Abstract
Cardiorespiratory functions in mammals are exquisitely sensitive to changes in arterial O2 levels. Hypoxia-inducible factors (e.g., HIF-1 and HIF-2) mediate transcriptional responses to reduced oxygen availability. We demonstrate that haploinsufficiency for the O2-regulated HIF-2α subunit results in augmented carotid body sensitivity to hypoxia, irregular breathing, apneas, hypertension, and elevated plasma norepinephrine levels in adult Hif-2α+/− mice. These dysregulated autonomic responses were associated with increased oxidative stress and decreased mitochondrial electron transport chain complex I activity in adrenal medullae as a result of decreased expression of major cytosolic and mitochondrial antioxidant enzymes. Systemic administration of a membrane-permeable antioxidant prevented oxidative stress, normalized hypoxic sensitivity of the carotid body, and restored autonomic functions in Hif-2α+/− mice. Thus, HIF-2α–dependent redox regulation is required for maintenance of carotid body function and cardiorespiratory homeostasis.
Keywords: blood pressure, control of ventilation, catecholamines
Hypoxia-inducible factors (HIFs) mediate transcriptional responses to reduced O2 availability (1). HIF-1, the first identified member of the HIF family, is comprised of an O2-regulated α subunit and a constitutively expressed β subunit (2). Complete deficiency of HIF-1α in Hif-1α−/− mice results in embryonic lethality at midgestation with major malformations of the central nervous system, heart, and vasculature (3). Hif-1α+/− mice, which are partially deficient in HIF-1α expression, develop normally and are indistinguishable from their WT littermates in the sedentary state with respect to autonomic functions, including blood pressure (BP), ventilatory responses to hypoxia or hypercapnia, and plasma catecholamine levels (4). However, Hif-1α+/− mice exhibit impaired O2 sensing by the carotid body (4, 5), the primary sensory organ for detecting hypoxemia (6, 7), and altered physiological adaptations to chronic hypoxia (5, 8).
The HIF-2α subunit, also known as endothelial PAS domain protein-1 (EPAS-1), shares 48% amino acid sequence identity with HIF-1α (9). As in the case of HIF-1α, continuous hypoxia leads to HIF-2α accumulation and subsequent dimerization with HIF-1β. Transcriptional activation by HIF-2 regulates some target genes in common with HIF-1 as well as other genes that are uniquely regulated by HIF-2 (10–12). Homozygous deficiency of HIF-2α is often lethal with the surviving Hif-2α–/− mice exhibiting multiple organ pathology and increased oxidative stress as a result of impaired induction of genes encoding major antioxidant enzymes (AOEs) (12).
Hif-2α+/− mice, like Hif-1α+/− mice, are phenotypically indistinguishable from WT littermates under sedentary conditions, but exhibit impaired responses to chronic hypoxia such as impaired pulmonary vascular remodeling, erythropoiesis, and retinal neovascularization (13–15). Although the carotid body is a prominent site of HIF-2α expression (16), the effects of Hif-2α haploinsufficiency on chemoreceptor responses to hypoxia have not been examined. Given the structural and functional similarities between HIF-1α and HIF-2α, we hypothesized that Hif-2α haploinsufficiency would alter the carotid body response to acute hypoxia and the ensuing autonomic responses. To test this possibility, carotid body and ventilatory responses to hypoxia, BP, and plasma catecholamine levels were measured in adult Hif-2α+/− and Hif-2α+/+ mice.
Results
Augmented Carotid Body Response to Hypoxia in Hif-2α+/− Mice.
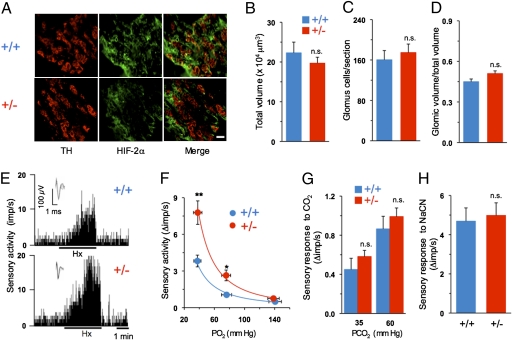
HIF-2α expression in carotid bodies from Hif-2α+/+ and Hif-2α+/− mice was analyzed by immunofluorescence. Sections were stained for tyrosine hydroxylase (TH), a marker of glomus cells, which are the putative O2-sensing cells in the carotid body, and HIF-2α (Fig. 1A, Left, Center). Many glomus cells expressed HIF-2α in both groups of mice as evidenced by colocalization with TH (Fig. 1A, Right). HIF-2α was also expressed in other carotid body cells, some of which appeared to be endothelial cells (Fig. 1A, Middle, Right). The intensity of HIF-2α expression was decreased in Hif-2α+/− mice. Morphometric analysis was performed to assess the effect of partial deficiency of HIF-2α on carotid body structure. There were no significant differences between genotypes with respect to total volume of the carotid bodies, number of glomus cells per section, or ratio of glomic volume to total volume (Fig. 1 B–D).
Fig. 1.
Analysis of carotid body structure and function. (A) Carotid body sections from Hif-2α+/+ and Hif-2α+/− littermate mice (denoted by +/+ and +/−, respectively) were stained with antibodies specific for TH, a marker of glomus cells, and HIF-2α. (Scale bar, 20 μm.) (B–D) Analysis (mean ± SEM) of total volume of the carotid body, number of glomus cells per section, and ratio of glomic volume/total volume from Hif-2α+/+ and Hif-2α+/− mice (n = 3 mice each). (E) Sensory response of isolated carotid bodies to hypoxia (Hx; PO2 ∼ 40 mm Hg). Integrated carotid body sensory activity presented as impulses per second (imp/s). Inset: Superimposed action potentials from the single fiber. (F) Carotid body responses to graded hypoxia from Hif-2α+/+ and Hif-2α+/− mice are shown as the difference in response between baseline and hypoxia (Δ imp/s). Data are mean ± SEM of 22 (Hif-2α+/+) and 16 (Hif-2α+/−) fibers from eight mice of each genotype. (G) CO2 response (mean ± SEM) from 15 (Hif-2α+/+) and 18 (Hif-2α+/−) fibers from seven mice in each group. (H) Carotid body response to 3 μg/mL cyanide (mean ± SEM) from 21 (Hif-2α+/+) and 15 (Hif-2α+/−) fibers from six mice in each group. *P < 0.05; **P < 0.01; n.s., not significant.
To assess the impact of partial HIF-2α deficiency on the carotid body response to hypoxia, carotid bodies were isolated and single-unit sensory discharge was recorded from the carotid sinus nerve. Examples of carotid body sensory responses to hypoxia from both groups of mice and plots of mean data for graded hypoxia are shown in Fig. 1 E and F, respectively. Carotid bodies from Hif-2α+/− mice manifested a significantly greater magnitude of hypoxic sensory response compared with WT littermates. In contrast, chemoreceptor responses to hypercapnia or cyanide, which are also potent carotid body stimulants, were comparable between genotypes (Fig. 1 G and H), indicating a selective alteration in the response to hypoxia in Hif-2α+/− mice.
Hif-2α+/− Mice Exhibit Irregular Breathing, Apnea, and Augmented Hypoxic Ventilatory Response.
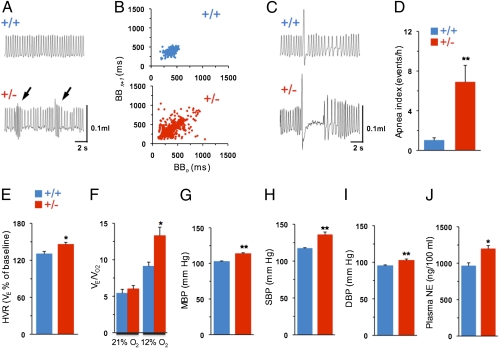
Breathing was monitored by plethysmography in unsedated Hif-2α+/+ and Hif-2α+/− mice. While breathing 21% O2, Hif-2α+/− mice exhibited irregular breathing with brief periods of hyperventilation followed by hypoventilation, whereas WT littermates exhibited a regular breathing pattern (Fig. 2A). Analysis of breath-to-breath (BB) interval (BBn) versus the subsequent interval (BBn+1) for 500 breaths in a mouse of each genotype during room air breathing is presented as Poincarè plots in Fig. 2B. Variability of BB intervals was greater in Hif-2α+/− than in Hif-2α+/+ mice. Analysis of the SD of BB intervals (17) showed that SD1 (representing the y axis) and SD2 (representing the x axis) were significantly greater in Hif-2α+/− compared with Hif-2α+/+ mice [SD1, 45.5 ± 4.4 ms (Hif-2α +/+) vs. 75.9 ± 9.4 ms (Hif-2α+/−), P < 0.01; SD2, 91 ± 10.6 ms (Hif-2α+/+) vs. 153 ± 18.9 ms (Hif-2α+/−), P < 0.01; n = 8 mice each]. In addition, Hif-2α+/− mice exhibited apnea (i.e., cessation of breathing) following sighs, a phenotype that was absent in Hif-2α+/+ mice (Fig. 2C). The incidence of postsigh apnea (duration of more than three breaths) was sevenfold higher in Hif-2α+/− compared with Hif-2α+/+ mice (Fig. 2D).
Fig. 2.
Breathing and BP in Hif-2α+/+ and Hif-2α+/− mice. (A) Breathing was monitored by whole-body plethysmography in unsedated mice. Example of irregular breathing with brief periods of hyperventilation (arrows) followed by hypoventilation in Hif-2α+/− and stable breathing in Hif-2α+/+ mice. (B) Poincaré plots of BBn versus BBn+1 for 500 breaths in a single mouse of each genotype. (C) Example of postsigh apnea in Hif-2α+/+ and its absence in Hif-2α+/− mice. (D) Mean ± SEM data for apnea index (number of postsigh apneas more than three breaths in duration). (E) HVR in Hif-2α+/+ and Hif-2α+/− mice (mean ± SEM). Data presented are percent change in minute ventilation (VE) from baseline. (F) Ratio of minute ventilation to O2 consumption (VE/VO2) during 21% and 12% O2 breathing (mean ± SEM). (G–J) Mean (MBP), systolic (SBP), and diastolic (DBP) BP and plasma NE levels in Hif-2α+/+ and Hif-2α+/− mice. Data presented as mean ± SEM from eight mice in each group. **P < 0.01; *P < 0.05.
Hypoxic ventilatory response (HVR) represents a major reflex triggered by the carotid body. HVR was determined in both genotypes by monitoring responses of the respiratory rate (RR) and tidal volume (VT) to hypoxia (i.e., 12% inspired O2), with sighs, sniffs, and movement-induced changes in breathing excluded from the analysis. Hif-2α+/− mice exhibited a significantly increased magnitude of HVR compared with WT littermates (Fig. 2E). The enhanced HVR in Hif-2α+/− mice was a result of significant elevations in RR as well as VT compared with control mice (Table S1). There were no significant differences between genotypes with respect to O2 consumption (VO2) or CO2 (VCO2) production during hypoxia (Table S1). The ratio of minute ventilation to O2 consumption (VE/VO2), which is a measure of convective requirement, was significantly increased during hypoxia in Hif-2α+/− compared with Hif-2α+/+ mice (Fig. 2F). In striking contrast, ventilatory responses to hypercapnia (i.e., 5% inspired CO2) were comparable between Hif-2α+/− and Hif-2α+/+ mice (Table S1).
Elevated BP and Plasma Norepinephrine Levels in Hif-2α+/− Mice.
Arterial BP was measured in unsedated Hif-2α+/+ and Hif-2α+/− mice. Mean BP was significantly increased in Hif-2α+/− (115 ± 1 mm Hg) compared with Hif-2α+/+ (103 ± 0.9 mm Hg) mice (P < 0.01; Fig. 2G), which was a result of increases in systolic and diastolic pressure (Fig. 2 H and I). Plasma norepinephrine (NE) levels were also significantly elevated in Hif-2α+/− mice compared with WT littermates (Fig. 2J).
Changes in Antioxidant Enzymes, Oxidative Stress, and Mitochondrial Electron Transport Chain Activity in Hif-2α+/− Mice.
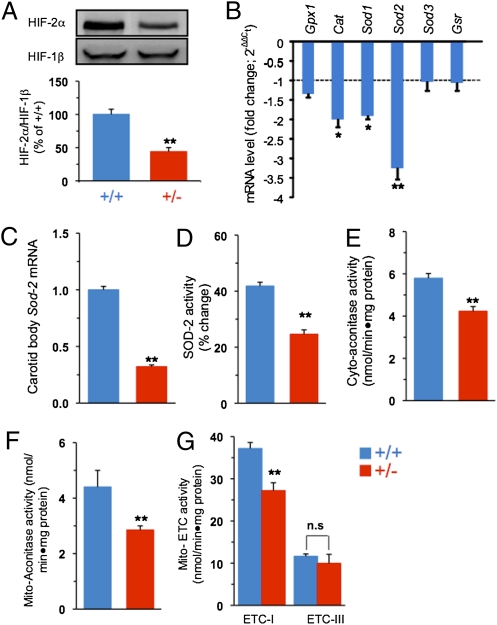
HIF-2 is a potent transcriptional activator of genes encoding AOEs (12). Analysis of mRNAs encoding AOEs in carotid bodies was technically difficult because of the limited availability of the tissue (mouse carotid body wet weight is 20–30 μg). However, the adrenal medulla, which also expresses high levels of HIF-2α (16), was chosen as an alternative tissue source. HIF-2α protein expression in the adrenal medulla was decreased by approximately 50% in Hif-2α+/− mice compared with WT littermates, whereas HIF-1β protein levels were similar (Fig. 3A). Analysis of AOE gene expression in adrenal medulla revealed that mRNAs encoding catalase (Cat) and superoxide dismutase (Sod1, Sod2) were significantly decreased in Hif-2α+/− mice, with Sod2 mRNA expression most severely reduced compared with WT littermates (Fig. 3B). Quantitative real-time RT-PCR assay demonstrated that Sod2 mRNA levels were also significantly decreased in carotid bodies from Hif-2α+/− compared with Hif-2α+/+ mice (Fig. 3C).
Fig. 3.
Redox abnormalities in Hif-2α+/− mice. (A) Top: Representative immunoblot of HIF-2α and HIF-1β proteins in adrenal medulla from Hif-2α+/+ and Hif-2α+/− mice. Bottom: Densitometric analysis (mean ± SEM; n = 5). (B) Levels of mRNAs encoding the AOEs glutathione peroxidase 1 (Gpx1), catalase (Cat), superoxide dismutase (Sod1, Sod2, and Sod3), and glutathione reductase (Gsr) in adrenal glands. The data represent mean ± SEM (n = 3) presented as fold change in Hif-2α+/− relative to Hif-2α+/+ mice. (C) Sod2 mRNA levels in carotid bodies from Hif-2α+/+ and Hif-2α+/− mice measured by quantitative real-time RT-PCR assay. Sod2 mRNA levels were normalized to 18S rRNA and are presented as fold change relative to Hif-2α+/+ mice. The data represent mean ± SEM (n = 4). (D–G) MnSOD, cytosolic (Cyto-aconitase) and mitochondrial (Mito-aconitase) aconitase, and mitochondrial ETC (Mito-ETC) complex I and III activities in adrenal medullae from Hif-2α+/+ and Hif-2α+/− mice (mean ± SEM; n = 5). **P < 0.01; *P < 0.05; n.s., not significant.
Sod2 encodes manganese superoxide dismutase (MnSOD), a major AOE in mitochondria. Analysis of mitochondrial fractions of adrenal medullae showed approximately 60% reduction in MnSOD enzyme activity in Hif-2α+/− compared with WT littermates (Fig. 3D). The reduced MnSOD activity was associated with significantly increased levels of cytosolic and mitochondrial reactive oxygen species (ROS), as evidenced by significantly decreased aconitase activity (Fig. 3 E and F), a sensitive biomarker of ROS (18). Mitochondrial electron transport chain (ETC) complex I activity, which is also a target for ROS (19, 20), was significantly decreased in Hif-2α+/− mice, whereas ETC complex III activity was unaffected (Fig. 3G).
Antioxidant Treatment Restores Carotid Body Response to Hypoxia and Cardiorespiratory Functions in Hif-2α+/− Mice.
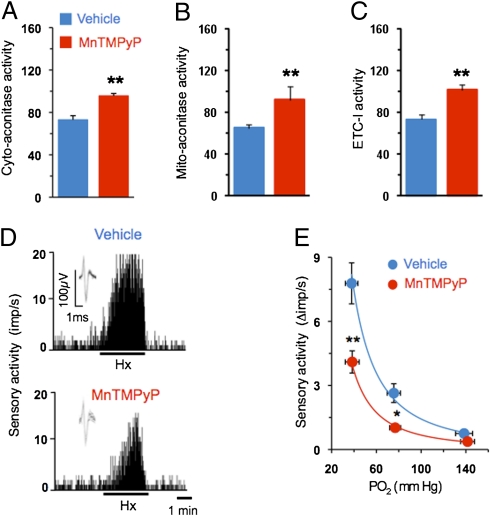
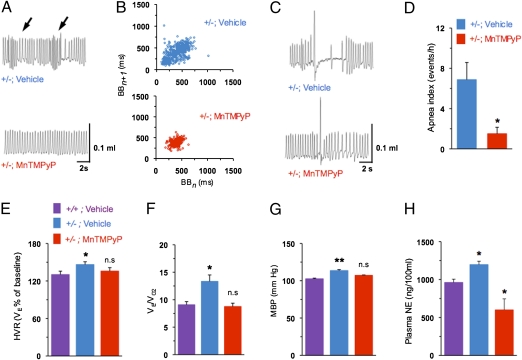
We next investigated whether preventing oxidative stress would correct autonomic functions and hypoxic sensitivity of the carotid body in Hif-2α+/− mice. To this end, mice from both genotypes were treated for 2 wk with manganese (III) tetrakis (1-methyl-4-pyridyl) porphyrin pentachloride (MnTMPyP), a potent membrane permeable antioxidant. Antioxidant treatment normalized cytosolic and mitochondrial aconitase as well as complex I activities in Hif-2α+/− mice (Fig. 4 A–C), confirming reversal of the increased oxidative stress. More importantly, the augmented hypoxic sensory response of the carotid body was absent in antioxidant-treated Hif-2α+/− mice (Fig. 4 D and E). The phenotype of irregular breathing, postsigh apneas, augmented HVR, elevated BP, and increased plasma NE levels was completely absent in antioxidant-treated Hif-2α+/− mice (Fig. 5). Antioxidant treatment, however, had no significant effects on carotid body response to hypoxia, HVR, or BP in WT littermate mice (P > 0.05).
Fig. 4.
Antioxidant treatment normalizes carotid body response to hypoxia in Hif-2α+/− mice. (A–C) Cytosolic (Cyto-aconitase) and mitochondrial (Mito-aconitase) aconitase and mitochondrial complex I (ETC-1) activities (mean ± SEM) in adrenal medullae from Hif-2α+/− mice (n = 6 each) treated with vehicle or MnTMPyP (5 mg/kg/d i.p. for 15 d). (D) Examples of isolated carotid body responses to hypoxia (Hx; PO2 ∼ 38 mm Hg) in Hif-2α+/− mice treated with vehicle or MnTMPyP. Integrated sensory activity is presented as impulses per second (imp/s). Superimposed action potentials from the single fiber are presented (Inset). (E) Carotid body responses to graded hypoxia from vehicle and MnTMPyP-treated Hif-2α+/− mice are shown as the difference in response between baseline and hypoxia (Δimp/s). Values shown are mean ± SEM of 16 (vehicle) and 18 (MnTMPyP) fibers from six mice each. *P < 0.05 and **P < 0.01.
Fig. 5.
Antioxidant treatment restores cardiorespiratory functions in Hif-2α+/− mice. (A) Representative tracings of breathing in Hif-2α+/− mice treated with vehicle or antioxidant MnTMPyP. Arrows denote periods of hyperventilation. (B) Poincaré plots of BBn versus BBn+1 for 500 breaths in vehicle- or MnTMPyP-treated Hif-2α+/− mice. (C and D) Examples showing postsigh apnea in vehicle-treated Hif-2α+/− mouse and its absence in MnTMPyP-treated Hif-2α+/− mouse and apnea index (mean ± SEM). (E and F) HVR and ratio of minute ventilation to O2 consumption (VE/VO2) during 21% and 12% O2 breathing (mean ± SEM) in Hif-2α+/− mice treated with vehicle or MnTMPyP. (G and H) Mean BP (MBP) and plasma NE levels in Hif-2α+/− mice treated with vehicle or MnTMPyP (mean ± SEM, n = 6; *P < 0.05; **P < 0.01; n.s., not significant).
Discussion
The present study demonstrates that mice with heterozygous deficiency of HIF-2α exhibit striking augmentation of the carotid body response to acute hypoxia and equally striking abnormalities in breathing as well as hypertension with elevated plasma NE levels. Although HIF-1α and HIF-2α are paralogues (1, 9, 10) that share some common functions, Hif-1α+/− mice exhibit neither hypertension nor abnormal breathing patterns (4). Moreover, carotid body sensitivity to hypoxia is severely impaired in Hif-1α+/− mice (4, 5), a finding that is diametrically opposed to the enhanced carotid body response to hypoxia in Hif-2α+/− mice. The striking differences between Hif-1α+/− and Hif-2α+/− mice suggest that HIF-2 plays an antagonistic rather than redundant role to that of HIF-1 in regulating oxygen sensing by the carotid body and cardiorespiratory homeostasis.
A major finding of the present study is that Hif-2α+/− mice displayed marked ventilatory instability while breathing room air and increased incidence of postsigh apneas. Periods of hyperventilation followed by hypoventilation are regarded as a hallmark of instability of the ventilatory control system (21). Hyperactive carotid body function has been implicated in triggering irregular breathing patterns (22). Indeed, carotid bodies from Hif-2α+/− mice showed striking enhancement of ventilatory responses to hypoxia, a well established reflex response triggered by the carotid body. The observed effects were selective to hypoxia because carotid body and ventilatory responses to CO2 in Hif-2α+/− mice were comparable to those in WT littermates. Although HIF-2α expression was reported previously in glomus cells of the carotid body (16, 23), its functional role has not been elucidated. Our results demonstrate that HIF-2α is essential for the maintenance of normal hypoxic sensitivity of the carotid body and that even partial deficiency of this protein results in excessive carotid body sensitivity to hypoxia, leading to ventilatory instability, apneas, and exacerbated chemoreceptor reflexes.
In addition to its effect on breathing, carotid body stimulation by hypoxia causes reflex activation of the sympathetic nervous system. Hif-2α+/− mice manifested a hyperactive sympathetic nervous system as evidenced by elevated plasma NE levels. Elevated sympathetic activity is likely to contribute to the hypertension observed in Hif-2α+/− mice. A previous study of young Hif-2α+/− mice reported no changes in plasma NE levels compared with Hif-2α+/+ mice (13). The differences between our data and those previously reported (13) may be a result of differences in the age of the mice (8 wk vs. 7–9 mo of age in our study) or their genetic background (mixed Swiss/129 Sv vs. F1 hybrid 129S6/SvEvTac:C57 BL/6J in the present study).
How might HIF-2α deficiency impact carotid body and cardiorespiratory functions? HIF-2 is a potent transcriptional regulator of AOEs that protect from oxidative stress (12, 24). The following findings demonstrate that down-regulation of AOEs and the resulting oxidative stress mediates heightened carotid body sensitivity to hypoxia and cardiorespiratory changes in Hif-2α+/− mice: (i) Cat, Sod1, and Sod2 mRNA levels were decreased; (ii) oxidative stress was increased as evidenced by decreased cytosolic and mitochondrial aconitase activity; and (iii) antioxidant treatment prevented oxidative stress and restored cardiorespiratory function as well as hypoxic sensitivity of the carotid body. The decreased Sod2 mRNA was associated with reduced MnSOD activity and elevated ROS levels in the mitochondria. Previous studies have shown that ROS are potent inhibitors of complex I activity (19, 20). Indeed, we found reduced activity of complex I, but not complex III, in Hif-2α+/− mice. The reduced complex I activity may further augment oxidative stress in Hif-2α+/− mice by generating more ROS. These observations demonstrate that oxidative stress, resulting from deficient HIF-2–dependent transcriptional activation of AOE genes, mediates the heightened carotid body sensitivity to hypoxia and cardiorespiratory abnormalities in Hif-2α+/− mice. Thus, HIF-2 functions to maintain redox homeostasis by activating transcription of genes encoding AOEs.
There are few precedents for genetic ablation of transcription factors causing cardiorespiratory abnormalities. PPARα and PPARγ homozygous-null mice develop hypertension in response to high-salt or high-fat diet, respectively (25), and Ahr-null mice exhibit elevated BP and cardiac hypertrophy along with other pathologic processes (26). Unlike these studies, our results demonstrate that partial deficiency of HIF-2α alone results in elevated BP and abnormal breathing in the absence of any environmental perturbations.
In humans, exaggerated carotid body response to hypoxia, elevated BP, and increased sympathetic activity occur as a consequence of recurrent apneas (i.e., repetitive brief cessations of breathing), which cause chronic intermittent hypoxia (CIH). Oxidative stress mediates cardiorespiratory responses and sleep disorders that occur in response to CIH (27, 28). We recently demonstrated that exposure of rats and cultured cells to CIH leads to depletion of HIF-2α and that restoring HIF-2α levels prevents oxidative stress and its pathological sequelae (29). In striking contrast, CIH leads to increased accumulation of HIF-1α (4, 30, 31) and, remarkably, Hif-1α+/− mice subjected to CIH do not exhibit the oxidative stress, increased catecholamines, hypertension, or carotid body changes that are observed in WT mice (4). Taken together, these results suggest that functional antagonism between HIF-1 and HIF-2 plays a fundamental role in redox regulation and the maintenance of cardiorespiratory homeostasis.
Materials and Methods
Preparation of Mice.
Experiments were approved by the institutional animal care and use committee of the University of Chicago and performed by individuals blinded to genotype of male, age-matched Hif-2α+/+ and Hif-2α+/− mice (12).
Carotid Body Sensory Activity.
Sensory activity from carotid bodies ex vivo was recorded as described (4). Clearly identifiable action potentials (two to three active units) were recorded from one of the nerve bundles with a suction electrode and “single” units were selected based on the height and duration of the individual action potentials by using a spike discrimination program (Spike Histogram Program; AD Instruments). The PO2 and PCO2 of the superfusion medium were determined by a blood gas analyzer (ABL 5; Radiometer).
Measurements of Respiratory Variables and BP.
Ventilation was monitored by whole-body plethysmograph, and O2 consumption and CO2 production were determined by the open-circuit method in unsedated mice (4, 5). Baseline ventilation was recorded for 2 h while the mice breathed 21% O2. Hypoxic (12% O2 with the balance N2) and hyperoxic hypercapnia (5% CO2/95% O2) gas challenges were given for 5 min. O2 consumption and CO2 production were measured at the end of the 5-min hypoxic challenge. All recordings were made at an ambient temperature of 25 ± 1 °C. BP was monitored by tail cuff method in unsedated mice by using a noninvasive BP system (AD Instruments) (5).
Immunohistochemistry.
Carotid bodies were harvested from anesthetized mice (urethane, 1.2 g/kg i.p.) perfused with heparinized saline solution followed by 4% paraformaldehyde. Fixation of carotid bodies, immunohistochemical analysis of HIF-2α and TH, and morphometric analysis were performed as described (5).
Western Blot Analysis.
Adrenal medullae were harvested from anesthetized mice and HIF-2α and HIF-1β proteins were analyzed by immunoblot assay (29). The following antibodies (Novus) were used: anti–HIF-2α polyclonal (1:500 dilution) and anti–HIF-1β monoclonal H1β234 (1:1,500 dilution).
Enzyme Activities.
Aconitase enzyme activity was determined in cytosolic and mitochondrial fractions of adrenal medulla (32). MnSOD activity was measured by using a superoxide dismutase assay kit (WST; S311–10; Dojondo Molecular Technologies) (28). Complex I (NADH-ubiquinone reductase) and complex III (ubiquinol–ferricytochrome c oxidoreductase) activities were measured as described (31).
Measurement of Plasma NE Levels.
Blood samples were collected from anesthetized mice by cardiac puncture and plasma NE was determined by HPLC combined with electrochemical detection using dihydroxybenzylamine as an internal standard (4).
Gene Expression Analysis.
AOE mRNA expression was analyzed in adrenal medullae by RT Profiler PCR Array for Oxidative Stress (SA Biosciences). The relative expression was determined by the ΔΔCt method whereby, first, the level of gene of interest (GOI) is normalized to a housekeeping gene (HKG):
where GOI is the gene of interest and HKG is the housekeeping gene. Fold change in gene expression is then determined as follows:
Sod2 mRNA was analyzed in the carotid bodies by quantitative real-time RT-PCR with mouse-specific primers detecting Sod2 mRNA or 18S rRNA with SYBR as a fluorogenic binding dye.
Chemicals.
MnTMPyP was obtained from Alexis Biochemicals.
Data Analysis.
In unsedated mice RR (breaths/min), VT (μL), minute ventilation [VE (mL/min) = RR × VT], O2 consumption [VO2 (mL/min)], and CO2 production [VCO2 (mL/min)] were analyzed. Breathing variability was calculated by Poincaré plots and analysis of SD1 and SD2 of BB intervals as described (33). Respiratory variables (RR and VT) were averaged for at least 20 consecutive breaths over a period of 5 min of inspired O2 and CO2 challenge. VT, VE, VO2, and VCO2 were normalized to body weight. Carotid body sensory activity (discharge from single units) was averaged during 3 min of baseline and during the 3 min of gas challenge and expressed as impulses per second. Biochemical and mRNA measurements were performed in three to five independent experiments, each in triplicate. Data are presented as mean ± SEM. Statistical significance was assessed by ANOVA or two-way ANOVA with repeated measures followed by Tukey test. P values lower than 0.05 were considered significant.
Acknowledgments
This research was supported by National Institutes of Health Grants HL-76537, HL-90554, and HL-86493 (to N.R.P.); the Department of Veterans Affairs (J.A.G.); and the Johns Hopkins Institute for Cell Engineering (G.L.S.). N.R.P. is a Harold H. Hines, Jr., Professor at the University of Chicago and G.L.S. is the C. Michael Armstrong Professor at Johns Hopkins University School of Medicine.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1100064108/-/DCSupplemental.
References
- 1.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 2.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyer NV, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 α. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng Y-J, et al. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kline DD, Peng YJ, Manalo DJ, Semenza GL, Prabhakar NR. Defective carotid body function and impaired ventilatory responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1 α. Proc Natl Acad Sci USA. 2002;99:821–826. doi: 10.1073/pnas.022634199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88:2287–2295. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- 7.Kumar P. Sensing hypoxia in the carotid body: From stimulus to response. Essays Biochem. 2007;43:43–60. doi: 10.1042/BSE0430043. [DOI] [PubMed] [Google Scholar]
- 8.Yu AY, et al. Impaired physiological responses to chronic hypoxia in mice partially deficient for hypoxia-inducible factor 1α. J Clin Invest. 1999;103:691–696. doi: 10.1172/JCI5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Ema M, et al. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1α regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94:4273–4278. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holmquist-Mengelbier L, et al. Recruitment of HIF-1α and HIF-2α to common target genes is differentially regulated in neuroblastoma: HIF-2α promotes an aggressive phenotype. Cancer Cell. 2006;10:413–423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 12.Scortegagna M, et al. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331–340. doi: 10.1038/ng1266. [DOI] [PubMed] [Google Scholar]
- 13.Brusselmans K, et al. Heterozygous deficiency of hypoxia-inducible factor-2α protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. J Clin Invest. 2003;111:1519–1527. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dioum EM, Clarke SL, Ding K, Repa JJ, Garcia JA. HIF-2α-haploinsufficient mice have blunted retinal neovascularization due to impaired expression of a proangiogenic gene battery. Invest Ophthalmol Vis Sci. 2008;49:2714–2720. doi: 10.1167/iovs.07-1469. [DOI] [PubMed] [Google Scholar]
- 15.Scortegagna M, et al. HIF-2α regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105:3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- 16.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- 18.Gardner PR. Aconitase: Sensitive target and measure of superoxide. Methods Enzymol. 2002;349:9–23. doi: 10.1016/s0076-6879(02)49317-2. [DOI] [PubMed] [Google Scholar]
- 19.Sadek HA, Szweda PA, Szweda LI. Modulation of mitochondrial complex I activity by reversible Ca2+ and NADH mediated superoxide anion dependent inhibition. Biochemistry. 2004;43:8494–8502. doi: 10.1021/bi049803f. [DOI] [PubMed] [Google Scholar]
- 20.Khan SA, et al. NADPH Oxidase 2 mediates intermittent hypoxia-induced mitochondrial complex I inhibition: Relevance to blood pressure changes in rats. Antioxid Redox Signal. 2011;11:2607–2619. doi: 10.1089/ars.2010.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cherniack NS, Longobardo GS. Mathematical models of periodic breathing and their usefulness in understanding cardiovascular and respiratory disorders. Exp Physiol. 2006;91:295–305. doi: 10.1113/expphysiol.2005.032268. [DOI] [PubMed] [Google Scholar]
- 22.Dunai J, Kleiman J, Trinder J. Ventilatory instability during sleep onset in individuals with high peripheral chemosensitivity. J Appl Physiol. 1999;87:661–672. doi: 10.1152/jappl.1999.87.2.661. [DOI] [PubMed] [Google Scholar]
- 23.Roux JC, Brismar H, Aperia A, Lagercrantz H. Developmental changes in HIF transcription factor in carotid body: Relevance for O2 sensing by chemoreceptors. Pediatr Res. 2005;58:53–57. doi: 10.1203/01.PDR.0000163390.78239.EA. [DOI] [PubMed] [Google Scholar]
- 24.Oktay Y, et al. Hypoxia-inducible factor 2α regulates expression of the mitochondrial aconitase chaperone protein frataxin. J Biol Chem. 2007;282:11750–11756. doi: 10.1074/jbc.M611133200. [DOI] [PubMed] [Google Scholar]
- 25.Obih P, Oyekan AO. Regulation of blood pressure, natriuresis and renal thiazide/amiloride sensitivity in PPARalpha null mice. Blood Press. 2008;17:55–63. doi: 10.1080/08037050701789278. [DOI] [PubMed] [Google Scholar]
- 26.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Prabhakar NR, Kumar GK, Nanduri J, Semenza GL. ROS signaling in systemic and cellular responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1397–1403. doi: 10.1089/ars.2007.1732. [DOI] [PubMed] [Google Scholar]
- 28.Veasey S. Insight from animal models into the cognitive consequences of adult sleep-disordered breathing. ILAR J. 2009;50:307–311. doi: 10.1093/ilar.50.3.307. [DOI] [PubMed] [Google Scholar]
- 29.Nanduri J, et al. Intermittent hypoxia degrades HIF-2α via calpains resulting in oxidative stress: Implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA. 2009;106:1199–1204. doi: 10.1073/pnas.0811018106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321–4328. doi: 10.1074/jbc.M407706200. [DOI] [PubMed] [Google Scholar]
- 31.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1α expression by intermittent hypoxia: Involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuan G, et al. Role of oxidative stress in intermittent hypoxia-induced immediate early gene activation in rat PC12 cells. J Physiol. 2004;557:773–783. doi: 10.1113/jphysiol.2003.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]