Abstract
Recent studies by Dorshkind, Yoder, and colleagues show that embryonic (E9) B-cell progenitors located in the yolk sac and intraembryonic hemogenic endothelium before the initiation of circulation give rise to B-1 and marginal zone B cells but do not give rise to B-2 cells. In studies here, we confirm and extend these findings by showing that distinct progenitors for B-1 and B-2 cells are present in the adult spleen. Furthermore, we show that the splenic B-cell progenitor population (lin–CD19+/B220lo/−/CD43–) that gives rise to B-1 cells is likely to be heterogeneous because, in some recipients, it also gives rise to B cells expressing the marginal zone phenotype (B220hiIgMhiIgDloCD21hi) and to some (CD19–CD5hi) T cells. In addition to the well-known function differences between B-1 and B-2, our studies demonstrate that substantial developmental differences separate these B-cell lineages. Thus, consistent with the known dependence of B-2 development on IL-7, all B-2 progenitors express IL-7R. However, >30% of the B-1 progenitors do not express this marker, enabling the known IL-7 independent development of B-1 cells in IL-7−/− mice. In addition, marker expression on cells in the early stages of the B-2 development pathway (CD19–/c-Kitlo/−/Sca-1lo/−) in adult bone marrow distinguish it from the early stages of B-1 development (CD19hi/c-Kit+/Sca-1+), which occur constitutively in neonates. In adults, in vivo inflammatory stimulation (LPS) triggers B-1 progenitors in spleen to expand and initiate development along this B-1 developmental pathway.
Keywords: spleen hematopoiesis, lymphopoiesis, lineage commitment, c-Kit
After many years of smoke but no clear fire, Dorshkind, Yoder, and colleagues (1) finally identified embryonic (E9) B-cell progenitors that give rise to B-1 and marginal zone (MZ) B cells in adoptive recipients, but do not give rise to B-2 cells. Located in the yolk sac and intraembryonic hemogenic endothelium before the initiation of circulation, these B-1 and MZ B progenitors predate the emergence of the well-known B-cell progenitors in the fetal liver, which collectively give rise to B-1, B-2, and MZ B cells (1–3). The fetal liver B-cell progenitors are detectable in the fetus from day 13 onwards. These progenitors start to give rise to mature IgM-bearing B cells shortly before birth and collectively continue to populate the B-cell compartments during neonatal life and beyond.
Many years ago, differences that we and others detected in the phenotypic, functional, and developmental pathways associated with B-1 and B-2 cells led us to propose that these cells belong to distinct developmental lineages (2, 3). For example, during the neonatal period, the progenitors that give rise to B-1 and MZ B cells largely settle in the spleen, where they generate substantial numbers of B-1 cells until the animals are weaned. B-2 progenitors are also detectable in neonatal spleen. However, the B-2 progenitors rise to predominance in adult bone marrow (BM), where they continue to churn out B-2 cells for the life of the animal. In contrast, B-1 cells largely inhabit the peritoneal and pleural cavities and largely persist by self-replenishment throughout adult life (4).
Consistent with this developmental pattern, when adult BM and fetal liver from allotype congenic donors are transferred together to lethally irradiated recipients, the fetal liver readily restores both B-1 and B-2 cells in the recipient (2, 3). In contrast, adult BM completely restores B-2 cells but gives rise to only a few (∼5%) B-1 cells (2, 5, 6). These findings, which provided some of the earliest evidence of lineage distinctions between B-1 and B-2 cells, also showed that some B-1 progenitors persist in adults. However, discussions of whether the BM-derived B-1 cells arose from independent progenitors or from developmental diversion of B-2 cells pushed the entire issue into the realm of experts, from whence it has only now been rescued by the recognition of distinct embryonic progenitors for B-1 and B-2 (1) and of a small subset of phenotypically distinct B-1 progenitors in adult BM (7–9).
Our studies here concern the related issue of whether the spleen retains its neonatal potential for regenerating lymphoid and other adult lineages. Surprisingly, despite the long history of using spleen cells to rescue lethally irradiated animals (10, 11), current thinking commonly relegates B-cell and other hematopoetic progenitors to BM. In essence, the spleen seems to have come to be viewed only as a secondary lymphoid organ (and as a clearance site for blood-borne pathogens and senescent erythrocytes). However, as we show here, the spleen in adult animals is a hearty source of hematopoetic progenitors, including those that give rise to B cells. These B-cell progenitors are subdivided between phenotypically distinct progenitors, only one of which give rise to B-1, but not B-2 when sorted and transferred into RAG1−/− recipients.
We show that the B-1 progenitors in adult spleen are very similar to the B-1 progenitors in neonatal spleen. However, although the progenitors in adults are largely quiescent, the progenitors in neonates actively generate B-1 cells. Thus, a proportion of the neonatal B-1 progenitors show markers of developmental progression, whereas these markers are not detectable on the adult progenitor population unless the donor has been stimulated with LPS 3 to 4 d previously.
In addition to confirming the developmental lineage distinctions between B-1 and B-2, the findings we present demonstrate that B-1 cells persist mostly by self-replenishment but that the animal maintains a reservoir of progenitors committed to B-1 development that can be called upon under stress to enlarge or replenish the B-1 population. We discuss these findings in relation to our recent studies characterizing memory responses and early development in B-1 cells (12) and to the overall importance of the B-1 system for protection against infection and for responsiveness to vaccination.
Results
Adult Lineage-Negative Spleen Cells Contain Independent Progenitors for B-1 and B-2 Cells.
During murine fetal life, hematopoiesis occurs mainly in liver and spleen. After birth, B-cell development (mainly B-1) continues in the spleen until weaning, when BM (which mainly generates B-2 cells) takes over the task (3). In adults, the B-2 population is maintained by de novo development of B-2 cells, whereas the B-1 population is largely, if not completely, maintained by self-renewal (4). Thus, as expected, transfer of FACS-sorted mature (Ig+) B cells from the spleen demonstrate that only the B-1 population is capable of self-renewal in RAG1−/− recipients (Fig. 1). In contrast, transfer of FACS-sorted lineage-negative (lin–) (Ig– /CD5–/CD11b–/Gr-1–) cells from the spleen demonstrate that the adult spleen contains progenitors capable of reconstituting both B-1 or B-2 cells by 4 to 6 wk after transfer (Fig. 1).
Fig. 1.
Adult lin– spleen cells contain progenitors for both B-1 and B-2 cells. Total spleen cells from adult BALB/c were FACS-sorted to deplete dead cells, T cells, and myeloid cells. Next, the remaining spleen cells (PI–/CD5–/CD11b–/Gr-1–) were further FACS-sorted into two fractions: Ig– cells (which contains early B-cell progenitors) and Ig+ cells (which contains mature B cells). Ig– and Ig+ fractions were intravenously transferred into sublethally irradiated RAG1−/− recipients. The Lower plots show PerC RAG1−/− recipients 4 wk after receiving either Ig– or Ig+ spleen fractions.
CD19 and B220 expression further subdivide the lin– cells and distinguish the splenic B-1 from B-2 progenitors (Fig. 2A). Thus, the B-1 progenitors are CD19+/B220lo/– and the B-2 progenitors, like B-2 progenitors in BM, are CD19–/B220lo/–. We determine the B-2 progenitor phenotype here by combining data in Figs. 1 and 2A [i.e., total lin– cells contain progenitors for B-2 cells (Fig. 1 and ref. 13)]. However, these progenitors are not found either in the lin– CD19+/B220lo/–, which contains B-1 progenitors, or in the lin– CD19–/B220hi subset, which does not contain any B-cell progenitors (Fig. 2A).
Fig. 2.
Adult lin–/CD19+/B220lo/– progenitors give rise to B-1 (both B-1a and B-1b) cells but not B-2 cells. (A) Total spleen cells from adult BALB/c were FACS-sorted to deplete dead cells, B cells, T cells, and myeloid cells. Next, the remaining spleen cells (PI–/Ig–/CD5–/CD11b–/Gr-1–) were further FACS-sorted into two fractions: lin–/CD19+/B220lo/– and lin–/CD19–/B220hi. Each fraction was transferred intravenously to sublethally irradiated RAG1−/− recipients. After 4 to 6 wk, lin–/CD19+/B220lo/– progenitors give rise to B-1 cells, but not B-2 cells (Upper Right). The lin–/CD19–/B220hi fraction does not give rise to B cells (Lower Left). (B) Phenotypic analysis of B-1 cells derived from adult splenic B-1 progenitors.
Consistent with the demonstration that B-1 progenitors found among early lymphoid progenitors, >90% of the Ig heavy-chain (IgH) loci in the splenic B-1 (lin–/CD19+/B220lo/–) progenitors are not rearranged, we found that they do not express Ig either on the cell surface (BCR) or internally and they do not show IgH rearrangement by detectable RT-PCR (Fig. S1). A small proportion (∼10%) of the sorted B-1 progenitors show intracellular IgM. Consistent with these findings, RT-PCR analysis indicates very low level IgH rearrangement (only detectable after 30 cycles of amplification) (Fig. S1). The data do not allow determination of whether cells responsible for this rearranged IgH signal are mature B-cell contaminants or developing B cells (B-1 or B-2).
B-1 Progenitors From Adult Spleen Give Rise to Both B-1a and B-1b Cells, Including B-1a That Express VH11+ or VH12+.
Previous reports indicate that adult BM contains some B-1 progenitors and that these largely give rise to B-1b (CD5–) (6). In contrast, the B-1 progenitors in the adult spleen give rise to both B-1a (CD5+) and B-1b (Fig. 2B) in the same proportions that these cells are represented in intact animals [i.e., B-1a > B-1b in peritoneal cavity (PerC)]. These B-1a and B-1b cells derived from the splenic progenitors express the typical B-1 phenotype (CD43+/IgMhi/B220lo/CD19hi) (Figs. 2B and 3B). As in intact PerC, a large proportion of the progenitor-derived B-1 express CD11b and a smaller proportion express VH11 or VH12 (Figs. 2B and 3B), two heavy-chain loci that tend to be highly expressed by B-1. Thus, B-1 progenitors (lin–/CD19+/B220lo/–) from the adult spleen reconstitute typical PerC B-1a and B-1b populations 4 wk after intravenous transfer into sublethally irradiated RAG−/− recipients.
Fig. 3.
Adult splenic B-1 progenitors give rise to typical peritoneal cavity (PerC) B-1 cells, including CD43+, CD11b+, and VH12+ mature B-1 cells. (A) Total spleen cells from adult BALB/c were FACS-sorted to deplete dead cells, T cells, and myeloid cells. Next, the remaining spleen cells (PI–/CD5–/CD11b–/Gr-1–) were gated to include only CD19+ cells. Next, total CD19+ cells were gated to exclude Ig+ (κ/λ) cells (Upper Right). (B) Finally, these FACS-sorted adult splenic B-1 progenitors (Igκ/λ–, CD19+) were transferred intravenously to sublethally irradiated RAG1−/− recipients. Plots show phenotypic analysis of mature B-1 cells derived from adult splenic B-1 progenitors.
In about one-third of our transfer experiments, the B-1 progenitor population from spleen also gave rise to splenic B cells expressing the MZ phenotype (B220hi/CD21hi/CD23−) (Fig. S2). More often, the B-1 progenitor population from spleen gives rise to a small number of Ig–/CD19–/CD5hi cells, whose phenotype suggests that they are T cells (Fig. S2) that perhaps belong to a specialized subset. However, we reiterate that this progenitor population did not give rise to any B cells expressing the follicular B-2 phenotype in any of the recipients in any of our transfer studies (Fig. 2).
Interestingly, B-1 cells, some T cells, and cells expressing the MZ B phenotype are found in adult IL-7−/− mice, which lack all other B and T cells (Fig. S2). Because lymphoid development terminates just shortly after birth in IL-7−/− animals (14), these findings support the idea that the developmental potential of the splenic progenitors in intact mice reflects the developmental potential of progenitors active during early lymphoid development.
Mature B-1 Cells Block de Novo B-1 Progenitor Maturation.
As we have shown, B-1 progenitors from the adult spleen readily develop into mature B-1 cells in RAG1−/− recipients. However, these B-1 progenitors (GFP+) fail to develop when transferred either to intact or sublethally irradiated BALB/c recipients (Fig. S3). Furthermore, these progenitors fail to develop when transferred into RAG1−/− recipients together with FACS-sorted allotype-congenic (IgMb) PerC B-1 cells (IgMhi/IgDlo/B220lo/CD19hi) (Fig. S3). Thus, the presence of mature PerC B-1 hampers the de novo maturation of splenic B-1 progenitors into mature PerC B-1. This finding is consistent with long-standing lore indicating that transfers of BM to lethally irradiated recipients tend to yield somewhat higher frequencies of B-1 cells when transferred alone, as opposed to being cotransferred with (allotype-congenic) mature PerC B-1.
In Vivo Inflammatory Stimuli Induce Expansion and Activation of Adult Splenic B-1 Progenitors.
Treating wild-type mice with 15 μg of LPS intravenously strikingly enriches B-1 progenitors in spleen 3 to 4 d after stimulation (Fig. 4). Strikingly, stimulation with this TLR4 agonist actually increases both the absolute number and the frequency of the progenitors (Figs. 4 and 5B). Thus, this well-known stimulatory treatment, which induces PerC B-1 migration and differentiation to plasma cells (14), has an even more intense effect on B-1 progenitors in that it selectively increases the frequency of these progenitors relative to the increase in spleen size.
Fig. 4.
In vivo inflammatory stimuli (LPS) induce expansion and activation of adult splenic B-1 progenitors. Adult BALB/c mice were injected intravenously with 15 μg of LPS. After 3 to 4 d, spleen cells were harvested and stained as described in Materials and Methods. For analysis, spleen cells were first gated to deplete dead cells, T cells, and myeloid cells. Next, the remaining cells were gated to include total CD19+. Total CD19+ cells were further gated to exclude Ig+ cells. The lin–/CD19+/B220lo/– B-1 progenitors were analyzed for the expression of c-Kit, Sca-1, and IL-7R.
Fig. 5.
A fraction of neonatal splenic B-1 progenitors express the essential activated phenotype (lin–/c-Kit+/CD19hi/B220lo/–) expressed by LPS-stimulated adult splenic B-1 progenitors. (A) Plots show the expression of c-Kit and CD19 on B-1 progenitors present in the spleen of neonate, adult, and LPS-stimulated adult BALB/c. (B) Absolute numbers of total B-1 progenitors or c-Kit+ fraction of B-1 progenitors (contained among total B-1 progenitors) present in the spleen of neonate, adult, and LPS-stimulated adult BALB/c adults or neonates BALB/c.
Surprisingly, a high proportion (30–40%) of the B-1 progenitors in LPS-stimulated spleen gain a unique phenotype characterized by the coexpression c-Kit, Sca-1, and IL-7R, along with high levels of CD19 and low levels of B220 (Fig. 4 and Table 1). The newly expressed markers are well-known on hematopoietic progenitors but are not previously known to be expressed on CD19hi cells (15).
Table 1.
Phenotypic profile of splenic B-1 progenitors
| Control | LPS-stimulated | |
| Intra/extra cell Ig | – | – |
| CD19 | + | + (Some CD19hi) |
| B220 | Low/– | Low/– |
| CD5 | – | – |
| CD43 | – | Not tested |
| IL-7R | IL-7R+ and IL-7R– | IL-7R+ and IL-7R– |
| Sca-1 | – | + |
| c-Kit | – | + |
| CD138 | Low | Majority are negative |
| CD11b | – | – |
| CD34 | – | Not tested |
Spleen cells from unstimulated or LPS-stimulated BALB/c were harvested, stained and total lin–/CD19+/B220lo/– B-1 progenitors were analyzed for the expression of several surface markers. Values described (+ or −) are based on the expression level of the median of fluorescence intensity (MFI). The value for the expression of each surface marker was obtained by subtracting the MFI of the full-stained cells from the MFI of fluorescence minus one (FMO) control (22). Cells that expressed MFI for a given marker similar or equal to FMO were considered negative. Data shown are representative of more than three experiments.
The expression of IL-7R in the B-1 development pathway would appear to contradict evidence showing that adult IL-7−/− mice have both B-1 and MZ B cells (16). However, we show here that at least a third of the splenic B-1 progenitors do not express IL-7R, even after LPS stimulation (Fig. 4). These findings suggest that the population of B-1 progenitors in spleen in wild-type animals is heterogeneous and may contain progenitors for two types of B-1 cells, only one of which requires IL-7 for development.
B-1 Progenitors Expressing the Adult B-1 Progenitor Phenotype Are Enriched in Neonatal Spleen and PerC.
Both the frequency and the absolute number of lin–/CD19+/B220lo/– B-1 progenitors are higher in spleen and PerC harvested from 2- to 4-d-old neonates (Fig. 6) than in similar cell populations harvested from these locations in adult mice. Pointedly, a proportion of the neonatal progenitors (∼15% at day 4) express the essential phenoptype of the activated B-1 progenitors found in LPS-stimulated adult mice (lin–/c-Kit+/CD19hi/B220lo/–) (Fig. 5), suggesting that this phenotype may reflect the presence of cells at early stages of differentiation both in the neonate and the LPS-stimulated spleen.
Fig. 6.
B-1 progenitors expressing the adult B-1 progenitor phenotype are enriched in neonatal spleen and PerC. (A) Spleen and PerC cells from neonates (2–4 d old) or adults BALB/c were harvested and stained as described. FACS plots show the frequency of B-1 progenitors present in the total CD19+ cells gated out from the Ig–/CD5–/CD11b–/Gr-1– population. (B) Absolute numbers of B-1 progenitors found in the PerC and spleen of adults or neonates BALB/c.
Discussion
In earlier studies, Dorshkind and colleagues (7) reported that a rare lin–/CD19+/B220lo/– subset isolated from adult BM reconstitutes B-1 cells but does not reconstitute B-2 cells. Recently, Dorshkind, Yoder, and colleagues (1) showed that progenitors in very early (E9) mouse embryos give rise to both B-1 and MZ B cells but not to B-2 cells. Together, these studies confirm the once controversial hypothesis that B-1 and B-2 belong to distinct developmental lineages (17, 18). Here, we independently confirm this hypothesis by showing that adult spleen contains phenotypically distinct progenitors that individually give rise to B-1 or B-2 cells when transferred to RAG1−/− recipients that lack all native B and T cells.
Currently, progenitors capable of repopulating B-1 cells in transfer recipients are not commonly thought to be present in adult spleen. However, a wide variety of earlier (10, 11) and recent (13) studies demonstrate clearly that spleen can reconstitute T and B cells in lethally irradiated recipients. Here, we show that lin– cells sorted from the spleen give rise to both B-1 and B-2 cells in RAG1−/− mice (sublethally irradiated) and that CD19+/B220lo/– progenitors sorted from this lin– population readily give rise to B-1 but not B-2 cells (Figs. 2 and 3). We have not further characterized the lin– progenitors that give rise to B-2. However, our findings demonstrate clearly that these are independent of the lin–/CD19+/B220lo/− splenic progenitors that give rise to B-1 cells.
Like the embryonic B-1 progenitors (1), the sorted adult splenic progenitor population that gives rise to B-1 cells can give rise to B cells expressing the CD21hi/B220hi/CD23– phenotype associated with MZ B cells (Fig. S2). However, because MZ B cells are only reconstituted in about 30% of the transfers, there is a strong possibility that the lin–/CD19+/B220lo/– progenitor population is heterogeneous and that the splenic progenitors for MZ B are actually distinct from the progenitors for B-1.
Consistent with this idea, we also observe some T-cell reconstitution in up to half of the recipients of the sorted lin–/CD19+/B220lo/– (Fig. S2). We suspect that these T cells, which are detectable (when present) in both recipient spleen and PerC, may be primitive (γδ) T cells comparable those demonstrated by Ikuta and Weissman (19) in the first wave of T-cell development. If so, then the lin–/CD19+/B220lo/– progenitors isolated from the adult spleen may either be heterogeneous or contain a common progenitor capable of giving rise to B-1, MZ B, and some T cells. In either case, our data suggest that these splenic progenitors are lineally related to very early hematopoietic progenitors that give rise to primitive lymphocyte subsets during fetal life.
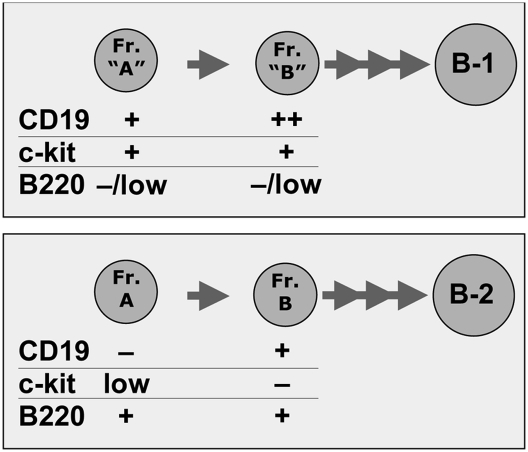
The unique phenotype expressed by B-1 progenitors reveals the existence of distinct developmental pathways for B-1 and B-2 lymphopoiesis. In the B-1 pathway, CD19 is expressed before B220 and CD43, whereas in the B-2 pathway, CD19 is expressed after B220 and CD43. Thus, the Hardy factions that define the ordered early development of B cells in the BM are applicable to early B-2 development (15) and must be modified to accommodate the early B-1 development pathway. The c-Kit expression also distinguished the two development pathways (Fig. 7). During B-2 development, c-Kit is highly expressed on hematopoietic stem cells, still clearly expressed on common lymphoid progenitors, expressed at very low levels in Hardy Fr. A, and is lost when CD19 turns on in Fr. B (15). In contrast, c-Kit is coexpressed with CD19 during the early stages of B-1 development, which occur in the neonate but not in the adult spleen unless spleens are taken from animals 3 to 4 d after LPS stimulation (Figs. 4 and 5). In this case, c-Kit is coexpressed with CD19, IL-7R, and Sca-1 (Fig. 4), suggesting that appropriate stimulation can trigger B-1 progenitors to initiate development in adult spleen along a pathway similar to the B-1 development pathway in neonates.
Fig. 7.
Early progenitors for B-1 and B-2 express different markers.
The idea that the B-1 progenitors in spleen may be held largely in a quiescent state during adult life, when the B-1 repertoire is typically maintained by self-renewal, is consistent with data presented here showing that splenic B-1 progenitors that develop de novo into mature B-1 cells in RAG1−/− recipients fail to initiate B-1 development in intact recipients (Fig. S3). However, as we have also shown, inflammatory signals (e.g., LPS stimulation) dramatically increase the frequency and absolute numbers of splenic B-1 progenitors and activate these to develop along the B-1 pathway (lin–/CD19hi/B220lo/−/c-Kit+) (Figs. 4 and 5). Therefore, the degree of quiescence of B-1 progenitors in adult animals may largely be expected to be a function of the immunogenic environment in which the animal is maintained.
Because the spleen is important to patrol the blood for blood-borne pathogens, it should not be surprising that certain antigens specifically trigger the differentiation of mature B-1 cells to antibody-producing plasma cells in spleen (14). However, more ground-breaking studies with this antigen now show that it triggers the development of antigen-specific B-1 memory cells that develop in the spleen, migrate to PerC, and persist indefinitely there until rechallenge in the context of a Toll-like receptor (TLR)-4 stimulations brings them back to develop into antibody-producing cells in the spleen (12, 19). Recent studies by Carsetti and colleagues (20) demonstrate that a Hoechst-excluding “side population” that can be sorted from the spleen contains B-1 progenitors that restore mucosal IgA production and, hence, provide key protection against gut infections. Studies presented here introduce a robust phenotype for these progenitors and distinguished them from B-2 progenitors in the spleen. In addition, our studies indicate that encounters with TLR-stimulating organisms induces quiescent B-1 progenitors in the adult spleen to differentiate to mature B-1 cells, and thus replenish the available pool of B-1 cells ready to develop into B-1 memory and plasma cells at the next pathogen encounter.
Overall, the studies we have presented demonstrate clearly that the adult spleen retains its fetal lymphopoiesis potential and contains B-cell progenitors that can collectively give rise to both B-1 and B-2 cells in RAG1−/− recipients. We have isolated the progenitors that give rise to B-1 cells from those that give rise to B-2 cells, and shown that they, or coisolated cells, can give rise in some recipients to B cells expressing the MZ phenotype and to some T cells. These findings suggest an early schism that divided the lymphoid development pathway, leading to B-1 development from that leading to B-2 cell development. As such, they support the idea that the mammalian immune system evolved in progressive layers that continue to contribute to immune function, albeit in modified form that accommodates the more sophisticated immune functions provided by more highly evolved layers (21).
Materials and Methods
Mice and Tissue Preparation.
BALB/c (IgMa allotype), CB.17 (IgMb allotype and either GFP+ or GFP–), C57BL/6, IL-7−/− (C57BL/6 background, kindly donated by Paulo Vieira, Département d'Immunologie, Institut Pasteur, Paris, France), and RAG1−/− mice (adults 6–10 wk old or neonates 2–4 d old) were purchased from Jackson Labs or bred locally. All mice were maintained at the Stanford Medical School Animal Care Facility. All experiments were conducted with Institutional Animal Care and Use Committee Approval. PerC cells were harvested by injecting 7 mL of staining medium (deficient RPMI plus 3% newborn calf serum) into PerC. Spleen was disrupted and resuspended to obtain single cell suspensions. All cell samples were resuspended at 25 × 106 cells/mL using custom RPMI medium 1640 deficient in biotin, l-glutamine, phenol red, riboflavin, and sodium bicarbonate (Invitrogen).
FACS.
Cell suspensions were preincubated with anti-CD16/CD32 mAb to block FcγRII/III and stained on ice for 15 min with the following fluorochrome-conjugated mAb in an 11-color staining combination: FITC-labeled anti-CD21 (7G6), or anti-CD34 (RAM34), or anti-Igκ and -λ light chains (187.1 and R26-46, respectively); PE-labeled anti-CD43 (S7), anti–I-A (AMS-32.1), anti-CD117 (c-Kit 2B8), or anti-IgMa (DS-1); PECy5-labeled anti-CD5 (53–7.3); PECy5.5-labeled anti-CD19 (1D3) or anti–Sca-1 (Ly-6A/E D7); PECy7-labeled anti-IgM (331) or anti-CD93 (AA4.1); APC-labeled anti-B220 (RA3-6B2), or anti-CD117 (c-Kit 2B8); Alexa700-labeled anti-IgD (11-26) or anti-IgMb (AF6-78.25); APCCy7-labeled anti-CD19 (1D3), or anti-B220 (RA3-6B2), or anti-CD11b (M1/70); Pacific Blue-labeled (Dump channel) anti-F4/80 (BM8), anti–Gr-1 (RB6-8C5), and anti-CD11b (M1/70); biotin-labeled anti-CD127 (IL-7Rα), or anti-Igκ and -λ light chains (187.1 and R26-46, respectively). Cells were then washed and stained again on ice for 15 min with streptavidin Qdot 605 (Invitrogen) to reveal biotin-coupled antibodies. Antibodies were either purchased (Invitrogen and BD Pharmingen) or conjugated in our laboratory. After washing, stained cells were resuspended in 10 μg/mL propidium iodide (PI), to exclude dead (i.e., PI− cells). Cells were analyzed and sorted on Stanford FACS facility instruments (Becton Dickinson LSRII or FACSAria). Data were collected for 0.2 to 1 × 106 cells. Staining protocols were designed with CytoGenie software (WoodSideLogic); data were analyzed with FlowJo software (TreeStar). To distinguish autofluorescent cells from cells expressing low levels of individual surface markers, we established upper thresholds for autofluorescence by staining samples with fluorescence-minus-one (FMO) control stain sets in which a reagent for a channel of interest is omitted (22).
Cell Transfer.
Spleens from BALB/c or CB.17 (GFP+) were harvested and stained as described. In sorting procedures, unless otherwise specified, cells were FACS-sorted to first exclude dead cells, T cells, and myeloid cells (i.e., PI–/CD5–/CD11b–/Gr-1–). The remaining cells were then further gated and FACS-sorted to purify specified cell populations. Purity among each sorted population constitutes >95%, as determined by a parallel full-stained population and reanalysis after sort. PBS buffer containing 0.1 to 2 × 104 adult splenic B-1 progenitors (lin–/CD19+/B220lo/−) or other cell populations (0.3–3 × 106 splenic lin–/Ig– or lin–/Ig+) were injected intravenously to sublethally irradiated RAG1−/− or BALB/c recipients. After 4 to 6 wk, total PerC and spleen cells from recipient mice were harvested, stained, and analyzed as described.
Acknowledgments
We thank Megan Phillips for outstanding technical help, John Mantovani and Claudia Weber for administrative support, and Roy Riblet for helpful discussions. This work was supported by National Institutes of Health Grant AI076434.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1019764108/-/DCSupplemental.
References
- 1.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2010;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. Adoptive transfer of murine B-cell lineages. Ann N Y Acad Sci. 1992;651:168–169. doi: 10.1111/j.1749-6632.1992.tb24610.x. [DOI] [PubMed] [Google Scholar]
- 3.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. doi: 10.1146/annurev.iy.11.040193.002441. [DOI] [PubMed] [Google Scholar]
- 4.Kantor AB, Stall AM, Adams S, Watanabe K, Herzenberg LA. De novo development and self-replenishment of B cells. Int Immunol. 1995;7(1):55–68. doi: 10.1093/intimm/7.1.55. [DOI] [PubMed] [Google Scholar]
- 5.Düber S, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114:4960–4967. doi: 10.1182/blood-2009-04-218156. [DOI] [PubMed] [Google Scholar]
- 6.Stall AM, Adams S, Herzenberg LA, Kantor AB. Characteristics and development of the murine B-1b (Ly-1 B sister) cell population. Ann N Y Acad Sci. 1992;651:33–43. doi: 10.1111/j.1749-6632.1992.tb24591.x. [DOI] [PubMed] [Google Scholar]
- 7.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 8.Esplin BL, Welner RS, Zhang Q, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tung JW, Mrazek MD, Yang Y, Herzenberg LA, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDevitt HO, Tyan ML. Genetic control of the antibody response in inbred mice. Transfer of response by spleen cells and linkage to the major histocompatibility (H-2) locus. J Exp Med. 1968;128(1):1–11. [PMC free article] [PubMed] [Google Scholar]
- 11.Kontiainen S, Mitchison NA. Blocking antigen-antibody complexes on the T-lymphocyte activation during in vitro incubation before adoptive transfer. Immunology. 1975;28:523–533. [PMC free article] [PubMed] [Google Scholar]
- 12.Cole LE, et al. Antigen-specific B-1a antibodies induced by Francisella tularensis LPS provide long-term protection against F. tularensis LVS challenge. Proc Natl Acad Sci USA. 2009;106:4343–4348. doi: 10.1073/pnas.0813411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morita Y, et al. Functional characterization of hematopoietic stem cells in the spleen. Exp Hematol. 2010 doi: 10.1016/j.exphem.2010.12.008. 10.1016/j.exphem.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 14.Yang Y, Tung JW, Ghosn EE, Herzenberg LA, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc Natl Acad Sci USA. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hardy RR, Kincade PW, Dorshkind K. The protean nature of cells in the B lymphocyte lineage. Immunity. 2007;26:703–714. doi: 10.1016/j.immuni.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 16.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194:1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzenberg LA. B-1 cells: The lineage question revisited. Immunol Rev. 2000;175:9–22. [PubMed] [Google Scholar]
- 18.Tung JW, Herzenberg LA. Unraveling B-1 progenitors. Curr Opin Immunol. 2007;19(2):150–155. doi: 10.1016/j.coi.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Ikuta K, et al. Development of gamma delta T-cell subsets from fetal hematopoietic stem cells. Ann N Y Acad Sci. 1992;651:21–32. doi: 10.1111/j.1749-6632.1992.tb24590.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosado MM, et al. From the fetal liver to spleen and gut: The highway to natural antibody. Mucosal Immunol. 2009;2:351–361. doi: 10.1038/mi.2009.15. [DOI] [PubMed] [Google Scholar]
- 21.Herzenberg LA, Kantor AB, Herzenberg LA. Layered evolution in the immune system. A model for the ontogeny and development of multiple lymphocyte lineages. Ann N Y Acad Sci. 1992;651:1–9. doi: 10.1111/j.1749-6632.1992.tb24588.x. [DOI] [PubMed] [Google Scholar]
- 22.Roederer M. Spectral compensation for flow cytometry: Visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]