Abstract
Hydrogen peroxide is thought to regulate cellular processes by direct oxidation of numerous cellular proteins, whereas antioxidants, most notably thiol peroxidases, are thought to reduce peroxides and inhibit H2O2 response. However, thiol peroxidases have also been implicated in activation of transcription factors and signaling. It remains unclear if these enzymes stimulate or inhibit redox regulation and whether this regulation is widespread or limited to a few cellular components. Herein, we found that Saccharomyces cerevisiae cells lacking all eight thiol peroxidases were viable and withstood redox stresses. They transcriptionally responded to various redox treatments, but were unable to activate and repress gene expression in response to H2O2. Further studies involving redox transcription factors suggested that thiol peroxidases are major regulators of global gene expression in response to H2O2. The data suggest that thiol peroxidases sense and transfer oxidative signals to the signaling proteins and regulate transcription, whereas a direct interaction between H2O2 and other cellular proteins plays a secondary role.
Numerous cellular processes, including transcription and signaling, are redox regulated, but the molecular basis for this regulation is not clear. Hydrogen peroxide (H2O2) is a key molecule that is involved in redox regulation (1–3). It is both a toxic compound that can cause oxidative stress (4) and a second messenger that is required for cell proliferation (5). Its signaling function is thought to result from direct oxidation of various cell signaling and regulatory components, and its toxicity from stochastic oxidative damage to proteins, lipids, and nucleic acids (6, 7).
Several classes of enzymes, such as catalases and peroxidases, have evolved that specifically act on H2O2 or other hydroperoxides as substrates. Prominent among them are thiol-dependent peroxidases, which belong to peroxiredoxin (Prx) and glutathione peroxidase (Gpx) protein families. Thiol peroxidase genes are present in all previously characterized organisms, suggesting that these enzymes serve important functions conserved throughout evolution. Prx and Gpx have been implicated in cell signaling due to their ability to reduce intracellular levels of hydroperoxides and to serve as floodgates of H2O2 signaling (8–10). However, studies have also revealed that Saccharomyces cerevisiae Gpx3/Hyr1/Orp1 can serve as an H2O2 sensor and activate the transcription factor Yap1 by forming a disulfide in this protein (11), and a Schizosaccharomyces pombe thiol peroxidase Tsa1 was found to stimulate signaling through a MAP kinase pathway (12, 13). S. pombe thiol peroxidase Tpx1 similarly regulates transcription factor Pap1 (14). In addition, the ability to transfer oxidizing equivalents was demonstrated for a mammalian GPx4 using a GFP-based redox sensor (15). It would be important to address the contribution of thiol peroxidases to stimulation and repression of redox regulation, particularly at a global, genome-wide scale.
In this work, we prepared a S. cerevisiae mutant lacking all eight thiol peroxidases. Surprisingly, this mutant was viable and could withstand significant oxidative stress. It responded to several redox stimuli by robust transcriptional reprogramming. However, it was unable to transcriptionally respond to hydrogen peroxide. The data suggested that thiol peroxidases transfer oxidative signals from peroxides to target proteins, thus activating various transcriptional programs. This study revealed a previously undescribed function of these proteins, in addition to their roles in removal of low levels of peroxides.
Results and Discussion
Yeast Cells Lacking All Thiol Peroxidases Are Viable and Can Withstand Redox Stresses.
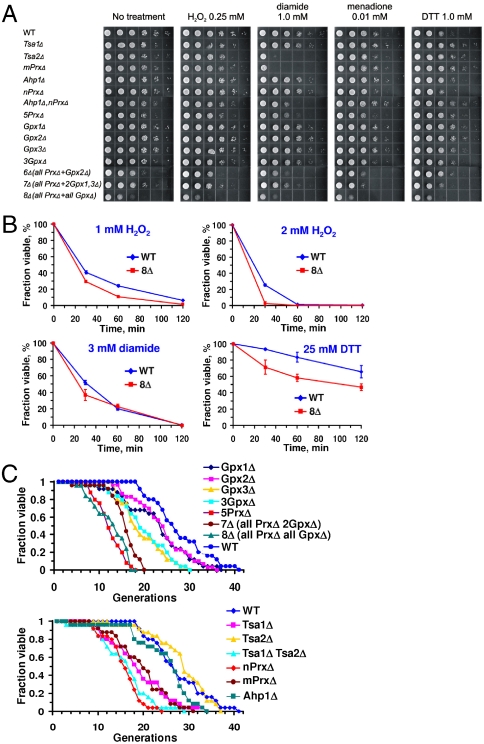
S. cerevisiae has five peroxiredoxins (Tsa1, Tsa2, Ahp1, nPrx, and mPrx) (16) and three glutathione peroxidases (Gpx1, Gpx2, and Gpx3) (17). We generated a series of mutants lacking multiple thiol peroxidases in different combinations (Fig. S1 and Table S1). These included several mutants that lacked seven (7Δ; three mutants with remaining Gpx2, Gpx3, or Tsa1) or all eight (8Δ) thiol peroxidase genes. The genome of the 8Δ strain was sequenced to 26.5× coverage on an Illumina platform, and the disruption of all eight thiol peroxidase genes was confirmed by DNA sequence analysis. All mutants lacking multiple thiol peroxidases, including 8∆, were viable, although their growth was affected compared to WT cells (Fig. 1A and Fig. S2). The mutant cells could withstand treatments with significant amounts of H2O2, diamide, DTT, and menadione, although some mutants were more sensitive than parental (WT) cells to these redox stresses (Fig. 1A and Fig. S2). Individual thiol peroxidases differentially contributed to this protection, with cells lacking multiple thiol peroxidases generally being more sensitive to stress. Removal of all eight thiol peroxidases also decreased cell growth in the absence of stressors (Fig. S2). We further compared viability of WT and multiple thiol peroxidase mutant cells following treatment with 1 or 2 mM H2O2, 3 mM diamide, or 25 mM DTT for 0.5–2 h and found smaller differences between WT and mutant strains (Fig. 1B). This observation suggests that the growth of 8Δ cells was inhibited by acute oxidative stress (Fig. 1A and Fig. S2), but cells were viable and could resume growth once stressors were removed (Fig. 1B). The unexpected oxidative stress resistance of 8∆ cells could be explained by the protective activities of catalase and cytochrome c peroxidase, which can remove hydrogen peroxide in thiol peroxidase mutant cells. Progressive removal of thiol peroxidases also resulted in lower life span (Fig. 1C). Taken together, these data suggested that the loss of thiol peroxidases decreased cell fitness and affected redox homeostasis. However, these enzymes were not essential, even when cells were treated with peroxide or other stressors.
Fig. 1.
Phenotypes and sensitivity of thiol peroxidase mutant strains to redox stresses. (A) Sensitivity of S. cerevisiae WT and different thiol peroxidase mutant cells to indicated concentrations of hydrogen peroxide, diamide, DTT, and menadione. Cells in series of 10× dilutions for each strain were grown on plates with indicated stress inducers for 48 h. (B) Viability of WT cells and 8Δ cells during 2-h treatment with indicated stressors. (C) Replicative life span of WT and thiol peroxidase mutant strains.
Thiol Peroxidase Null Cells Are Unable to Activate and Repress Gene Expression in Response to H2O2.
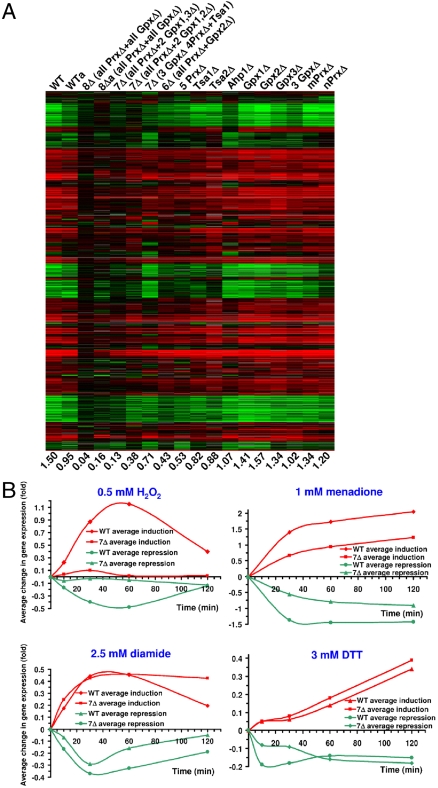
In S. cerevisiae, a large number of genes respond to H2O2 (18). Using cDNA microarrays with 6,692 gene features, we found that expression of 1,144 genes was induced and 574 genes repressed more than 2-fold upon incubation of WT cells with 0.5 mM H2O2 for 30 min (Fig. 2A). On average, gene expression was changed in WT cells 1.5-fold under these conditions (Fig. 2A and Fig. S3).
Fig. 2.
Disruption of H2O2-dependent regulation of gene expression in yeast cells lacking multiple thiol peroxidase genes. (A) Regulation of gene expression by H2O2. Changes in gene expression of WT and indicated mutant thiol peroxidase strains in response to 0.5 mM H2O2 treatment (30 min) are shown for all genes that are either induced or repressed in at least one strain (WT or mutant) used in the study. Numbers below the columns show average values of changes in gene expression (the sum of activation and repression values divided by the total number of yeast genes as described in Materials and Methods) for each dataset. WTa and 8Δa refer to the corresponding H2O2-treated and untreated cells grown under anaerobic conditions. Repression is indicated by green and induction by red colors, and their intensities are graded as log2 of the fold increase/decrease in gene expression. (B) Time course of average changes in gene expression in WT and 7Δ cells in response to 0.5 mM H2O2, 2.5 mM DTT, 2.5 mM diamide, and 1 mM menadione treatments.
The main known function of thiol peroxidases is to scavenge hydroperoxides. Accordingly, these proteins may be expected to decrease the transcriptional response to H2O2 by lowering the cellular peroxide levels. Therefore, deletion of the peroxidase genes would be expected to stimulate gene expression in response to H2O2. However, in contrast to this prediction, the response to H2O2 was inhibited in cells lacking multiple thiol peroxidases. This effect was especially pronounced in the mutants that lacked six or more thiol peroxidase genes (Fig. 2A and Fig. S3) and was observed at both 0.1 and 0.5 mM H2O2 (Fig. S4). Moreover, cells lacking seven (i.e., all except Gpx2, all except Gpx3, and all except Tsa1) or all eight thiol peroxidases essentially lost the ability to regulate gene expression in response to H2O2. For example, 8Δ cells had a 37-fold reduced response to H2O2 treatment compared to WT cells. Importantly, both activation and repression of gene expression were inhibited (Fig. 2A and Fig. S3).
Thiol Peroxidase Null Cells Transcriptionally Respond to Stresses Other Than H2O2.
To determine if the observed transcriptional effect was specific to H2O2 or if the mutant cells also lost the ability to respond to other stresses, we examined the response of WT and 7Δ (all thiol peroxidases except Gpx2) cells to several other redox stressors, including DTT, diamide, and menadione (Fig. 2B). DTT is a strong reductant that causes reductive stress and induces unfolded protein response (18), whereas menadione is a superoxide generator, and diamide is an oxidant that generates nonspecific disulfide bonds. WT cells responded to all treatments. We also found that 7Δ cells responded to DTT and diamide similarly to WT cells; however, they did not respond to H2O2 treatment. Thus, deletion of multiple thiol peroxidase genes specifically disrupted the H2O2-induced transcriptional reprogramming without affecting the ability of cells to respond to other redox stresses. The response of mutant cells to menadione was twofold lower than that in WT cells (Fig. 2B), but this effect could be explained by the fact that this compound generates superoxide, which is further converted to H2O2 (to which these cells do not respond).
Thiol Peroxidase Null Cells Do Not Respond to Varying H2O2 Concentrations and Treatment Times.
To examine the possibility that the response to H2O2 in the thiol peroxidase mutants was delayed or accelerated rather than the regulation was abolished, we analyzed gene expression in WT and 7Δ cells at 10, 30, 60, and 120 min after addition of H2O2 (Fig. 2B). In WT cells, gene expression (both activation and repression) changed by 30 min, peaked at 60 min, and diminished at 120 min. However, 7Δ cells did not respond to H2O2 at any time points. Likewise, we examined the regulation of gene expression in WT and 8Δ cells at different concentrations of H2O2 (0.05, 0.5, 1, 2, and 5 mM; 30-min treatment) (Fig. S5). In WT cells, activation and repression were most pronounced at 0.5 mM H2O2, but at concentrations above 1 mM the response diminished (Fig. 1B). However, the response of 8Δ cells was low at any H2O2 concentration. To test if other redox proteins functionally linked to thiol peroxidases were involved in the H2O2 response, we examined yeast cells deficient in cytochrome c peroxidase (Ccp1) or sulfiredoxin (Srx1). Both mutants responded to H2O2 similarly to WT cells (Fig. S6).
The data presented so far are consistent with a model wherein thiol peroxidases were required for the transfer of the H2O2 signal to other cellular components for transcriptional reprogramming. Moreover, this requirement was not limited to certain gene groups. Thus, thiol peroxidases appeared to function as global mediators (rather than inhibitors) of gene expression in response to H2O2.
Thiol Peroxidase-Dependent Repression of Ribosomal Protein Gene Expression.
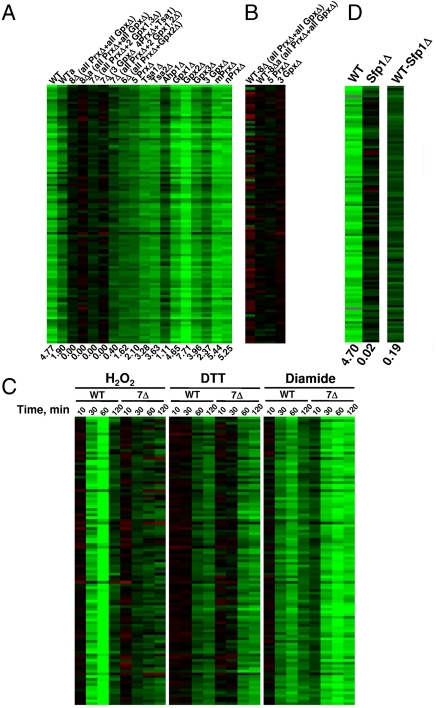
As a representative example, we analyzed expression of cytosolic ribosomal protein genes in WT and thiol peroxidase mutant cells. Ribosomal protein genes (i) were not down-regulated by H2O2 in mutant cells (Fig. 3A), (ii) exhibited normal expression levels in untreated mutant cells compared to WT cells (Fig. 3B), (iii) were down-regulated by H2O2, diamide, and DTT in WT cells, and (iv) were down-regulated by DTT and diamide (but not by H2O2) in mutant cells (Fig. 3C). These data argue for the specific thiol peroxidase-dependent regulation of ribosomal protein gene expression.
Fig. 3.
Thiol peroxidase-dependent transcriptional regulation of ribosomal proteins by H2O2. (A) Changes in gene expression of cytosolic ribosomal proteins upon treatment of WT and thiol peroxidase mutant cells with 0.5 mM H2O2 for 30 min. (B) Changes in gene expression of cytosolic ribosomal proteins between untreated WT and mutant cells. (C) Time course of gene expression changes of cytosolic ribosomal proteins upon treatment of WT and 7Δ cells with 0.5 mM H2O2, 2.5 mM diamide. or 2.5 mM DTT for indicated time periods. (D) Changes in gene expression for cytosolic ribosomal proteins upon treatment of WT and Sfp1 mutant cells with H2O2. Right column compares gene expression in WT and Sfp1Δ cells.
Expression of ribosomal protein genes is regulated by transcription factor Sfp1 (19). We tested the response of Sfp1 mutant cells to H2O2 (Fig. 3D) and found no repression of ribosomal protein genes, suggesting that Sfp1 is a H2O2- and thiol peroxidase-regulated transcription factor. An additional related cluster of genes that failed to respond to H2O2 treatment in 8∆ cells includes genes involved in rRNA modification and translation (Figs. S7 and S8). Similar to cytosolic ribosomal protein genes, rRNA modification and translation genes were not activated or repressed in response to H2O2. However, their responses to DTT and diamide treatments in mutant cells were similar to those in WT cells. Interestingly, although cytosolic translational machinery was transcriptionally repressed by H2O2 in a thiol peroxidase-dependent manner (Fig. 3 and Figs. S7 and S8), mitochondrial ribosomal protein genes were induced (Fig. S9). A similar effect was observed for genes coding for ubiquitin-dependent protein degradation components (Fig. S10). Further examination of responses in individual thiol peroxidase mutants suggested that thiol peroxidases could compensate for each other in mediating the repression of cytosolic ribosomal and translation-related proteins. Nevertheless, the contributions of individual thiol peroxidases to the peroxide-dependent regulation varied (Fig. 3A and Fig. S8).
Thiol Peroxidase Null Cells Do Not Show Elevated Levels of Reactive Oxygen Species (ROS).
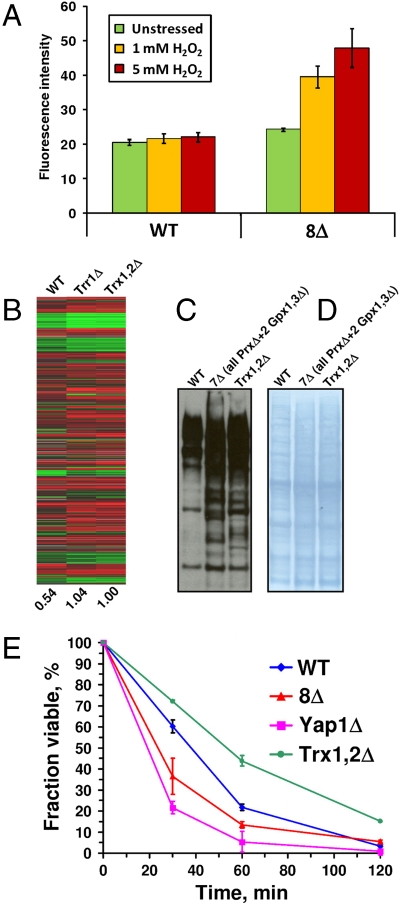
Thiol peroxidases have been suggested to serve as key enzymes in antioxidant defense. If so, a possibility had to be considered that the deletion of these enzymes led to oxidative stress (and therefore resulted in the H2O2 response even in the absence of stress) and that treatment with H2O2 did not further change or exacerbate the expression profile or response. Because the definition of oxidative stress is complex, we examined this possibility in a number of ways, as described in several sections below. First, we analyzed ROS levels in WT and mutant cells by monitoring 2′,7′-dichlorofluorescein (DCF) fluorescence (Fig. 4A). Little difference was found between WT and 8Δ cells in the absence of stress; however, ROS levels were 2-fold higher in multiple thiol peroxidase mutant cells following 1–5 mM H2O2 treatment (Fig. 4A). These data suggest that 8Δ cells were not in a state of severe peroxide stress that could preclude their response to H2O2 treatment. The increase in ROS levels in 8Δ cells after the addition of H2O2 could not explain the loss of the transcriptional response because 8Δ cells could transcriptionally respond to other stresses (Fig. 2B). In addition, in the presence of 0.1 mM H2O2, ROS were not increased in 8Δ cells, yet transcriptional response was inhibited (Fig. S4).
Fig. 4.
ROS in thiol peroxidase mutant cells and the peroxide response in thioredoxin mutant strains. (A) ROS in WT and 8Δ cells were assayed by DCF fluorescence. Cells were treated with indicated concentrations of peroxide for 30 min. (B) Changes in gene expression in WT, Trx1,2Δ. and Trr1Δ cells in response to H2O2 treatment. Yeast strains in SY2626 background were used in this experiment. (C) Analysis of oxidized cysteine residues in yeast proteins in WT and indicated mutant strains. Reduced cysteine residues in yeast proteins were blocked with iodoacetamide, and the oxidized residues were reduced with DTT, alkylated with a biotinylated iodoacetamide, and visualized with streptavidin-conjugated antibodies. (D) Protein loading control for the data shown in C. (E) Viability of WT, 8Δ, Yap1Δ, and Trx1,2Δ cells following treatment with 1 mM H2O2 for indicated periods of time.
Thioredoxin and Thioredoxin Reductase Null Cells Respond to H2O2 Treatment.
The reduced state of most or all thiol peroxidases in the yeast cytosol and nucleus is maintained by thioredoxins Trx1 and Trx2, which in turn are reduced by NADPH-dependent thioredoxin reductase Trr1. We tested if deletion of both Trxs or of Trr1 also disrupts regulation of gene expression by H2O2, but instead found that these mutants had an increased response to H2O2 (Fig. 4B). Stimulation of activation and repression of gene expression in response to H2O2 was also previously seen in the Trr1 mutant cells treated with lower concentrations of H2O2 (20). Thus, we observed an important difference between thiol peroxidases on the one side and their reductants on the other. The data suggest that deletion of Trxs or Trr1 leads to accumulation of oxidized forms of thiol peroxidases and therefore results in an increased response to H2O2.
Thioredoxin and Thiol Peroxidase Mutants Show Similar Levels of Cysteine Oxidation.
Because Trxs, Trr1, and thiol peroxidases function in the same pathway to transfer electrons from NADPH to H2O2, further comparison of these mutant strains offered us an opportunity to better understand the unique role of thiol peroxidases in the peroxide-dependent transcriptional regulation. We examined levels of oxidized cysteine residues in protein extracts from unstressed 7Δ and Trx1,2Δ cells. Although both strains had somewhat elevated cysteine oxidation compared to WT cells, we found no significant differences between these mutant cells (Fig. 4 C and D). These data further support the idea that redox stress cannot explain the specific block in the H2O2 response by thiol peroxidase null cells.
During the course of these studies, we made another interesting observation: Trx1,2Δ cells were more resistant to H2O2 treatment in the cell viability assay than even WT cells (Fig. 4E). It is possible that Trx1,2Δ cells had an increased capacity for H2O2 regulation due to elevation in oxidized thiol peroxidases. This finding is not contradictory with the current literature (20, 21). Indeed, the double thioredoxin mutant grows slowly in the presence of H2O2; however, its viability, determined as the number of colonies formed following the treatment and transfer of cells to a new medium, was not affected. Overall, our data support the idea that Trr1 and Trxs inhibit the transcriptional response to H2O2 (by reducing thiol peroxidases), whereas thiol peroxidases stimulate peroxide-dependent regulation of gene expression (by oxidizing target proteins).
Antioxidant Compounds and Anaerobic Growth Do Not Restore the Ability of 8Δ Cells to Respond to Peroxide.
As an additional test, we examined the H2O2-dependent changes in gene expression of WT and 8Δ cells under anaerobic conditions (Fig. 2A), as well as aerobically in the presence of antioxidant compounds, 5 mM L-proline and 5 mM N-acetylcysteine (NAC) (Fig. S11). The difference in gene expression between WT and 8Δ cells (Fig. S11, Right) was significantly decreased by anaerobiosis and proline/NAC treatment; however, these conditions and treatments did not restore the ability of thiol peroxidase mutant cells to respond to H2O2. These data once again argue that peroxide stress is not a reason for the inability of the mutant strains to respond to H2O2.
Antioxidants Inhibit Rather Than Stimulate Growth of 8Δ Cells.
We tested if expression of a bacterial thiol peroxidase, Escherichia coli 2-Cys peroxiredoxin BCP (Fig. S12), or treatment of the thiol peroxidase mutant strain with 5 mM NAC (Fig. S13) could decrease the elevated cysteine oxidation in multiple thiol peroxidase mutants and found that they could not. In addition, we examined if antioxidants (5 mM NAC and 2 mM DTT) could normalize the growth of thiol peroxidase mutant cells. Surprisingly, the growth of 8Δ cells was inhibited by both compounds (Fig. S14). For example, whereas the growth of WT cells was not affected by NAC, the NAC-treated 8Δ cells showed no growth until 20 h. Moreover, 8Δ cells did not grow in the presence of 2 mM DTT (Fig. S14), even though this compound did not affect viability of these cells (during brief exposure) to much higher concentrations (Fig. 1B and Fig. S2). Although 5 mM NAC did not change the overall levels of disulfides in WT and mutant cells (Fig. S13), our observations support the idea that DTT- and NAC-dependent inhibition of growth of 8∆ cells was due to the reduction of disulfides in regulatory proteins. It is possible that the increased levels of nonspecific cysteine oxidation in multiple thiol peroxidase mutant cells is a molecular response that protects cells from stress caused by deletion of thiol peroxidase genes.
Roles of Yap1, Skn7, Msn2, and Msn4 in the Global Response to H2O2.
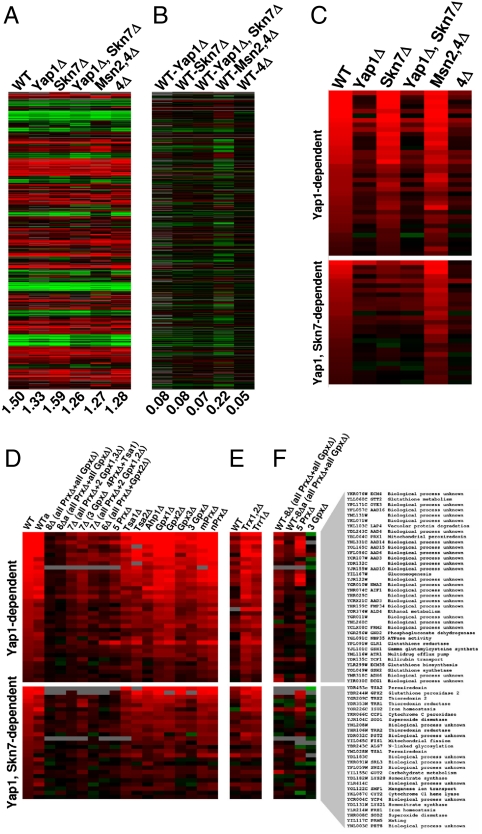
In S. cerevisiae, four transcription factors, Yap1, Skn7, Msn2, and Msn4, are known to regulate gene expression in response to H2O2, but they can also be activated by other stresses. We deleted these genes individually or in combination and tested the transcriptional response of the resulting cells to H2O2. These mutant cells, including the mutant that lacked all four transcription factors (4Δ), were viable and responded to H2O2 similarly to WT cells (Fig. 5A and Fig. S15); however, sensitivity to H2O2 was higher in Yap1Δ and 4Δ cells compared to WT cells. For example, expression of ribosomal protein genes was inhibited by H2O2 in these cells (Fig. S16). Comparison of expression profiles between WT and cells lacking the four transcription factors showed little difference (Fig. 5B). Reversible cysteine oxidation in the mutant cells was also similar to that in WT cells under equivalent treatment conditions (i.e., with and without H2O2) (Fig. S17). These results suggest that the global response to H2O2 is not mediated exclusively by these four redox transcription factors. Our data also allowed us to better define the sets of genes dependent on these transcription factors (Fig. 5 C and D). In particular, we identified genes uniquely dependent on Yap1 as well as genes dependent on both Yap1 and Skn7 (Fig. 5C).
Fig. 5.
Role of oxidative stress transcription factors in the H2O2 response. (A) Regulation of gene expression by H2O2 in cells lacking single and multiple transcription factors Yap1, Skn7, Msn2, and Msn4. (B) Comparison of gene expression between untreated WT and indicated mutant cells. The values of average changes in gene expression are shown under each column. (C) Changes in gene expression in response to H2O2 treatment of Yap1- and Yap1/Skn7-dependent genes in WT and transcription factor mutant cells. This set was generated by filtering the entire set of genes responsive to H2O2 treatment as described in Materials and Methods. (D) Changes in gene expression, in response to H2O2 treatment of Yap1- and Yap1/Skn7-dependent genes, in WT and thiol peroxidase mutant cells. D shows a filtered version of data from Fig. 2A. (E) Same experiment performed with Trr1Δ and Trx1,2Δ cells and isogenic WT cells. E shows a filtered version of data from Fig. 4B. (F) Changes in gene expression of Yap1- and Yap1/Skn7-dependent genes between WT and indicated mutant cells. Yap1- and Yap1/Skn7-dependent genes are shown on the right.
Expression of several, but not all, Yap1- and Yap1/Skn7-dependent genes was elevated in the untreated 8Δ cells compared to WT controls (Fig. 5D). It is known that Yap1 can be activated by general stresses, including treatment with diamide, and this regulation may be independent of thiol peroxidases (22). We examined changes in the expression of Yap1- and Yap1/Skn7-dependent genes in WT and 7Δ cells upon treatment with diamide, menadione, or DTT at different time points (Fig. S18). The analysis of the response to menadione and diamide suggested that Yap1/Skn7 dependent genes could still be fully regulated in 7Δ cells. On the other hand, the DTT response showed an opposite effect for WT and 7Δ cells. This observation suggested that disulfide bonds in Yap1/Skn7 transcription factors may have formed nonspecifically in the oxidative environment of 7Δ cells and that such disulfide bonds could not be reoxidized under experimental conditions, because formation of physiological disulfide bonds was not possible in the 7Δ mutant.
Hydrogen Peroxide-Sensing Cysteines Are Intact in 8Δ Cells.
We tested the response of WT and 8Δ cells to the combined H2O2 and diamide treatment. H2O2 did not modify the response of WT and 8Δ cells to diamide (Fig. S19). Moreover, transcriptional responses to H2O2 and diamide significantly overlapped in WT cells and the response to H2O2 in WT cells also overlapped with the diamide response in 8Δ cells (Figs. S19, S20, and S21). Diamide can form disulfide bonds in proteins nonspecifically, and this observation explains the overlap between H2O2 and diamide responses and suggests that both compounds have a similar set of targets. These data support the hypothesis that 8Δ cells do not respond to H2O2 because they lack thiol peroxidases, which form regulatory disulfides in signaling proteins. Diamide can directly form such disulfides, and therefore the response to diamide in 8Δ cells is similar to those of H2O2 and diamide in WT cells.
A Model of Thiol Peroxidase-Dependent Regulation of Transcription.
We showed that thiol peroxidase null cells are unable to sense H2O2 and carry out peroxide-dependent transcriptional reprogramming. The peroxide response was not observed at any time points following peroxide treatments nor at any peroxide concentrations. Neither anaerobic conditions nor antioxidants could restore it. Yet, thiol peroxidase mutant cells showed robust transcriptional responses to other redox treatments. We propose that these findings can be explained by disruption of the signaling network from peroxide to transcription factors. Thiol peroxidases emerge as global regulators of gene expression. Overall, the data suggest that thiol peroxidases exhibit two functions associated with H2O2 reduction: (i) They transmit oxidative signals to upstream (with respect to electron flow) effectors, such as transcription factors and signaling and regulatory molecules, and (ii) they provide antioxidant defense by reducing hydroperoxides. Previously, it has been difficult to distinguish these functions experimentally because by reducing peroxides, thiol peroxidases fulfill two roles: oxidation of target proteins and antioxidant defense.
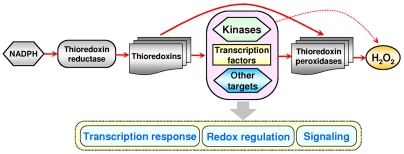
Our model of gene regulation by H2O2 (Fig. 6) is illustrated using the example of the NADPH-dependent Trx system. In the absence of stress, thiol peroxidases are kept in the reduced state by Trxs and other thiol-disulfide oxidoreductases. Upon addition (or intracellular generation) of low concentrations of H2O2, this compound specifically oxidizes thiol peroxidases, whereas oxidation of other cellular proteins by H2O2 is minimal. High specificity of these enzymes toward H2O2 (23) makes them excellent candidates for sensing H2O2 and other hydroperoxides and allows them to effectively compete for H2O2 with other proteins containing reactive cysteines. Thiol peroxidases then oxidize regulatory and signaling proteins, resulting in the transcriptional response and signaling programs. The examples of Gpx3-dependent activation of Yap1 in response to H2O2 (11, 24) and of Tsa1-dependent activation of a stress-activated MAP kinase (12, 13) are supportive of our model and illustrate a molecular mechanism, by which thiol peroxidases may transfer oxidative signals to regulate gene expression.
Fig. 6.
A model of redox regulation of gene expression. Shown is the flow of reducing equivalents (red arrows) in the thioredoxin system from NADPH to hydrogen peroxide and the role of thiol peroxidases in this process. Thiol peroxidases are initially oxidized by H2O2 and then oxidize transcription factors, kinases, and other target proteins in yeast cells. Oxidation of these targets elicits transcriptional response, redox regulation, signaling pathways, and other programs (shown by a vertical gray arrow). Direct oxidation of signaling and regulatory proteins by H2O2 is minimal (red dotted arrow).
Although regulation due to a direct target oxidation by H2O2 also likely exists, the key difference between our model (Fig. 6) and previously suggested models of redox regulation is that H2O2 does not need to interact with other cellular proteins to a significant extent in order to regulate gene expression or other peroxide-dependent programs. The model also explains specificity of H2O2 transcriptional regulation and points to basic mechanisms of redox signaling and redox regulation of cellular processes. Finally, we found that thiol peroxidases regulate the H2O2 transcriptional response in S. cerevisiae. We suggest that thiol peroxidases, via thiol-based redox coupling with cellular proteins, may also control different signaling and regulatory programs in other organisms, including mammals.
Materials and Methods
A detailed SI Materials and Methods is located in SI Appendix.
Yeast Strains.
The yeast strains used in this study are shown in Table S1. Single and multiple thiol peroxidase mutants were prepared by mating the strain lacking five peroxiredoxin genes (GY14) with the strain lacking three glutathione peroxidase genes (GY25, GY30) in BY4741 background. The transcription factor mutant strains were prepared by a one-step gene disruption method.
Spot Assays.
Overnight cultures were adjusted to OD600 = 1, and 10 μL of serial dilutions (10-fold each) were spotted on SD solid medium that contained stressors at indicated concentrations. Cells were grown for 2 d.
Yeast Aging Assays.
After growing for 2 d on fresh plates, 35 undivided daughter cells were collected and arranged on yeast YPD plates using a dissecting microscope. New buds from these original daughter cells were separated and discarded as they formed. This process continued until cells stopped dividing.
cDNA Microarray Analyses.
Cells were grown to 0.3–0.5 OD600 in 200 mL of YPD medium, treated for indicated times with indicated compounds, harvested by centrifugation, and kept at -80 °C. Total RNA was isolated, and mRNA was prepared by amplification and used to prepare cDNA probes by reverse transcription with incorporation of Cy3-dCTP or Cy5-dCTP. DNA microarray data were K-mean clustered with CLUSTER and visualized using TreeView. Average levels of gene activation and repression were estimated as described in SI Materials and Methods.
Detection of Reversibly Oxidized Cysteine Residues in WT and Mutant Yeast Strains.
Each strain was grown in YPD to OD600 = 1. Reduced cysteines were modified with iodoacetamide under denaturing conditions. Then, remaining oxidized cysteines were reduced with DTT and modified with biotinylated iodoacetamide. The levels of oxidized cysteines (in the initial samples) were detected by Western blotting using streptavidin-conjugated antibodies.
Viability Assays.
Each strain was grown in YPD to OD600 = 0.5 and treated with indicated stress agents for various time periods. Cells were washed with YPD medium and serial dilutions were plated on YPD agar plates. Colony numbers were counted after 3 d.
ROS Analyses.
Yeast cells were grown to OD600 = 0.5, washed twice with PBS, resuspended in PBS to 108 cells/mL, and loaded with 5 μM 2′-7′-dichlorodihydrofluorescein diacetate. Cells were treated with 1 mM or 5 mM H2O2 for 30 min. DCF fluorescence was analyzed by flow cytometry.
Genome Sequence of 8Δ Cells.
Genomic DNA was isolated from 8Δ cells and sequenced on an Illumina platform. Reads were assembled into the genome with MAQ genome assembler.
Acknowledgments.
We thank S. V. Avery (University of Nottingham, Nottingham, United Kingdom) and G. F. Merrill (Oregon State University, Corvallis, OR) for providing yeast strains, A. Bird for help with microarrays, and the University of Utah Microarray Core Facility for excellent technical assistance. This work was supported by National Institutes of Health (NIH) Grant GM065204 (to V.N.G.). J.C.R. was supported by NIH Grant GM083292 (to D.R.W.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010721108/-/DCSupplemental.
References
- 1.D’Autréaux B, Toledano MB. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol. 2007;8:813–824. doi: 10.1038/nrm2256. [DOI] [PubMed] [Google Scholar]
- 2.Fourquet S, Huang ME, D’Autreaux B, Toledano MB. The dual functions of thiol-based peroxidases in H2O2 scavenging and signaling. Antioxid Redox Sign. 2008;10:1565–1576. doi: 10.1089/ars.2008.2049. [DOI] [PubMed] [Google Scholar]
- 3.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: A key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE. 2000;2000:pe1. doi: 10.1126/stke.2000.53.pe1. [DOI] [PubMed] [Google Scholar]
- 4.Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- 5.Sundaresan M, Yu ZX, Ferrans VJ, Irani K, Finkel T. Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science. 1995;270:296–299. doi: 10.1126/science.270.5234.296. [DOI] [PubMed] [Google Scholar]
- 6.Finkel T. Oxidant signals and oxidative stress. Curr Opin Cell Biol. 2003;15:247–254. doi: 10.1016/s0955-0674(03)00002-4. [DOI] [PubMed] [Google Scholar]
- 7.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: Signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 8.Rhee SG, Kang SW, Chang TS, Jeong W, Kim K. Peroxiredoxin, a novel family of peroxidases. IUBMB Life. 2001;52:35–41. doi: 10.1080/15216540252774748. [DOI] [PubMed] [Google Scholar]
- 9.Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300:650–653. doi: 10.1126/science.1080405. [DOI] [PubMed] [Google Scholar]
- 10.Kang SW, et al. Mammalian peroxiredoxin isoforms can reduce hydrogen peroxide generated in response to growth factors and tumor necrosis factor-alpha. J Biol Chem. 1998;273:6297–6302. doi: 10.1074/jbc.273.11.6297. [DOI] [PubMed] [Google Scholar]
- 11.Delaunay A, Pflieger D, Barrault MB, Vinh J, Toledano MB. A thiol peroxidase is an H2O2 receptor and redox-transducer in gene activation. Cell. 2002;111:471–481. doi: 10.1016/s0092-8674(02)01048-6. [DOI] [PubMed] [Google Scholar]
- 12.Veal EA, et al. A 2-Cys peroxiredoxin regulates peroxide-induced oxidation and activation of a stress-activated MAP kinase. Mol Cell. 2004;15:129–139. doi: 10.1016/j.molcel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Veal EA, Day AM, Morgan BA. Hydrogen peroxide sensing and signaling. Mol Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Vivancos AP, et al. A cysteine-sulfinic acid in peroxiredoxin regulates H2O2-sensing by the antioxidant Pap1 pathway. Proc Natl Acad Sci USA. 2005;102:8875–8880. doi: 10.1073/pnas.0503251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gutscher M, et al. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J Biol Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong CM, Siu KL, Jin DY. Peroxiredoxin-null yeast cells are hypersensitive to oxidative stress and are genomically unstable. J Biol Chem. 2004;279:23207–23213. doi: 10.1074/jbc.M402095200. [DOI] [PubMed] [Google Scholar]
- 17.Avery AM, Avery SV. Saccharomyces cerevisiae expresses three phospholipid hydroperoxide glutathione peroxidases. J Biol Chem. 2001;276:33730–33735. doi: 10.1074/jbc.M105672200. [DOI] [PubMed] [Google Scholar]
- 18.Gasch AP, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11:4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marion RM, et al. Sfp1 is a stress- and nutrient-sensitive regulator of ribosomal protein gene expression. Proc Natl Acad Sci USA. 2004;101:14315–14322. doi: 10.1073/pnas.0405353101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carmel-Harel O, et al. Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol Microbiol. 2001;39:595–605. doi: 10.1046/j.1365-2958.2001.02255.x. [DOI] [PubMed] [Google Scholar]
- 21.Garrido EO, Grant CM. Role of thioredoxins in the response of Saccharomyces cerevisiae to oxidative stress induced by hydroperoxides. Mol Microbiol. 2002;43:993–1003. doi: 10.1046/j.1365-2958.2002.02795.x. [DOI] [PubMed] [Google Scholar]
- 22.Azevedo D, Tacnet F, Delaunay A, Rodrigues-Pousada C, Toledano MB. Two redox centers within Yap1 for H2O2 and thiol-reactive chemicals signaling. Free Radical Bio Med. 2003;35:889–900. doi: 10.1016/s0891-5849(03)00434-9. [DOI] [PubMed] [Google Scholar]
- 23.Hofmann B, Hecht HJ, Flohe L. Peroxiredoxins. Biol Chem. 2002;383:347–364. doi: 10.1515/BC.2002.040. [DOI] [PubMed] [Google Scholar]
- 24.Wood MJ, Storz G, Tjandra N. Structural basis for redox regulation of Yap1 transcription factor localization. Nature. 2004;430:917–921. doi: 10.1038/nature02790. [DOI] [PubMed] [Google Scholar]