several cryptic splice donor and acceptor sites within the STOP sequences created stable alternatively spliced transcripts. We note that in our model system, alternative splicing of the HdhSTOP allele was not detected. Moreover, we have used the same strategy to generate a conditional gene repair allele of the huntingtin-associated protein 1 gene (Hap1) by insertion of our neostop cassette within intron 1 and we have not detected aberrantly spliced RNA transcripts (manuscript in preparation). It is possible that placing the neo cassette upstream of the STOP sequences suppresses the activity of cryptic splice acceptor sites upstream of the donor splice site within the STOP cassette. Interestingly, although the potential exists to generate high levels of a truncated RNA transcript spanning exon 1 and terminating within the first intron, we failed to detect such transcripts originating from the Hdh mutant allele by northern blotting (Fig. 3A). However, a small amount of RT–PCR product containing Hdh exon 1 sequence was obtained following 30 cycles of PCR amplification using HdhSTOP/– cDNA as template (Fig. 3B). It is possible that the absence of a functional intron within the HdhSTOP primary transcript was sufficient to reduce significantly the level of a truncated transcript (24). Following Cre-mediated excision of the neostop cassette, expression from the HdhΔSTOP allele was restored to wild-type level. Thus, retention of a single loxP site within the first intron did not affect significantly HdhΔSTOP gene expression.
The gene targeting strategy described in this study provides a useful complement to conventional and conditional knockout strategies. In conventional knockouts, the experimental goal of designing mutations that will prevent gene expression from the targeted locus has been achieved by inserting a drug resistance gene into an exon critical for gene function, by making a suitable deletion and/or by inserting a reporter gene in-frame with the coding sequence of the targeted gene (1). These strategies have provided a powerful means to interfere with and/or follow the expression of targeted genes in the mouse. However, conventional knockout strategies frequently result in unanticipated consequences such as incomplete gene inactivation (25,26), and interference with neighboring gene expression (27–29). The strategy described here bypasses some of these potential problems and has several unique advantages over traditional approaches. First, combining the STOP cassette with the neo cassette increases the likelihood of generating a functional null and not a silent or hypomorphic mutation. Second, insertion of the neostop cassette within intron 1 of the target gene can be a safer alternative to deletion strategies that may remove control elements associated with closely spaced neighboring genes. Third, all of the isolated genomic sequence can be used as flanking homology for gene targeting, thus maximizing the frequency of homologous recombination (e.g. >30 versus 11% for targeting at the Hdh locus using the conditional gene repair or deletion/replacement targeting vector, respectively) (30). Fourth, the potential to delete the neostop cassette using a cell lineage-specific cre transgenic line provides a way to rescue gene expression in specific cell lineages and bypass embryonic lethality (6,7). Absence of Hdh expression in the extraembryonic portion of the conceptus during mouse development results in lethality shortly after gastrulation (31). Rescue of Hdh expression in the visceral endoderm and/or the trophoblast of HdhSTOP/– mutants using appropriate cre transgenic lines should provide additional information regarding the requirement for Hdh expression in specific extraembryonic cell lineages.
We note that a single targeting vector containing the floxed neostop cassette and a third loxP site can be used to generate ES cells carrying a null allele, a conditional gene inactivation allele or a conditional gene repair allele (Fig. 5). In this strategy, the floxed neostop cassette is located within intron 1 and a third loxP site is inserted upstream of the target gene’s promoter elements. Two homologous recombination products can be generated, each depending upon where the crossover events occur during recombination. If recombination in the 5′ flanking genomic sequence occurs between the proximal loxP site and the 5′ loxP site flanking the neostop cassette, a conditional gene repair allele will be generated (Fig. 5B). However, if recombination in the 5′ flanking genomic sequence occurs upstream of the proximal loxP site, a targeted allele carrying all three loxP sites will be generated (Fig. 5C). The frequency of obtaining these two kinds of recombination products will depend upon the relative distances between the three loxP sites (the greater the separation between the proximal loxP and the floxed neostop cassette, the greater the proportion of targeted clones carrying the conditional repair allele) (32). ES cells carrying a targeted allele with all three loxP sites can then be used to generate a null allele and conditional gene inactivation allele by transient transfection with a Cre expression plasmid (33). The relative proportion of each recombination product cannot be predicted in advance and will depend upon on the distance between each loxP site and surrounding sequence context, and also on the duration and magnitude of Cre expression (34).
Figure 5.
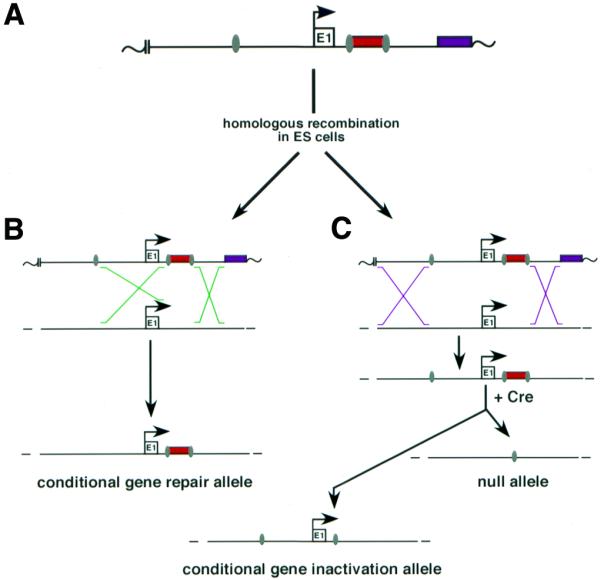
Diagram of a strategy to generate null, conditional inactivation and conditional gene repair alleles starting with the same targeting construct. (A) Two loxP sites (gray ovals) are shown flanking the neostop cassette (red rectangle) inserted within the target gene’s first intron and a third loxP site is located upstream of exon 1 (E1) and promoter elements. Transcriptional orientation is indicated with an arrow, and a thymidine kinase gene (for negative selection of transfected ES cells) is indicated with a purple rectangle. Plasmid sequences are indicated with wavy lines, and the restriction site used to linearize the target vector prior to electroporation is indicated with two vertical parallel lines. (B) Recombination occurring within the homology between the first two loxP sites and within the 3′ flanking homology will generate ES cells carrying a targeted conditional gene repair allele. (C) Recombination occurring within the flanking homology upstream of the first loxP site and within the 3′ homology will, in contrast, generate a targeted allele carrying all three loxP sites. In the presence of Cre, recombination between the first and third loxP sites will generate a null allele, while recombination between the loxP sites flanking the neostop cassette will generate a conditional gene inactivation allele (Discussion).
In summary, we have combined the reliable transcriptional silencing properties of the STOP cassette with the potential promoter interfering properties of the neo gene cassette to provide an alternative method to generate a conditional gene repair allele. The potential to create several different mutant alleles from the same initial targeting construct also provides an efficient alternative to existing targeting strategies.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Thomas Kolar for technical assistance, Monica Mendelsohn for help with blastocyst injections, Paula Dietrich for critical reading of the manuscript, and Argiris Efstratiadis for helpful discussion and support.
References
- 1.Muller U. (1999) Ten years of gene targeting: targeted mouse mutants, from vector design to phenotype analysis. Mech. Dev., 82, 3–21. [DOI] [PubMed] [Google Scholar]
- 2.Copp A.J. (1995) Death before birth: clues from gene knockouts and mutations. Trends Genet., 11, 87–93. [DOI] [PubMed] [Google Scholar]
- 3.Sauer B. (1998) Inducible gene targeting in mice using the Cre/lox system. Methods, 14, 381–392. [DOI] [PubMed] [Google Scholar]
- 4.Sauer B. and Henderson,N. (1988) Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc. Natl Acad. Sci. USA, 85, 5166–5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dietrich P., Dragatsis,I., Xuan,S., Zeitlin,S. and Efstratiadis,A. (2000) Conditional mutagenesis in mice with heat shock promoter-driven cre transgenes. Mamm. Genome, 11, 196–205. [DOI] [PubMed] [Google Scholar]
- 6.Nagy A. (2000) Cre recombinase: the universal reagent for genome tailoring. Genesis, 26, 99–109. [PubMed] [Google Scholar]
- 7.Rossant J. and Nagy,A. (1995) Genome engineering: the new mouse genetics. Nature Med., 1, 592–594. [DOI] [PubMed] [Google Scholar]
- 8.Meyers E.N., Lewandoski,M. and Martin,G.R. (1998) An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nature Genet., 18, 136–141. [DOI] [PubMed] [Google Scholar]
- 9.Mohn A.R., Gainetdinov,R.R., Caron,M.G. and Koller,B.H. (1999) Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell, 98, 427–436. [DOI] [PubMed] [Google Scholar]
- 10.Nagy A., Moens,C., Ivanyi,E., Pawling,J., Gertsenstein,M., Hadjantonakis,A.K., Pirity,M. and Rossant,J. (1998) Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr. Biol., 8, 661–664. [DOI] [PubMed] [Google Scholar]
- 11.Partanen J., Schwartz,L. and Rossant,J. (1998) Opposite phenotypes of hypomorphic and Y766 phosphorylation site mutations reveal a function for Fgfr1 in anteroposterior patterning of mouse embryos. Genes Dev., 12, 2332–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White J.K., Auerbach,W., Duyao,M.P., Vonsattel,J.P., Gusella,J.F., Joyner,A.L. and MacDonald,M.E. (1997) Huntingtin is required for neurogenesis and is not impaired by the Huntington’s disease CAG expansion. Nature Genet., 17, 404–410. [DOI] [PubMed] [Google Scholar]
- 13.Wolpowitz D., Mason,T.B., Dietrich,P., Mendelsohn,M., Talmage,D.A. and Role,L.W. (2000) Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron, 25, 79–91. [DOI] [PubMed] [Google Scholar]
- 14.Lakso M., Sauer,B., Mosinger,B.J., Lee,E.J., Manning,R.W., Yu,S.H., Mulder,K.L. and Westphal,H. (1992) Targeted oncogene activation by site-specific recombination in transgenic mice. Proc. Natl Acad. Sci. USA, 89, 6232–6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakita T., Taya,C., Katsume,A., Kato,J., Yonekawa,H., Kanegae,Y., Saito,I., Hayashi,Y., Koike,M. and Kohara,M. (1998) Efficient conditional transgene expression in hepatitis C virus cDNA transgenic mice mediated by the Cre/loxP system. J. Biol. Chem., 273, 9001–9006. [DOI] [PubMed] [Google Scholar]
- 16.Drago J., Padungchaichot,P., Wong,J.Y., Lawrence,A.J., McManus,J.F., Sumarsono,S.H., Natoli,A.L., Lakso,M., Wreford,N., Westphal,H. et al. (1998) Targeted expression of a toxin gene to D1 dopamine receptor neurons by cre-mediated site-specific recombination. J. Neurosci., 18, 9845–9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour S.L., Thomas,K.R. and Capecchi,M.R. (1988) Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature, 336, 348–352. [DOI] [PubMed] [Google Scholar]
- 18.Zeitlin S., Liu,J.P., Chapman,D.L., Papaioannou,V.E. and Efstratiadis,A. (1995) Increased apoptosis and early embryonic lethality in mice nullizygous for the Huntington’s disease gene homologue. Nature Genet., 11, 155–163. [DOI] [PubMed] [Google Scholar]
- 19.Levine M.S., Klapstein,G.J., Koppel,A., Gruen,E., Cepeda,C., Vargas,M.E., Jokel,E.S., Carpenter,E.M., Zanjani,H., Hurst,R.S. et al. (1999) Enhanced sensitivity to N-methyl-D-aspartate receptor activation in transgenic and knockin mouse models of Huntington’s disease. J. Neurosci. Res., 58, 515–532. [PubMed] [Google Scholar]
- 20.Hogan B., Beddington,R., Costantini,F. and Lacy,E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 21.Persichetti F., Ambrose,C.M., Ge,P., McNeil,S.M., Srinidhi,J., Anderson,M.A., Jenkins,B., Barnes,G.T., Duyao,M.P., Kanaley,L. et al. (1995) Normal and expanded Huntington’s disease gene alleles produce distinguishable proteins due to translation across the CAG repeat., Mol. Med., 1, 374–383. [PMC free article] [PubMed] [Google Scholar]
- 22.Jacks T., Shih,T.S., Schmitt,E.M., Bronson,R.T., Bernards,A. and Weinberg,R.A. (1994) Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nature Genet., 7, 353–361. [DOI] [PubMed] [Google Scholar]
- 23.Forlino A., Porter,F.D., Lee,E.J., Westphal,H. and Marini,J.C. (1999) Use of the Cre/lox recombination system to develop a non-lethal knock- in murine model for osteogenesis imperfecta with an alpha1(I) G349C substitution. Variability in phenotype in BrtlIV mice. J. Biol. Chem., 274, 37923–37931. [DOI] [PubMed] [Google Scholar]
- 24.Choi T., Huang,M., Gorman,C. and Jaenisch,R. (1991) A generic intron increases gene expression in transgenic mice. Mol. Cell. Biol., 11, 3070–3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li E., Bestor,T.H. and Jaenisch,R. (1992) Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell, 69, 915–926. [DOI] [PubMed] [Google Scholar]
- 26.Muller U., Cristina,N., Li,Z.W., Wolfer,D.P., Lipp,H.P., Rulicke,T., Brandner,S., Aguzzi,A. and Weissmann,C. (1994) Behavioral and anatomical deficits in mice homozygous for a modified beta-amyloid precursor protein gene. Cell, 79, 755–765. [DOI] [PubMed] [Google Scholar]
- 27.Fiering S., Epner,E., Robinson,K., Zhuang,Y., Telling,A., Hu,M., Martin,D.I., Enver,T., Ley,T.J. and Groudine,M. (1995) Targeted deletion of 5′HS2 of the murine beta-globin LCR reveals that it is not essential for proper regulation of the beta-globin locus. Genes Dev., 9, 2203–2213. [DOI] [PubMed] [Google Scholar]
- 28.Ohno H., Goto,S., Taki,S., Shirasawa,T., Nakano,H., Miyatake,S., Aoe,T., Ishida,Y., Maeda,H., Shirai,T. et al. (1994) Targeted disruption of the CD3 eta locus causes high lethality in mice: modulation of Oct-1 transcription on the opposite strand. EMBO J., 13, 1157–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olson E.N., Arnold,H.H., Rigby,P.W. and Wold,B.J. (1996) Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell, 85, 1–4. [DOI] [PubMed] [Google Scholar]
- 30.Hasty P., Rivera-Perez,J. and Bradley,A. (1991) The length of homology required for gene targeting in embryonic stem cells. Mol. Cell. Biol., 11, 5586–5591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dragatsis I., Efstratiadis,A. and Zeitlin,S. (1998) Mouse mutant embryos lacking huntingtin are rescued from lethality by wild-type extraembryonic tissues. Development, 125, 1529–1539. [DOI] [PubMed] [Google Scholar]
- 32.Vazquez J.C., Nogues,C., Rucker,E.B. and Piedrahita,J.A. (1998) Factors affecting the efficiency of introducing precise genetic changes in ES cells by homologous recombination: tag-and-exchange versus the Cre-loxp system. Transgenic Res., 7, 181–193. [DOI] [PubMed] [Google Scholar]
- 33.Taniguchi M., Sanbo,M., Watanabe,S., Naruse,I., Mishina,M. and Yagi,T. (1998) Efficient production of Cre-mediated site-directed recombinants through the utilization of the puromycin resistance gene, pac: a transient gene- integration marker for ES cells. Nucleic Acids Res., 26, 679–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu H., Kessler,J. and Shen,J. (2000) Heterogeneous populations of ES cells in the generation of a floxed Presenilin-1 allele. Genesis, 26, 5–8. [PubMed] [Google Scholar]