Abstract
The dense collagen network in tumors significantly reduces the penetration and efficacy of nanotherapeutics. We tested whether losartan—a clinically approved angiotensin II receptor antagonist with noted antifibrotic activity—can enhance the penetration and efficacy of nanomedicine. We found that losartan inhibited collagen I production by carcinoma-associated fibroblasts isolated from breast cancer biopsies. Additionally, it led to a dose-dependent reduction in stromal collagen in desmoplastic models of human breast, pancreatic, and skin tumors in mice. Furthermore, losartan improved the distribution and therapeutic efficacy of intratumorally injected oncolytic herpes simplex viruses. Finally, it also enhanced the efficacy of i.v. injected pegylated liposomal doxorubicin (Doxil). Thus, losartan has the potential to enhance the efficacy of nanotherapeutics in patients with desmoplastic tumors.
Keywords: drug delivery, matrix modifier, thrombospondin-1, transforming growth factor β, transport
Although nanotherapeutics have offered new hope for cancer treatment, their clinical efficacy is modest (1–4). This is partly because their penetration is hindered, especially in fibrotic tumors, where the small interfibrillar spacing in the interstitium retards the movement of particles larger than 10 nm (5–8). Pegylated liposomal doxorubicin (Doxil), approved by the Food and Drug Administration, and oncolytic viruses, currently in multiple clinical trials, represent two nanotherapeutics whose size (∼100 nm) hinders their intratumoral distribution and therapeutic effectiveness (9). Matrix modifiers such as bacterial collagenase, relaxin, and matrix metalloproteinase-1 and -8 have been used to modify the collagen or proteoglycan network in tumors and have improved the efficacy of intratumorally (i.t.) injected oncolytic viruses (8, 10–13). However, these agents may produce normal tissue toxicity (e.g., bacterial collagenase) or increase the risk of tumor progression (e.g., relaxin, matrix metalloproteinases).
Losartan (14)—approved to control hypertension in patients—does not have many of these safety risks. Furthermore, in addition to its antihypertensive properties, losartan is also an antifibrotic agent that has been shown to reduce the incidence of cardiac and renal fibrosis (15, 16). The antifibrotic effects of losartan are caused, in part, by the suppression of active transforming growth factor-β1 (TGF-β1) levels via an angiotensin II type I receptor (AGTR1)-mediated down-regulation of TGF-β1 activators such as thrombospondin-1 (TSP-1) (15–19). Using a dose that has minimal effects on mean arterial blood pressure (MABP), we show that losartan reduces collagen I levels in four tumor models—a spontaneous mouse mammary carcinoma (FVB MMTV PyVT), an orthotopic pancreatic adenocarcinoma (L3.6pl), and s.c. implanted fibrosarcoma (HSTS26T) and melanoma (Mu89). Losartan also improves the intratumoral penetration of nanoparticles injected i.t. or i.v.
Based on these results, we tested how losartan would affect the distribution and efficacy of oncolytic herpes simplex viruses (HSV) administered i.t.—a widely used method of administration in patients for gene therapy (20–22)—and the efficacy of i.v. administered Doxil. Losartan improved the efficacy of both i.t. injected oncolytic HSV and i.v. administered Doxil. The results from our intratumoral experiments show that losartan enhances nanoparticle penetration in the interstitial space by improving interstitial transport. Additionally, the results from our i.v. studies indicate that losartan improves the efficacy of systemically administered nanotherapeutics to highly fibrotic solid tumors, such as pancreatic adenocarcinomas. Altogether, these results suggest that losartan, a Food and Drug Administration–approved antihypertensive drug, could potentially be used to improve the efficacy of various nanotherapeutics in multiple tumor types.
Results
Losartan Inhibits Collagen I Synthesis by Carcinoma-Associated Fibroblasts.
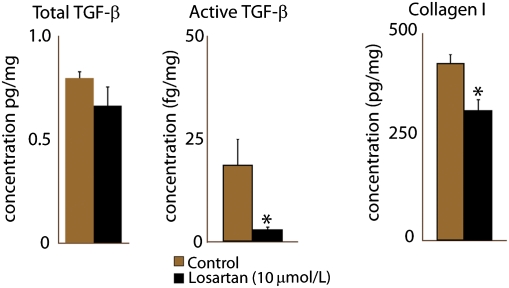
We first determined the effect of losartan on the expression and activation of TGF-β1 and collagen I production by mammary carcinoma-associated fibroblasts (CAFs) (Fig. 1). Losartan did not affect the levels of total TGF-β1, but significantly (P < 0.05) reduced active TGF-β1 levels by 90%. It also significantly decreased the in vitro synthesis of collagen I by 27% (P < 0.04). Because collagen in tumors is mostly produced by CAFs, we next determined how losartan would affect the collagen content in tumors.
Fig. 1.
Losartan reduces TGF-β1 activation and collagen I production in carcinoma-associated fibroblasts in vitro. Cells were treated with 10 μmol/L losartan for 24 h. Losartan reduced by 90% the active TGF-β1 levels, whereas total TGF-β1 levels were unaffected. There was a corresponding 27% decrease in collagen I levels. The reduction in active TGF-β1 and collagen I was statistically significant (Student's t test, *P < 0.05).
Losartan Decreases Collagen I in Tumors in a Dose-Dependent Manner.
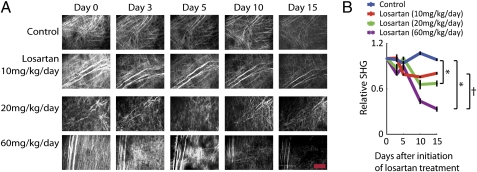
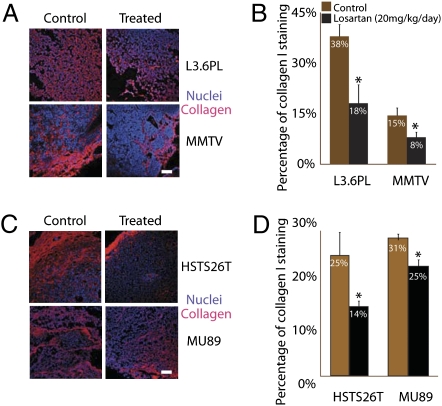
To determine the dose–response of losartan on intratumoral collagen levels, we injected 10, 20, and 60 mg·kg−1·d−1 i.p. and performed second-harmonic generation (SHG) imaging of fibrillar collagen in HSTS26T tumors in dorsal skin fold chambers (Fig. 2) and collagen I immunostaining of tumor sections (Fig. 3). Collagen I and other fibril-forming collagens (e.g., collagen III, V) could contribute to SHG signal intensity. However, because collagen I is the predominant collagen type in most soft tissues (23), it is likely the main source of the SHG signal. Additionally, in human pancreatic tumors, collagen I is the main fibrillar collagen, with significantly lower levels of collagen V (24). Losartan doses of 20 and 60 mg·kg−1·d−1 significantly reduced intratumoral SHG signal intensity, whereas the lowest dose of 10 mg·kg−1·d−1 did not have a significant effect on SHG signal intensity (Fig. 2 A and B). The injection of losartan at 60 and 20 mg·kg−1·d−1 also significantly reduced the collagen I immunostaining in HSTS26T tumors by 65% and 42%, respectively (Fig. S1). Although treatment with the 60 mg·kg−1·d−1 dose led to the highest reduction in collagen I, we did not use this dose because it significantly reduced the MABP by 35 mmHg (P < 0.04; Fig. S2). Consequently, we chose the 20 mg·kg−1·d−1 dose for further study because after 2 wk of losartan treatment it only reduced the MABP by 10 mmHg (Fig. S2), thus maintaining the MABP within the normal range (70–95 mmHg) for severe combined immunodeficient mice (25). It also had no effect on mouse weight (average of 26 ± 1 g for treated vs. 26 ± 1 g for control). The 20 mg·kg−1·d−1 dose also decreased collagen I immunostaining in three other tumor types—FVB MMTV PyVT, L3.6pl, and Mu89—by 47% (P < 0.05), 50% (P < 0.03), and 20% (P < 0.02), respectively (Fig. 3 A–D).
Fig. 2.
Losartan reduces collagen production in tumors. (A) Over a period of 2 wk, there was a dose-dependent reduction in collagen levels assessed by SHG imaging in losartan-treated HSTS26T tumors (10, 20, and 60 mg·kg−1·d−1). (Scale bar, 200 μm.) (B) At the end of 15 d, losartan doses of 10, 20, and 60 mg·kg−1·d−1 decreased the SHG levels by 20%, 33%, and 67%, respectively. There was a statistically significant difference (*) between the control group and the two higher doses (20 and 60 mg·kg−1·d−1). There was also a statistically significant difference (†) between the 20 and 60 mg·kg−1·d−1 groups.
Fig. 3.
Losartan reduces collagen levels in tumors. (A) Collagen I (red) and nuclei (blue) immunostaining in tumor sections in L3.6pl and MMTV control and losartan (20 mg·kg−1·d−1)-treated tumors. (Scale bar, 100 μm.) (B) The 2-wk losartan treatment at 20 mg·kg−1·d−1 significantly reduced the collagen I immunostaining in L3.6pl and FVB MMTV PyVT by 50% (*P < 0.03) and 47% (*P < 0.05), respectively. (C) Collagen I (red) and nuclei (blue) immunostaining in tumor sections in HSTS26T and Mu89 control and losartan (20 mg·kg−1·d−1)-treated tumors. Note that there is no reduction in collagen I immunostaining at 200 μm from the edge of HSTS26T tumors. This phenomenon is less obvious in treated Mu89 tumors, where there is some persistent staining both at the edge and in central tumor areas. (Scale bar, 100 μm.) (D) Losartan significantly reduced the collagen I immunostaining in HSTS26T and Mu89 by 42% (*P < 0.02) and 20% (*P < 0.05), respectively.
Losartan Decreases TSP-1 Expression in Tumors.
TSP-1 is a key regulator of TGF-β1 activation, and losartan has been shown to reduce TSP-1 expression and TGF-β1 activation in mouse models of Marfan's syndrome and muscular dystrophy (19). The measurement of protein levels in homogenized HSTS26T tumors showed that losartan did not affect total TGF-β1 levels but significantly reduced TSP-1, active TGF-β1, and collagen I levels (Fig. S3). Losartan also decreased TSP-1 immunostaining in HSTS26T (73%, P < 0.04) and Mu89 (24%, P < 0.03) (Fig. S4). In both Mu89 and HSTS26T tumors, the immunostaining patterns for TSP-1 and collagen I were closely matched (Fig. 3C; Fig. S4). We found high levels of TSP-1 and collagen I in the tumor margin, whereas losartan induced obvious reductions in TSP-1 and collagen I levels in the tumor center (Fig. 3C; Fig. S4). These data indicate that the reduction in collagen I levels could result in part from the decreased activation of TGF-β1 due to the losartan-induced reduction in TSP-1 expression.
Losartan Improves the Intratumoral Distribution of Nanoparticles and Nanotherapeutics.
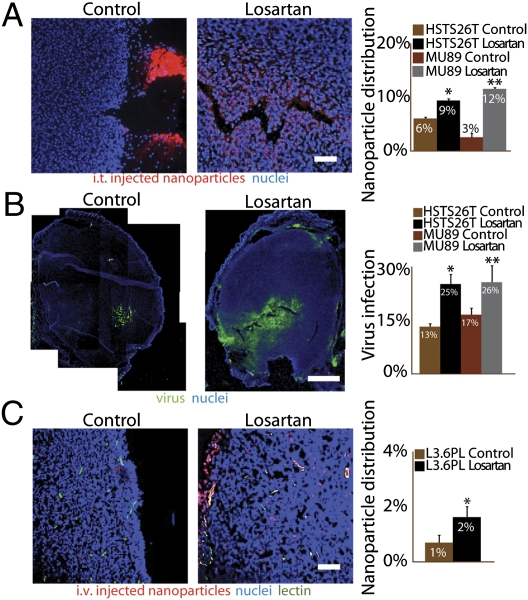
Based on our previous studies on the tumor interstitial matrix (6, 8), we hypothesized that a decrease in collagen content would improve the intratumoral distribution of nanoparticles. We therefore measured the intratumoral distribution of fluorescent polystyrene nanoparticles (100-nm diameter) in three different tumor types—HSTS26T, Mu89, and L3.6pl—after an i.t. or i.v. injection. In mice injected i.t. with nanoparticles, losartan improved nanoparticle accumulation and penetration in the tumor center (Fig. 4A) (HSTS26T, P < 0.001; Mu89, P < 0.001). Conversely, there was little or no nanoparticle accumulation in the center of control tumors. Most of the injected nanoparticles in control tumors were found in the tumor margin and around the needle insertion point (Fig. 4A). We also determined the effects of losartan on the intratumoral distribution of oncolytic HSV. In both HSTS26T and Mu89, losartan significantly increased the intratumoral spread of HSV injected intratumorally (Fig. 4B). Whereas these data show that losartan increases the distribution of large nanoparticles, we also found that in HSTS26T it increased interstitial diffusion of IgG (Fig. S5) and the mean interstitial matrix pore radius—from 9.91 ± 0.43 nm to 11.78 ± 0.41 nm, calculated based on IgG diffusion data (26).
Fig. 4.
Losartan increases delivery of nanoparticles and nanotherapeutics. (A) Distribution of i.t. injected 100-nm-diameter nanoparticles in HSTS26T tumors. Losartan significantly increased (*, **P < 0.001) the distribution of i.t. injected nanoparticles in both tumor types (1.5-fold in HSTS26T and 4-fold in Mu89). An analysis of the distribution pattern shows control tumors with fewer intratumoral nanoparticles (red) and a majority of nanoparticles that backtracked out of the needle track and accumulated at the tumor surface. In contrast, treated tumors have a significant number of intratumoral nanoparticles. (Scale bar, 100 μm.) (B) Distribution of viral infection 24 h after the intratumoral injection of HSV-expressing green fluorescent protein. HSV infection (green) in control tumors is limited to the cells in close proximity to the injection site, whereas losartan-treated tumors have a more extensive spread of HSV infection. (Scale bar, 1 mm.) Losartan significantly increased (*, **P < 0.05) the virus spread in HSTS26T and Mu89 tumors. (C) Distribution of i.v. injected 100-nm-diameter nanoparticles in L3.6pl tumors. The nanoparticles (red) are localized around perfused vessels (green). There is a twofold increase (*P < 0.05) in nanoparticle content in losartan-treated tumors compared with control tumors. (Scale bar, 100 μm.)
We then tested the effect of losartan on blood vessel perfusion and the intratumoral distribution of i.v. injected nanoparticles in mice with orthotopic pancreatic tumors (L3.6pl). Losartan did not significantly change the fraction of perfused vessels in tumors (Fig. S6A). However, the intratumoral accumulation and penetration of beads away from blood vessels were significantly higher in losartan-treated tumors (Fig. 4C; Fig. S6B). These results indicate that losartan improves the transport and distribution of both i.t. and i.v. injected nanoparticles.
Losartan Improves the Efficacy of Doxil and Oncolytic HSV.
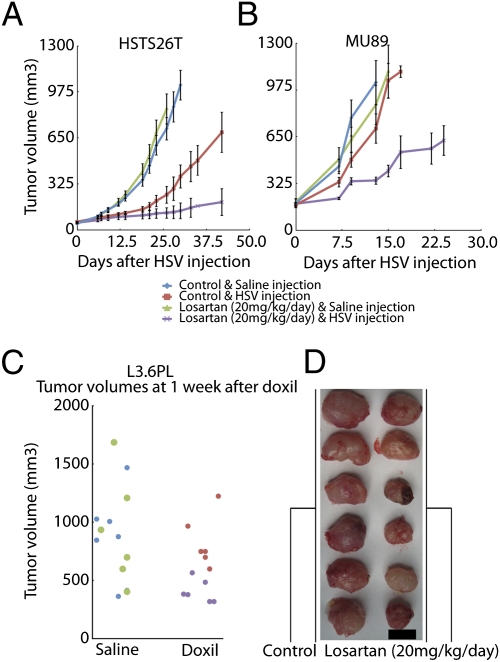
We then determined whether losartan could improve the efficacy of i.t. injected oncolytic HSV and i.v. injected Doxil. The effect of losartan combined with the i.t. injection of HSV was determined in HSTS26T and Mu89 tumors. The administration of losartan alone did not affect the tumor growth rate (Fig. 5 A and B). However, when animals were treated with losartan for 2 wk before i.t. injection of HSV, losartan significantly delayed (P < 0.001) the growth in both Mu89 and HSTS26T tumors. Interestingly, the volume of HSTS26T tumors remained stable for up to 9 wk in 50% of mice treated with losartan and HSV. On the other hand, the growth delay in Mu89 tumors was only transient; 4 wk after the virus injection, all of the tumors were threefold larger than the starting treatment size.
Fig. 5.
Losartan significantly delays the growth of tumors treated with Doxil or HSV. (A and B) Mice bearing HSTS26T (A) and Mu89 (B) tumors were treated for 2 wk with either losartan or saline before the i.t. injection of HSV. Losartan alone did not affect the growth of Mu89 or HSTS26T tumors. The growth delay was significantly longer (P < 0.001) in HSTS26T tumors treated with losartan and HSV compared with tumors treated with HSV alone. The i.t. injection of HSV did not delay the growth of Mu89 tumors, but the combined losartan and HSV treatment significantly retarded (P < 0.001) the growth of Mu89 tumors. (C) Mice that received losartan treatment before i.v. Doxil infusion (purple dots) have significantly smaller (P < 0.001) tumors than those that received Doxil alone (red dots) in L3.6pl tumors. Note that there is no difference in tumor size between saline- (blue) and losartan-treated (green) mice. (D) The image shows a clear difference in size between control tumors (left column) and losartan-treated tumors (right column) at 1 wk after Doxil infusion. (Scale bar, 1 cm.)
To test whether losartan would increase the efficacy of a nanotherapeutic injected i.v., mice with orthotopic pancreatic tumors (L3.6pl) were treated with Doxil and losartan. Four weeks after tumor implantation and 2 wk after initiation of losartan treatment (20 mg·kg−1·d−1), we treated mice with a subtherapeutic dose of Doxil (4 mg/kg, i.v.). After 7 d, losartan or Doxil alone did not affect the mean tumor volume (Fig. 5C). However, in mice treated with losartan and Doxil, the tumors were significantly smaller (P < 0.001) than in mice that received Doxil alone (Fig. 5 C and D).
Pattern of Collagen Distribution Governs the Effectiveness of Losartan.
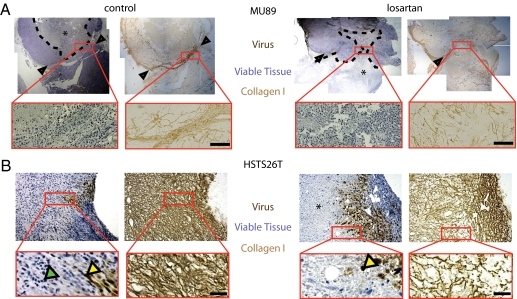
To investigate the differences in response between HSTS26T and Mu89 to the losartan-HSV combination therapy, we determined the HSV infection and necrosis patterns 21 d after the i.t. injection of HSV. First, we noticed striking differences between the collagen structure in Mu89 (Fig. 6A) and HSTS26T (Fig. 6B) tumors. These differences altered the virus propagation in the two tumor types. In Mu89 tumors, the collagen fiber network was well-organized and formed finger-like projections into the tumor (Figs. 6A and 7A). These projections divided the tumor into distinct compartments which could not be crossed by HSV particles, and thus the virus infection and resulting necrosis were restricted to the infected compartments (Fig. S7A). Losartan treatment disrupted the collagen projections to some extent but did not completely eliminate them (Fig. 6A). As a result, there was some cross-over of virus particles between compartments in losartan-treated Mu89 tumors. In contrast, in HSTS26T tumors, the dense collagen network was more diffuse, less fibrillar, and less compartmentalized (Figs. 6B and 7B). The dense collagen network seemed to slow down virus propagation but did not completely impede it, resulting in increased virus propagation and a more diffuse pattern of necrosis in this tumor (Fig. S7A).
Fig. 6.
Relationship between collagen structure and virus infection and necrosis. (A) In Mu89 tumors, collagen bundles are seen around the tumor margin. Occasionally, these bundles project into the tumor (black arrowheads) and divide the tumor into separate compartments. These compartments seem to confine movement of HSV, evident from the containment of the necrotic region (*) within the region bounded by collagen bundles. When these tumors were treated with losartan, the collagen bundles at the margins of the tumor remained intact but the projections became less organized (inset). This presumably allowed virus propagation and necrosis to extend across the boundaries. (Scale bar, 100 μm.) (B) In HSTS26T tumors, the dense mesh-like collagen network confined virus infection to the immediate area surrounding the injection point. With losartan treatment, there was a reduction in the density of the network that presumably allowed virus particles to infect a larger area and thus more tumor cells. Green and yellow arrowheads indicate viable and virus-infected cells, respectively. (Scale bar, 40 μm.)
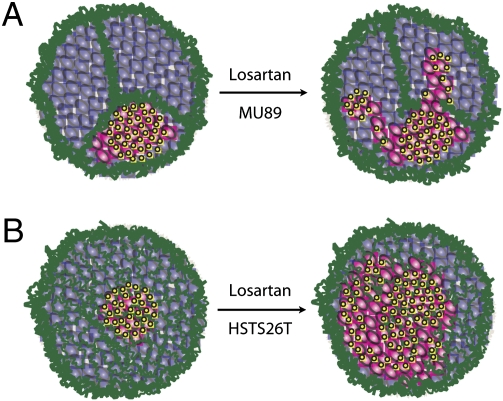
Fig. 7.
Schematic of virus distribution and infection in Mu89 and HSTS26T tumors. The schematics show how the different collagen network structures affect virus propagation and distribution. The collagen fibers (green) restrict the movement of virus particles (yellow) and the infection (pink) of noninfected (purple) cancer cells. (A) In Mu89 tumors, collagen bundles divide the tumor into isolated regions that cannot be traversed by virus particles. Losartan treatment destabilizes the collagen bundles and allows virus particles to move from one region to another. (B) In HSTS26T tumors, the collagen structure is a mesh-like sieve. Virus particles can still propagate through the sieve but do not extend very far from the injection site. Losartan treatment significantly destabilizes the mesh structure in the internal regions of the tumor and allows the virus to propagate and infect a larger area.
Discussion
The renin-angiotensin-aldosterone system (RAAS) plays an important role in the regulation and production of extracellular matrix components (27–29). Angiotensin II in particular has been shown to stimulate collagen production via both TGF-β1–dependent and –independent pathways (30). As a result, losartan and other RAAS inhibitors can reduce the levels of collagen I and III and basement membrane collagen IV in various experimental models of fibrosis (31, 32) and reverse renal and cardiac fibrosis in hypertensive patients (33, 34). Using four different tumor types, we show that losartan also inhibits collagen I production in tumors.
Other matrix modifiers, such as bacterial collagenase, relaxin, and matrix metalloproteinase-1 and -8, have been used to modify the collagen or proteoglycan network in tumors and have improved the efficacy of oncolytic virus injected intratumorally (8, 10–13). However, these agents may produce normal tissue toxicity (e.g., bacterial collagenase) or increase the risk of tumor progression (e.g., relaxin, matrix metalloproteinases). In contrast, losartan (14) has limited side effects and has been shown to reduce the incidence of metastasis in some tumor types (35).
Our findings strongly support the hypothesis that a reduction in collagen content by losartan improves interstitial transport and the intratumoral distribution of nanoparticles and nanotherapeutics. We also discovered that the organization of the collagen fibrillar network affects nanoparticle distribution. This was striking because of significant differences in the structural organization of fibrillar collagen I between Mu89 and HSTS26T. In Mu89 tumors, thick bundles of fibrillar collagen I surround the tumor margins and form finger-like projections which subdivide the tumor mass into isolated compartments and confine the viral infection to the injection site/isolated compartments (Fig. 7A). In contrast, HSTS26T tumors have a mesh-like collagen structure which hinders the virus spread but does not restrict viral particles to the injection site (Fig. 7B). The slower growth rate of HSTS26T compared with Mu89 tumors could also explain in part the enhanced efficacy of losartan combined with HSV in HSTS26T tumors. Our data also suggest that not only the collagen content but also the collagen network organization plays an important role in limiting the penetration of large therapeutics in tumors.
Pancreatic cancer patients treated with cytotoxic agents have a very high frequency of relapse with a 5-y survival of less than 5% (36). The poor vascular supply and increased fibrotic content of pancreatic tumors most likely play a significant role in limiting the delivery and efficacy of cytotoxics (37). We show—in a mouse orthotopic model of human pancreatic cancer (L3.6pl)—that losartan increases both the intratumoral dispersion and extravascular penetration distance of i.v. injected nanoparticles. The increased distribution and extravasation of nanoparticles suggest that losartan might not only improve interstitial transport—as shown with the i.t. injections of nanoparticles and virus—but also transvascular transport. When used alone, losartan did not affect the growth of pancreatic tumors or the weight of treated mice. However, losartan combined with Doxil reduced the tumor sizes by 50% compared with Doxil treatment alone. These results suggest that losartan increased the tumor penetration and distribution and enhanced the efficacy of Doxil injected i.v. in orthotopic pancreatic carcinomas in mice.
The effects of losartan are not limited to the interstitial space. Modifications to the RAAS can also inhibit angiogenesis (38) or alter tumor blood flow (39, 40). Losartan blockade of AGTR1 can also reduce the production of vascular endothelial growth factor (VEGF) by cancer cells and the expression of VEGFR1 in endothelial cells, and inhibit tumor angiogenesis and growth (41, 42). In the present study, losartan did not affect tumor growth or the vascular density in HSTS26T tumors. Losartan can also reduce the proliferation of tumor cells expressing AGTR1 (43). We did not find a decrease in cancer cell proliferation (Fig. S8) or tumor size in the human melanoma Mu89, which expresses AGTR1 (Fig. S9). The difference between our study and other studies might be due to differences in dosage. For example, in previous studies, the dose of losartan was up to 15-fold higher than in our experiments (41). We believe that a low dose of losartan will allow for a more clinically translatable protocol and avoid hypotensive complications.
Patients receiving RAAS antagonists have reduced incidence of breast and lung cancer (44). Several mechanisms have been proposed to explain the antitumor properties of RAAS antagonists (45–48). AGTR1 signaling has been shown to increase the proliferation of stromal and tumor cells and the transcription of inflammatory cytokines and chemokines that promote cancer cell migration and dissemination (49). The reduction in active TGF-β1 levels by RAAS antagonists could also reduce metastasis (50). Consequently, in addition to improving the delivery of antitumor agents, losartan may also inhibit tumor progression and metastasis.
To use losartan as an adjunct in the treatment of cancer patients, it is important to consider dosing and treatment schedules along with potential side effects. Results from our dose- and time-dependent studies suggest a minimum of 2 wk of losartan administration before antitumor treatment. To obtain maximum effects in patients, it might be prudent to initiate losartan treatment 2 wk before and continue it during the entire antitumor treatment schedule. Because long-term losartan therapy in hypertensive patients has been shown to have limited and manageable side effects and many antitumor agents (e.g., anti-VEGF drugs) have been shown to increase blood pressure (45), extended losartan cotherapy could be beneficial to cancer patients. For clinical studies, we suggest treating patients with a dose of 2 mg·kg−1·d−1, which is similar to that used for the treatment of patients with Marfan's syndrome (51).
Although losartan and angiotensin II receptor blockers have limited side effects, losartan therapy is not recommended for patients with known renal disease. Losartan can induce renal insufficiency in patients with renal microvascular or macrovascular disease or congestive heart failure (52). Hyperkalemia can also occur in patients with poor renal function or patients who are concomitantly receiving potassium supplements or potassium-sparing diuretics. Finally, angioedema caused by high levels of circulating angiotensin II can occur in patients treated with losartan (52).
It is also important to consider tumor resistance to losartan therapy after extended treatment. Tumor drug resistance is thought to occur at many levels, including increased drug efflux, drug inactivation, evasion from apoptosis, and alterations in target pathways (53). Because losartan is not an antitumor agent, any potential resistance may result from other mechanisms. Given that TGF-β1 activation is induced by different agents such as matrix metalloproteinases and integrins in addition to TSP-1, tumor resistance to losartan could result from changes in TGF-β1 activation and signaling. Fortunately, long-term losartan therapy after myocardial infarction is not associated with a reduction in antifibrotic properties (54). It will be important to determine whether these results can be reproduced in tumors.
In conclusion, we show that losartan reduces the stromal collagen content in tumors and improves the penetration and therapeutic efficacy of nanoparticles (Doxil, HSV) delivered both intratumorally and intravenously. Losartan also exhibits vasoactive and antimetastatic properties that could increase its clinical application. Furthermore, because losartan is already approved for clinical use, it represents a safe and effective adjunct for improving the efficacy of nanotherapeutics in cancer patients.
Materials and Methods
A more detailed description of techniques is presented in SI Materials and Methods. Briefly, CAFs isolated from human breast cancer biopsies were treated with losartan for 24 h before measurement of collagen and cytokine levels. Protein assays were done with commercial ELISA kits. All animal experiments were done with the approval of the Institutional Animal Care and Use Committee of Massachusetts General Hospital. Losartan was administered i.p. at concentrations of 10, 20, or 60 mg·kg−1·d−1 for up to 2 wk. Mice were treated with HSV (i.t.) and Doxil (i.v. via tail vein) after 2 wk of losartan treatment. Excised tumors were either snap-frozen for biochemical analysis or fixed in paraformaldehyde and embedded in paraffin or optimum cutting temperature compound for immunohistochemistry.
Acknowledgments
We acknowledge Drs. N. Kirkpatrick, D. Lacorre, and R. Guang for help in planning experiments, Dr. T. Stylianopoulos for help with manuscript preparation, and Dr. L. Fisher (National Institute of Dental Research) for kindly providing the LF-67 antibody. We thank Eve Smith and Sylvie Roberge for their technical assistance. This work was supported by US National Cancer Institute grants to R.K.J. (P01CA80124 and R01CA85140) and Y.B. (R01CA98706) and a Department of Defense Breast Cancer research fellowship to B.D.-F. (W91ZSQ7342N607) and innovator award to R.K.J. (W81XWH-10-1-0016).
Footnotes
Conflict of interest statement: R.K.J. received commercial research grants from Dyax, AstraZeneca, and MedImmune; consultant fees from AstraZeneca/MedImmune, Dyax, Astellas-Fibrogen, Regeneron, Genzyme, Morphosys, and Noxxon Pharma; and a speaker honorarium from Genzyme. R.K.J. owns stock in SynDevRx. No reagents or funding from these companies was used in these studies. There is no significant financial or other competing interest in the work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1018892108/-/DCSupplemental.
References
- 1.Jain RK, Stylianopoulos T. Delivering nanomedicine in solid tumors. Nat Rev Clin Oncol. 2010;7:653–664. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: An emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 3.Peer D, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–760. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 4.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 5.Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60:2497–2503. [PubMed] [Google Scholar]
- 6.Pluen A, et al. Role of tumor–host interactions in interstitial diffusion of macromolecules: Cranial vs. subcutaneous tumors. Proc Natl Acad Sci USA. 2001;98:4628–4633. doi: 10.1073/pnas.081626898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ramanujan S, et al. Diffusion and convection in collagen gels: Implications for transport in the tumor interstitium. Biophys J. 2002;83:1650–1660. doi: 10.1016/S0006-3495(02)73933-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown E, et al. Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation. Nat Med. 2003;9:796–800. doi: 10.1038/nm879. [DOI] [PubMed] [Google Scholar]
- 9.Nemunaitis J, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 10.McKee TD, et al. Degradation of fibrillar collagen in a human melanoma xenograft improves the efficacy of an oncolytic herpes simplex virus vector. Cancer Res. 2006;66:2509–2513. doi: 10.1158/0008-5472.CAN-05-2242. [DOI] [PubMed] [Google Scholar]
- 11.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007;67:10664–10668. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 12.Ganesh S, et al. Relaxin-expressing, fiber chimeric oncolytic adenovirus prolongs survival of tumor-bearing mice. Cancer Res. 2007;67:4399–4407. doi: 10.1158/0008-5472.CAN-06-4260. [DOI] [PubMed] [Google Scholar]
- 13.Kim J-H, et al. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006;98:1482–1493. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 14.Johnston CI. Angiotensin receptor antagonists: Focus on losartan. Lancet. 1995;346:1403–1407. doi: 10.1016/s0140-6736(95)92411-6. [DOI] [PubMed] [Google Scholar]
- 15.Habashi JP, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn RD, et al. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204–210. doi: 10.1038/nm1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavoie P, et al. Neutralization of transforming growth factor-β attenuates hypertension and prevents renal injury in uremic rats. J Hypertens. 2005;23:1895–1903. doi: 10.1097/01.hjh.0000182521.44440.c5. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain JS. ACE inhibitor bulks up muscle. Nat Med. 2007;13:125–126. doi: 10.1038/nm0207-125. [DOI] [PubMed] [Google Scholar]
- 19.Dietz HC. TGF-β in the pathogenesis and prevention of disease: A matter of aneurysmic proportions. J Clin Invest. 2010;120:403–407. doi: 10.1172/JCI42014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu JC, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–6747. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 21.Senzer NN, et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J Clin Oncol. 2009;27:5763–5771. doi: 10.1200/JCO.2009.24.3675. [DOI] [PubMed] [Google Scholar]
- 22.Breitbach CJ, Reid T, Burke J, Bell JC, Kirn DH. Navigating the clinical development landscape for oncolytic viruses and other cancer therapeutics: No shortcuts on the road to approval. Cytokine Growth Factor Rev. 2010;21:85–89. doi: 10.1016/j.cytogfr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Gelse K, Pöschl E, Aigner T. Collagens—Structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Mollenhauer J, Roether I, Kern HF. Distribution of extracellular matrix proteins in pancreatic ductal adenocarcinoma and its influence on tumor cell proliferation in vitro. Pancreas. 1987;2:14–24. doi: 10.1097/00006676-198701000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kristjansen PE, Boucher Y, Jain RK. Dexamethasone reduces the interstitial fluid pressure in a human colon adenocarcinoma xenograft. Cancer Res. 1993;53:4764–4766. [PubMed] [Google Scholar]
- 26.Nugent LJ, Jain RK. Extravascular diffusion in normal and neoplastic tissues. Cancer Res. 1984;44:238–244. [PubMed] [Google Scholar]
- 27.Cook KL, Metheny-Barlow LJ, Tallant EA, Gallagher PE. Angiotensin-(1-7) reduces fibrosis in orthotopic breast tumors. Cancer Res. 2010;70:8319–8328. doi: 10.1158/0008-5472.CAN-10-1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Vita J, et al. Angiotensin II activates the Smad pathway in vascular smooth muscle cells by a transforming growth factor-β-independent mechanism. Circulation. 2005;111:2509–2517. doi: 10.1161/01.CIR.0000165133.84978.E2. [DOI] [PubMed] [Google Scholar]
- 29.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-β pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 30.Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-β-dependent and -independent Smad pathways: The role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 31.Toblli JE, et al. Effects of angiotensin II subtype 1 receptor blockade by losartan on tubulointerstitial lesions caused by hyperoxaluria. J Urol. 2002;168:1550–1555. doi: 10.1016/S0022-5347(05)64519-3. [DOI] [PubMed] [Google Scholar]
- 32.Boffa JJ, et al. Regression of renal vascular and glomerular fibrosis: Role of angiotensin II receptor antagonism and matrix metalloproteinases. J Am Soc Nephrol. 2003;14:1132–1144. doi: 10.1097/01.asn.0000060574.38107.3b. [DOI] [PubMed] [Google Scholar]
- 33.Lim DS, et al. Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation. 2001;103:789–791. doi: 10.1161/01.cir.103.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khalil A, et al. Angiotensin II type 1 receptor antagonist (losartan) down-regulates transforming growth factor-β in experimental acute pyelonephritis. J Urol. 2000;164:186–191. [PubMed] [Google Scholar]
- 35.Arafat HA, et al. Antihypertensives as novel antineoplastics: Angiotensin-I-converting enzyme inhibitors and angiotensin II type 1 receptor blockers in pancreatic ductal adenocarcinoma. J Am Coll Surg. 2007;204:996–1005. doi: 10.1016/j.jamcollsurg.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 36.Li J, Wientjes MG, Au JL-S. Pancreatic cancer: Pathobiology, treatment options, and drug delivery. AAPS J. 2010;12:223–232. doi: 10.1208/s12248-010-9181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujita M, et al. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- 39.Jain R, Ward-Hartley K. Tumor blood flow—Characterization, modifications and role in hyperthermia. IEEE Trans Son Ultrason. 1984;31:504–526. [Google Scholar]
- 40.Zlotecki RA, Boucher Y, Lee I, Baxter LT, Jain RK. Effect of angiotensin II induced hypertension on tumor blood flow and interstitial fluid pressure. Cancer Res. 1993;53:2466–2468. [PubMed] [Google Scholar]
- 41.Otake AH, et al. Inhibition of angiotensin II receptor 1 limits tumor-associated angiogenesis and attenuates growth of murine melanoma. Cancer Chemother Pharmacol. 2010;66:79–87. doi: 10.1007/s00280-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 42.Noguchi R, et al. Synergistic inhibitory effect of gemcitabine and angiotensin type-1 receptor blocker, losartan, on murine pancreatic tumor growth via anti-angiogenic activities. Oncol Rep. 2009;22:355–360. [PubMed] [Google Scholar]
- 43.Rhodes DR, et al. AGTR1 overexpression defines a subset of breast cancer and confers sensitivity to losartan, an AGTR1 antagonist. Proc Natl Acad Sci USA. 2009;106:10284–10289. doi: 10.1073/pnas.0900351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lever AF, et al. Do inhibitors of angiotensin-I-converting enzyme protect against risk of cancer? Lancet. 1998;352:179–184. doi: 10.1016/S0140-6736(98)03228-0. [DOI] [PubMed] [Google Scholar]
- 45.Ager EI, Neo J, Christophi C. The renin-angiotensin system and malignancy. Carcinogenesis. 2008;29:1675–1684. doi: 10.1093/carcin/bgn171. [DOI] [PubMed] [Google Scholar]
- 46.Lindberg H, Nielsen D, Jensen BV, Eriksen J, Skovsgaard T. Angiotensin converting enzyme inhibitors for cancer treatment? Acta Oncol. 2004;43:142–152. doi: 10.1080/02841860310022346. [DOI] [PubMed] [Google Scholar]
- 47.Miyajima A, et al. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62:4176–4179. [PubMed] [Google Scholar]
- 48.Rosenthal T, Gavras I. Angiotensin inhibition and malignancies: A review. J Hum Hypertens. 2009;23:623–635. doi: 10.1038/jhh.2009.21. [DOI] [PubMed] [Google Scholar]
- 49.Deshayes F, Nahmias C. Angiotensin receptors: A new role in cancer? Trends Endocrinol Metab. 2005;16:293–299. doi: 10.1016/j.tem.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Jakowlew SB. Transforming growth factor-β in cancer and metastasis. Cancer Metastasis Rev. 2006;25:435–457. doi: 10.1007/s10555-006-9006-2. [DOI] [PubMed] [Google Scholar]
- 51.Brooke BS, et al. Angiotensin II blockade and aortic-root dilation in Marfan's syndrome. N Engl J Med. 2008;358:2787–2795. doi: 10.1056/NEJMoa0706585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sica DA, Gehr TW, Ghosh S. Clinical pharmacokinetics of losartan. Clin Pharmacokinet. 2005;44:797–814. doi: 10.2165/00003088-200544080-00003. [DOI] [PubMed] [Google Scholar]
- 53.Longley DB, Johnston PG. Molecular mechanisms of drug resistance. J Pathol. 2005;205:275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 54.Schieffer B, et al. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]