Abstract
DNA mismatch repair (MMR) is a multifunctional process that promotes genetic stability and suppresses carcinogenesis. Correction of DNA replication errors is its major function. Despite the importance of MMR, its functioning in eukaryotes is not well understood. Here we report that human mismatch correction reactions in cell-free extracts occur during concomitant nick-dependent nucleosome assembly shaped by the replication histone chaperone CAF-I. Concomitant nucleosome assembly protects the discontinuous mismatch-containing strands from excessive degradation by MMR machinery. Such protection is also demonstrated in a defined purified system that supports both mismatch correction and CAF-I-dependent histone H3–H4 deposition reactions. In addition, we find that the mismatch recognition factor MutSα suppresses CAF-I-dependent histone H3–H4 deposition in a mismatch-dependent manner. We suggest that there is active crosstalk between MMR and replication-dependent nucleosome assembly during the correction of DNA replication errors and, as a result, the nascent mismatch-containing strands are degraded in a controlled manner.
Keywords: CAF-I histone chaperone, hereditary nonpolyposis colorectal cancer, MutLalpha endonuclease, DNA repair, replication errors
MMR is a strongly conserved process that guards genetic stability and suppresses carcinogenesis by correcting replication errors, removing mismatches during homologous recombination, suppressing homeologous recombination, and initiating cell signaling and apoptotic responses upon detection of DNA damage of several classes (reviewed in refs. 1–3). It removes both single base–base mismatches and small insertions/deletions.
MMR is best understood in Escherichia coli, where the sequence of biochemical events that lead to the correction of DNA replication errors has been identified (3). Significant progress has also been made in understanding the correction of DNA replication errors in eukaryotes. Naturally occurring strand breaks (nicks and gaps) in the daughter strands suffice to direct human MMR to remove a DNA replication error (4, 5). The current understanding of eukaryotic MMR suggests that it consists of mismatch recognition, incision, excision, and DNA synthesis steps (6). Eukaryotic correction of a DNA replication error is initiated by recognition of the mismatch by the primary mismatch recognition factor MutSα. In the next step, MutSα, the PCNA clamp, and the RFC clamp loader activate MutLα endonuclease to incise the daughter strand in the vicinity of the error in an ATP-dependent reaction (7). Function of RFC in the activation of MutLα endonuclease is limited to loading PCNA on the nicked DNA, whereas the loaded PCNA directs MutLα endonuclease to incise the discontinuous strand (8). An incision introduced by MutLα 5′ to the error is used by MutSα-activated Exo1 as the entry site for a 5′-to-3′-directed excision that removes the error along with a stretch of surrounding DNA in a reaction modulated by the RPA ssDNA-binding protein (7, 9). The resulting gap is then filled by DNA polymerase δ holoenzyme (10, 11). In the absence of Exo1, eukaryotic correction of DNA replication errors can proceed without the excision step by relying on the strand-displacement activity of DNA polymerase δ, which removes a mismatch in a reaction initiated from a 5′ MutLα incision (12). Although the described view of eukaryotic correction of replication errors is supported by both biochemical and genetic data, more research is needed to advance this view and to investigate whether there are complementary and alternative mechanisms of mismatch correction.
Eukaryotic MMR operates in the nucleosomal environment, but the impact of the nucleosomal organization on eukaryotic MMR is not understood. Nuclear DNA is packaged into nucleosomes by various histone chaperones during diverse DNA transactions such as replication and transcription, but nucleosome assembly follows the same path: (H3-H4)2 tetramer is initially loaded onto DNA and two H2A–H2B dimers are then added to the (H3-H4)2 tetramer (13). The correction of DNA replication errors occurs on newly replicated DNA that is packaged into nucleosomes by the histone chaperone CAF-I (14–16). In this work, we present evidence that CAF-I-dependent nucleosome assembly controls the mismatch-provoked degradation of the discontinuous strands during the correction of DNA replication errors.
Results
CAF-I-Dependent Control of Degradation of the Discontinuous Strands During Mismatch Repair in Cell-Free Extracts.
A biochemical analysis revealed that mismatch correction reactions with 90–180-μg HeLa cytosolic extract or 90-μg HeLa nuclear extract are accompanied by mismatch-provoked degradation of the discontinuous strands, but that this degradation is suppressed in reactions with 135–180-μg HeLa nuclear extract (Fig. 1 A–C). For example, the reactions with 135-μg HeLa cytosolic extract correct 20% of available mispaired G bases (Fig. 1C) and provoked 16% degradation of the indirectly labeled discontinuous strands (bracketed area of Fig. 1A, and Fig. 1B), whereas the 135-μg HeLa nuclear extract reactions repair 43% of mispairs and degrade only 5% of the discontinuous strands. Comparing these results suggests that mismatch correction in HeLa cytosolic extracts is accompanied by excessive degradation of the discontinuous mismatch-containing strands, and such degradation is suppressed in reactions with larger amounts of HeLa nuclear extract.
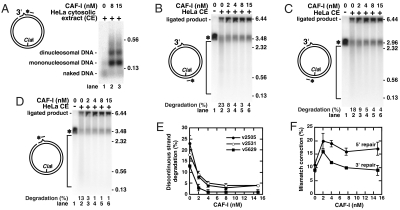
Fig. 1.
Mismatch correction and nucleosome assembly in HeLa cell-free extracts. Mismatch-correction reactions with 3′ G-T or control 3′ A-T DNA and the indicated amounts of HeLa nuclear or cytosolic extract were performed and analyzed as described in Materials and Methods. (A) Recovered DNA products of the indicated mismatch correction reactions were cleaved with ClaI, separated under denaturing conditions in a 0.8% agarose gel, transferred to a nylon membrane, and hybridized with a 32P-labeled probe complementary to the discontinuous strand position 2505–2526. The diagram on the left of the image shows the relative positions of the 3′ strand break, the G-T mispair (sharp bump), a 32P-labeled probe (dashed asterisk), and the unique ClaI site in the 3′ G-T DNA. The asterisk specifies the position of the indirectly labeled ClaI fragment of the discontinuous strand detected in the control reaction carried out in the absence of any extract or purified proteins. In the extract reactions, the discontinuous strand is subjected to mismatch correction and/or ligation; ligation generates the 6.44-kb ligated product. The mismatch-correction reaction proceeds via mismatch-provoked degradation of the discontinuous strand that generates smaller fragments located in the area indicated by the bracket. Degradation of the discontinuous strand in a particular reaction expressed as a percentage of the total label and representing the mean of three experiments is indicated below the reaction lane. (B) Degradation of the discontinuous strand of 3′ G-T (▪ and □) or 3′ A-T (• and ◯) DNA as a function of the concentration of HeLa nuclear (□ and ◯) or cytosolic (▪ and •) extract. Data are from images like the one shown in A. (C) Dependence of mismatch correction of 3′ G-T DNA in HeLa nuclear (□) or cytosolic (▪) extract on the amount of extract used in the reactions. The data in B and C are presented as the means ± 1 SD, n = 3. (D) Assembly of nucleosomes on 3′ G-T or cc G-T DNA during mismatch correction in HeLa nuclear and cytosolic extracts. DNA products of the indicated mismatch correction reactions were subjected to micrococcal nuclease cleavage and separation in a 1.5% native gel, and Southern hybridization with a 32P-labeled probe complementary to f1MR59 minus strand position 5733–5756 (35). 32P hybridizations were visualized with a GE Storm PhosphorImager system. (E) Detection of CAF-I in HeLa nuclear and cytosolic extracts by Western analysis. Proteins of HeLa nuclear and cytosolic extracts (20 μg each) were separated in a denaturing SDS-gel, transferred to a PVDF membrane and incubated with rabbit CAF-I p150 antibodies (Santa Cruz), followed by detection of the immune complexes with an ECL Plus kit (GE HealthCare).
The excessive mismatch-dependent degradation of discontinuous strands in the mismatch-correction reactions in HeLa cytosolic extract (Fig. 1 A and B) is similar to that observed in the reconstituted four-protein incision system consisting of MutLα endonuclease, MutSα, the PCNA clamp, and the RFC clamp loader (7). This commonality suggested to us that both the cytosolic extract and the reconstituted system might lack a nuclear factor that protects the discontinuous mismatch-containing strands from uncontrolled degradation. We considered that tight binding of such a factor to DNA in the vicinity of a mismatch might prevent an incision complex containing MutLα from sliding away and from subsequent incision of the discontinuous strand at remote sites. Histone octamer, the protein component of nucleosomes, is among the eukaryotic factors that bind DNA with high affinity. We therefore tested if assembly of nucleosomes is responsible for controlling the degradation of discontinuous strands during mismatch-correction reactions. Nucleosome assembly was probed by cleavage with micrococcal nuclease, and the results demonstrated that increased nucleosome assembly is observed in the same nuclear-extract reactions (Fig. 1D) in which the uncontrolled degradation of the discontinuous strands is suppressed (Fig. 1 A and B). Remarkably, increased nucleosome assembly depended largely on the presence of a strand break (Fig. 1D). These results therefore suggest that nick-directed nucleosome assembly might participate in controlling the mismatch-provoked degradation of discontinuous strands.
Because the major replication-dependent histone chaperone CAF-I assembles nucleosomes in a nick-dependent manner (17) and is largely lacking in cytosolic extracts (18) (Fig. 1E), we hypothesized that CAF-I-dependent nucleosome assembly might be involved in control of the mismatch-dependent degradation of the discontinuous strands. In complete accord with previous observations (14, 17, 19), we find that supplementation of HeLa cytosolic extract mismatch correction reactions with a highly purified recombinant CAF-I (Fig. S1A) results in the formation of nucleosomes on the nicked, mismatch-containing DNA molecules (Fig. 2A and Fig. S2A).
Fig. 2.
Involvement of CAF-I in control of degradation of the 3′ G-T discontinuous strand in mismatch-correction reactions in HeLa cytosolic extract. Analyses of mismatch correction and nucleosome assembly of 3′ G-T DNA in HeLa cytosolic extract (90 μg) supplemented or not with purified recombinant CAF-I were performed as described in the legend to Fig. 1. (A) Assembly of nucleosomes on 3′ G-T DNA in HeLa cytosolic extract depends on the presence of CAF-I. Products of micrococcal nuclease cleavage of the indicated reactions were visualized by Southern hybridization with a 32P-labeled probe complementary to discontinuous-strand position 5733–5756. (B–D) CAF-I controls degradation of the discontinuous strand of 3′ G-T DNA in HeLa cytosolic extracts. The products of the mismatch correction reactions were analyzed by Southern hybridization with a 32P-labeled probe complementary to discontinuous-strand position 2505–2526 (B), 2531–2552 (C), or 5629–5652 (D). (E) Degradation of the discontinuous strand as a function of CAF-I concentration. The data are from images like those shown in B–D. (F) Mismatch correction of 3′ G-T DNA (▪) and 5′ G-T DNA (◯) as a function of CAF-I concentration. The data in E and F are the means ± 1 SD, n≥2.
We then inquired whether the presence of the purified CAF-I has any effect on mismatch-provoked degradation of the discontinuous strands of the 3′ and 5′ G-T DNA. After ClaI cleavage and separation in denaturing agarose gels, the products of the mismatch-correction reactions were subjected to Southern hybridizations with 32P-labeled probes complementary to different sequences of the discontinuous strand (Fig. 2 B–D and Fig. S2 B and C). Analysis of the resulting images indicated that the degradation products of 0.13–3-kb size, visualized with a 32P-labeled probe complementary to a discontinuous strand DNA sequence immediately 3′ to the ClaI site, are present at 18–23% of the total in the HeLa cytosolic extract reactions lacking purified CAF-I but are only at 3–4% in the reactions containing 4–15 nM purified CAF-I (Fig. 2E and Fig. S2D). Similarly, 0.13–3-kb degradation products, detectable with a 32P-labeled probe complementary to a discontinuous-strand sequence immediately 5′ to the ClaI site, were at 18–22% in the reactions without CAF-I, whereas the level of these products was suppressed to 4–8% by the addition of 8–15 nM CAF-I (Fig. 2E and Fig. S2D). Furthermore, a Southern analysis with a 24-nt 32P-labeled probe complementary to a discontinuous-strand sequence containing the mispaired G shows that the presence of 4–15 nM CAF-I suppresses level of the degradation from 13% to 1% (Fig. 2D). The results of these experiments therefore support the hypothesis that CAF-I-dependent nucleosome assembly is involved in the control of the mismatch-provoked degradation of the discontinuous strands during mismatch correction in HeLa cytosolic extract. We also tested whether the presence of CAF-I impacts the efficiency of 3′- and 5′-nick-directed mismatch correction in the HeLa cytosolic extract reactions. The results suggest that CAF-I has no or little stimulating effect on 3′-nick-directed mismatch correction but modestly increases 5′-nick-directed mismatch correction by about 50% (Fig. 2F).
293T cells lack MMR and MutLα due to methylation of the MLH1 promoter, but adding purified MutLα to 293T cytosolic extract efficiently complements the MMR deficiency (20) (Fig. S3). To extend our observations obtained with HeLa cytosolic extract, we conducted mismatch-correction reactions in 293T cytosolic extracts in the presence or absence of purified CAF-I. Our control reactions showed that the indirectly end-labeled region of the 3′ G-T discontinuous strands is degraded by only 5% in the absence of purified MutLα and that this MMR-independent degradation is not affected by the presence of 7.5-nM CAF-I (Fig. S3A, lanes 14–15). Consistent with our previous observations (Fig. 2 and Fig. S2), considerable MutLα-dependent degradation of the indirectly end-labeled discontinuous strands is observed in 293T cytosolic reactions lacking purified CAF-I, and the addition of CAF-I strongly suppresses this degradation (Fig. S3 A and B). Furthermore, we observed that CAF-I supplementation increases 3′ and 5′ nick-directed mismatch correction up to twofold (Fig. S3 C–E) and is necessary for nick-directed assembly of nucleosomes (Fig. S3F). The combined results of these experiments further support our hypothesis that CAF-I-dependent nucleosome assembly is involved in the control of mismatch-provoked degradation of discontinuous strands.
MutSα Represses CAF-I-Dependent Histone H3–H4 Deposition in a Mismatch-Dependent Manner.
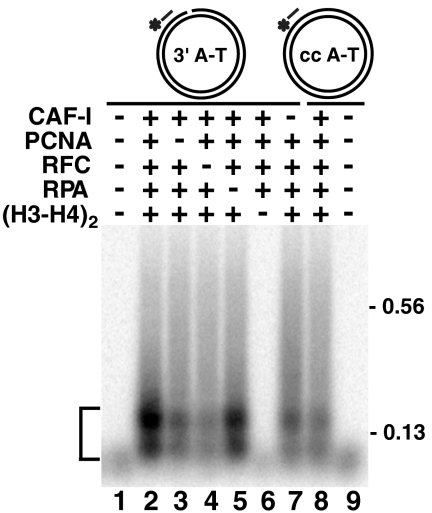
In order to understand how CAF-I affects the mismatch-provoked degradation of discontinuous strands, we carried out reconstitution studies. Deposition of a histone (H3-H4)2 tetramer protects a 73–146-bp DNA fragment from micrococcal nuclease cleavage (21), enabling us to use this feature to detect the deposition. Because PCNA recruits CAF-I to newly replicated DNA (22) and RFC loads PCNA onto nicked DNA, we investigated whether CAF-I-dependent histone (H3-H4)2 deposition occurs in a purified system that contains purified human CAF-I, histone (H3-H4)2 tetramer, PCNA, RFC, and RPA proteins and nicked DNA. In these experiments, 73–146-bp DNA fragments that resist micrococcal nuclease cleavage due to the histone deposition were visualized with Southern hybridization (Fig. 3). The results of these experiments suggest that CAF-I-dependent histone (H3-H4)2 tetramer deposition on a nicked DNA occurs in reactions also containing PCNA and RFC (Fig. 3, lanes 2 and 5). The omission/substitution experiments suggest that the observed histone deposition requires the presence of CAF-I, histone (H3-H4)2 tetramer, RFC, and a DNA strand break and largely depends on PCNA (Fig. 3 and Table S1).
Fig. 3.
CAF-I-dependent histone H3–H4 deposition in a defined system. Histone (H3-H4)2 tetramer deposition reactions and their analysis were performed as described in Materials and Methods. The reactions contained the specified protein components and 3′ A-T DNA (lanes 1–7) or cc A-T DNA (lanes 8–9). DNA products generated by micrococcal nuclease cleavage were visualized with a 32P-labeled probe complementary to the minus strand of the two DNAs at position 5225–5248. The brackets indicate positions of DNA species protected from micrococcal nuclease by histone (H3-H4)2 tetramer deposition.
Because the correction of DNA replication errors and CAF-I-dependent nucleosome assembly are two postreplicative processes that are likely to co-occur on newly synthesized DNA, we tested whether the mismatch-recognition factor MutSα impacts reconstituted histone (H3-H4)2 tetramer deposition in a mismatch-dependent manner. Visualization of the histone-deposition products with a 32P-labeled probe complementary to the discontinuous strand sequence located 59 nt 5′ from the mismatched G base suggests that MutSα suppresses deposition in the vicinity of mismatch in a concentration- and mismatch-dependent manner (Fig. 4A and Fig. S4A). Similarly, MutSα decreases histone deposition on a small DNA region containing the G-T mismatch (Fig. 4B and Fig. S4B). However, MutSα has no effect on histone deposition occurring approximately 400 nt 3′ from the mismatch (Fig. 4C and Fig. S4C). These results are consistent with a model in which MutSα interferes with the assembly of nucleosomes on DNA regions containing a mismatch.
Fig. 4.
MutSα inhibits CAF-I-dependent histone H3-H4 deposition in the vicinity of a mismatch. Histone deposition reactions containing 3′ G-T DNA (lanes 1–6) or 3′ A-T DNA (lanes 7–12) and the indicated concentrations of MutSα were performed and analyzed as in Fig. 3. Histone deposition products were visualized by hybridization with a 32P-labeled probe complementary to the discontinuous strand position 5690–5713 (A), 5629–5652 (B), or 5225–5248 (C).
CAF-I-Dependent Control of Mismatch-Provoked Degradation of the Discontinuous Strands in a Purified System.
The availability of the minimal histone-deposition (Fig. 3) and mismatch-correction reactions (11) permitted us to investigate whether CAF-I-dependent histone H3–H4 deposition, the first step in replication-dependent nucleosome assembly, can control the mismatch-provoked degradation of the discontinuous strands in a defined system. In accordance with earlier studies (11, 12), we find that the seven-protein MMR system consisting of MutSα, MutLα, RFC, PCNA, Exo1, RPA, and DNA polymerase δ is proficient in 3′-nick-directed mismatch correction (Fig. 5D). In addition, we observe a strong MutSα-dependent degradation of the discontinuous mismatch-containing strands in the reconstituted reaction (Fig. 5 A and B and Fig. S5A, lanes 8 and 9). This is a result of the enzymatic actions of MutLα endonuclease and Exo1 (7, 9, 23). The addition of CAF-I to the seven-protein system has no effect on the degradation of the discontinuous strands (Fig. 5 A and B, lanes 3 and 9, and Fig. 5C) or on mismatch correction (Fig. 5D). Supplementation of the seven-protein MMR system with both CAF-I and the (H3-H4)2 tetramer protects the discontinuous mismatch-containing strands from excessive degradation (Fig. 5 A–C) and weakly stimulates mismatch correction (Fig. 5D). These two effects require the presence of both CAF-I and the (H3-H4)2 tetramer and depend on the concentration of the histone tetramer (Fig. 5). The results of these reconstitution experiments indicate that the controlled degradation of the discontinuous strands observed in the cytosolic extracts supplemented with purified CAF-I (Fig. 2 and Figs. S2 and S3) can be mediated by CAF-I-dependent histone H3-H4 deposition.
Fig. 5.
The impact of CAF-I-dependent histone H3-H4 deposition on mismatch correction in a defined system. Reconstituted reactions containing 3′ G-T DNA and the indicated proteins were performed and analyzed as described in Materials and Methods. (A–C) CAF-I-dependent histone H3-H4 deposition protects the discontinuous mismatch-containing strands from excessive degradation by MMR. Recovered products of the mismatch correction reactions were cleaved with ClaI, separated in a denaturing 0.8% agarose gel, transferred to a nylon membrane, and hybridized with 32P-labeled probe v2505 (A) or v2531 (B), complementary to discontinuous-strand positions 2505–2526 and 2531–2552, respectively. (C) The effect of the presence of CAF-I and H3–H4 tetramer on degradation of discontinuous mismatch-containing strands. Data are from images like those in A and Fig. S5. Degradation occurring in the reconstituted reactions with (▪ and •) or without (□ and ◯) 15-nM CAF-I was determined by Southern analyses with 32P-labeled probe v2505 (▪ and □) or v2531 (• and ◯). (D) Mismatch correction in the defined system in the presence of CAF-I and H3–H4 tetramer. The reactions containing MutSα, MutLα, RFC, PCNA, Exo1, RPA, DNA polymerase δ, and 3′ G-T DNA were in the presence (▪) or the absence (□) of CAF-I (15 nM) and the indicated concentrations of the histone tetramer. The data in C and D are the means ± 1 SD, n≥2.
MMR of DNA in Nucleosomes.
Because newly replicated DNA subjected to mismatch correction is likely to contain naked regions and subnucleosomal assemblies surrounded by assembled nucleosomes (24, 25), it is important to understand whether MMR can function on DNA in nucleosomes. Mismatch-containing nucleosomal DNAs were reconstituted by salt dialysis (26) of 5′ G-T DNA and purified HeLa chromatin histones (Fig. S1B) mixed at different molar ratios. (A 1∶31 molar ratio of 5′ G-T DNA to HeLa histone octamers saturates the DNA with nucleosomes.) Probing the dialyzed DNA–protein complexes with micrococcal nuclease confirms that nucleosomes were reconstituted (Fig. S6A). Next, we used the assembled nucleosomal DNAs as substrates in mismatch-correction reactions in 293T cytosolic extracts supplemented with purified MutLα. The results of these experiments suggest that MMR can function in the presence of subsaturating amounts of nucleosomes, whereas saturation of mismatch-containing DNA with nucleosomes represses mismatch correction (Fig. S6B).
Discussion
CAF-I was discovered as a factor that assembles newly replicated DNA into nucleosomes in cytosolic extracts of human 293 cells (14). CAF-I is essential for the viability of human cells (16) but not Saccharomyces cerevisiae (27). Given that the core histones are indispensable for eukaryotic organisms, the absence of a growth defect in S. cerevisiae cac1-3 mutants encoding the three CAF-I subunits suggests that these yeast mutants can assemble nucleosomes during replication, probably by relying on the mechanisms that normally function during transcription (13). Although yeast CAF-I is not an essential protein, it is required for the suppression of gross chromosome rearrangements (28). In this work, we provide several lines of evidence that human CAF-I-dependent nucleosome assembly is responsible for controlling the degradation of the discontinuous mismatch-containing strands during mismatch correction. First, significant mismatch-dependent degradation of discontinuous strands is observed in HeLa cytosolic extract containing only trace amounts of CAF-I (Fig. 1). Second, supplementation of the cytosolic extracts with purified CAF-I abolishes most mismatch-provoked degradation of the discontinuous strands but does not suppress mismatch correction (Fig. 2 and Figs. S2 and S3), suggesting that the degradation occurring in the absence of CAF-I is uncontrolled and unnecessary. Third, the absence of significant degradation of discontinuous mismatch-containing strands in HeLa nuclear extract correlates with the presence of both CAF-I protein and nick-directed nucleosome assembly (Fig. 1), the latter being known to depend on CAF-I (17). Fourth, CAF-I-dependent histone (H3-H4)2 tetramer deposition protects the discontinuous mismatch-containing strand from excessive degradation by MMR in a defined purified system (Fig. 5). In addition to controlling degradation, CAF-I-dependent nucleosome assembly modestly stimulates mismatch correction (up to twofold) (Fig. 2 and Figs. S2 and S3). However, we believe that this effect might be of limited importance for MMR because the stimulation is weak and probably will matter only in the presence of suboptimal concentrations of the MMR proteins. Consistent with this view, we observed that the magnitude of this stimulation becomes progressively weaker with increasing MutLα concentration (Fig. S3 C and D).
Our current hypothesis to explain the impact of CAF-I on mismatch correction is that nucleosomes assembled by CAF-I serve as roadblocks that prevent the MMR incision complex from sliding freely along mismatch-containing DNA, which is necessary for MutLα to be able to cleave the discontinuous strand at sites located far from the mismatch (Figs. 2 and 5 and Figs. S2 and S3). Because Exo1 excision and DNA synthesis by polymerase δ initiated from remote MutLα incision sites require more time for completion than those initiated from nearby sites, and because the remote incisions compete with the near incisions as starting points of Exo1 excision and subsequent DNA polymerization, it is hardly surprising that protection of discontinuous mismatch-containing strands by CAF-I-dependent nucleosome assembly weakly stimulates mismatch correction.
In the reconstituted systems, MutLα endonuclease cleaves the discontinuous mismatch-containing strand at numerous sites scattered along its complete 6.44-kb length (7, 29) (Fig. 5 A and B). This incision pattern differs from that observed in nuclear extracts of HeLa and H6 cells in which MutLα endonuclease incises the discontinuous strand within a small, approximately 600-bp region surrounding a mismatch (7). The basis of this different cleavage pattern is not understood, but protein factors that regulate MutLα endonuclease activity in the nuclear extracts may be missing from the purified system. Results obtained in this study provide evidence that CAF-I-dependent nucleosome assembly is the basis for the difference in the incision pattern of MutLα endonuclease (Fig. 5).
Previous investigations of replication-dependent nucleosome assembly have implicated the histone H3–H4 chaperone CAF-I and the PCNA clamp in packaging the newly synthesized DNA into nucleosomes (14, 19, 22). We have now shown that, in addition to CAF-I, PCNA, and histone (H3-H4)2 tetramer, CAF-I-dependent tetramer deposition also requires RFC, which loads PCNA onto DNA (Fig. 3 and Table S1). These requirements define a minimal set of proteins for this reaction. Surprisingly, we observed that a low level of tetramer deposition takes place in the absence of PCNA. Previous work showed that PCNA is essential for CAF-I-dependent nucleosome assembly (19). Thus, we think that the weak PCNA-independent histone deposition is normally suppressed by the cellular environment lacking in our reconstituted system and/or is not sufficient to support nucleosome assembly during DNA replication. Our results showing that MutSα prevents CAF-I-dependent histone (H3-H4)2 tetramer deposition in the vicinity of a mismatch (Fig. 4 and Fig. S4) suggest the existence of crosstalk between MMR and replication-dependent nucleosome assembly. The simplest interpretation of this observation is that MutSα clamps bound to and/or moving away from a mispair (30, 31) sterically occlude DNA surrounding the mispair from being assembled into nucleosomes by CAF-I.
We determined that the presence of the saturating amounts of preassembled nucleosomes suppresses nick-directed mismatch correction in a cell-free extract (Fig. S6). This observation is not very surprising, given that nucleosomes inhibit DNA sliding (32, 33) and mismatch recognition (32) by MutSα. We suggest that one of the roles of the nucleosomal organization is to promote genetic stability by preventing MMR-dependent strand breakage during and after S phase.
In summary, our results suggest that postreplicative mismatch correction and CAF-I-dependent nucleosome assembly co-occur on the newly replicated DNA and that there is active communication between the two processes. As a result, the mismatch-containing daughter strands are degraded in a controlled manner. The abilities of MutSα to suppress CAF-I-dependent nucleosome assembly in the vicinity of mismatch (Fig. 4 and Fig. S4) and to passively disassemble a nucleosome in a mismatch-dependent manner (34) are likely to contribute to MMR functioning during concomitant CAF-I-dependent nucleosome assembly.
Materials and Methods
Circular 6.44-kb DNA substrates for the mismatch-correction reactions, referred to as 3′-G-T, 3′-A-T, 5′-G-T, 5′-A-T, cc G-T and cc A-T DNAs, were prepared on the basis of f1 phages as described (7, 35). The 3′-G-T and 3′-A-T DNAs contain a strand break 141 base pairs 3′ to a G-T mispair and an A-T pair, respectively. A strand break in the 5′ G-T and A-T substrates is located 128 base pairs 5′ to a G-T mispair and an A-T pair, respectively. The cc G-T and cc A-T DNAs contain a G-T mispair or an A-T pair, respectively, and do not have a strand break. Correction of a G-T mispair in the 3′-G-T, 5′-G-T, and cc G-T substrates, using the viral strand as the DNA template, restores HindIII restriction endonuclease recognition sites, permitting the use of HindIII sensitivity to score mismatch-correction (4). Cell-free extracts and purified proteins were prepared as described in SI Text.
Mismatch-correction reactions were carried out according to the previously developed procedures (4, 7, 12) at 37 °C for 10 min. Mismatch-correction reactions including cell-free extracts were performed in 80-μl mixtures containing 20 mM HEPES-NaOH (pH 7.4), 91 mM KCl, 19 mM NaCl, 5 mM MgCl2, 5 mM potassium phosphate (pH 7.5), 3 mM ATP, 2 mM dithiothreitol (DTT), 0.2 mg/ml bovine serum albumin (BSA), 0.3 nM 5′- or 3′-nicked DNA (0.1 μg), 0.1 mM each of the four dNTPs (dATP, dGTP, dCTP, and dTTP), and the specified amount of cell-free extract. When indicated, the cell-free-extract reactions were supplemented with purified CAF-I and the reactions containing 293T cytosolic extract were also supplemented with purified MutLα. Mismatch-correction reactions in the reconstituted system were performed in 40-μl mixtures containing 20 mM HEPES-NaOH (pH 7.4), 121 mM KCl, 19 mM NaCl, 5 mM MgCl2, 3 mM ATP, 2 mM DTT, 0.4 mg/ml BSA, 1% (v/w) glycerol, 0.6 nM 3′-nicked DNA (0.1 μg), 0.1 mM each of the four dNTPs, 12.5 nM MutSα, 2.5 nM MutLα, 15 nM PCNA, 2 nM RFC, 25 nM RPA, 0.6 nM Exo1, 0.6 nM DNA polymerase δ, 15 nM CAF-I, and 1–15 nM (H3-H4)2 tetramer. In control reconstituted reactions, one or several indicated proteins were omitted. Mismatch-correction reactions in the extract and reconstituted systems were stopped, processed, and analyzed as described in SI Text.
Analysis of CAF-I-dependent nucleosome assembly in mismatch-correction reactions in cell-free extracts was performed as described in SI Text. CAF-I-dependent histone H3–H4 deposition reactions were performed at 37 °C in 40-μl mixtures containing 20 mM HEPES-NaOH (pH 7.4), 121 mM KCl, 19 mM NaCl, 5 mM MgCl2, 3 mM ATP, 2 mM DTT, 0.4 mg/ml BSA, 1% (v/w) glycerol, 0.6 nM 3′-nicked DNA or cc A-T DNA, 15 nM CAF-I, 15 nM (H3-H4)2 tetramer, 15 nM PCNA, 2 nM RFC, 25 nM RPA. When indicated, the deposition reactions were also supplemented with 6–25 nM MutSα. In 10 min of the incubation, 35-μl fractions of the histone-deposition reactions were supplemented with a 5-μl mixture containing micrococcal nuclease and CaCl2 so that their final concentrations were 0.3 U/μl and 2.5 mM, respectively, and the reaction temperature was switched to 21–23 °C. Micrococcal nuclease cleavage was carried out for 5 min and stopped by the addition of a 4-μl mixture containing 0.5% SDS, 70 mM EDTA, 2.5 mg/ml Proteinase K, and 40% glycerol, followed by incubation at 50 °C for 15 min. DNAs of the stopped reactions were separated in 1.5% agarose gels in 1xTAE, followed by Southern hybridization analyses performed as described in SI Text.
Acknowledgments.
We thank Paul Modrich for generously providing the human and insect cell lines and the overproduction constructs for MutSα, MutLα, RFC, Exo1, and DNA polymerase δ; Bruce Stillman and Mark Wold for giving us kind permission to use the human PCNA and RPA plasmids; Jan Drake and Paul Modrich for critical reading of the manuscript; and Blaine Bartholomew for his advice on preparing HeLa histones and nucleosomal DNA. This work was supported by startup funds and by the Central Research Committee of the Southern Illinois University School of Medicine.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015914108/-/DCSupplemental.
References
- 1.Stojic L, Brun R, Jiricny J. Mismatch repair and DNA damage signalling. DNA Repair. 2004;3:1091–1101. doi: 10.1016/j.dnarep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Kunkel TA, Erie DA. DNA Mismatch Repair. Annu Rev Biochem. 2005;74:681–710. doi: 10.1146/annurev.biochem.74.082803.133243. [DOI] [PubMed] [Google Scholar]
- 3.Iyer RR, Pluciennik A, Burdett V, Modrich PL. DNA mismatch repair: Functions and mechanisms. Chem Rev. 2006;106:302–323. doi: 10.1021/cr0404794. [DOI] [PubMed] [Google Scholar]
- 4.Holmes J, Clark S, Modrich P. Strand-specific mismatch correction in nuclear extracts of human and Drosophila melanogaster cell lines. Proc Natl Acad Sci USA. 1990;87:5837–5841. doi: 10.1073/pnas.87.15.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas DC, Roberts JD, Kunkel TA. Heteroduplex repair in extracts of human HeLa cells. J Biol Chem. 1991;266:3744–3751. [PubMed] [Google Scholar]
- 6.Modrich P. Mechanisms in eukaryotic mismatch repair. J Biol Chem. 2006;281:30305–30309. doi: 10.1074/jbc.R600022200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kadyrov FA, Dzantiev L, Constantin N, Modrich P. Endonucleolytic function of MutLalpha in human mismatch repair. Cell. 2006;126:297–308. doi: 10.1016/j.cell.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 8.Pluciennik A, et al. PCNA function in the activation and strand direction of MutLalpha endonuclease in mismatch repair. Proc Natl Acad Sci USA. 2010;107(37):16066–16071. doi: 10.1073/pnas.1010662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genschel J, Modrich P. Mechanism of 5’-directed excision in human mismatch repair. Mol Cell. 2003;12:1077–1086. doi: 10.1016/s1097-2765(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 10.Longley MJ, Pierce AJ, Modrich P. DNA polymerase δ is required for human mismatch repair in vitro. J Biol Chem. 1997;272:10917–10921. doi: 10.1074/jbc.272.16.10917. [DOI] [PubMed] [Google Scholar]
- 11.Constantin N, Dzantiev L, Kadyrov FA, Modrich P. Human mismatch repair: Reconstitution of a nick-directed bidirectional reaction. J Biol Chem. 2005;280:39752–39761. doi: 10.1074/jbc.M509701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadyrov FA, et al. A possible mechanism for exonuclease 1-independent eukaryotic mismatch repair. Proc Natl Acad Sci USA. 2009;106:8495–8500. doi: 10.1073/pnas.0903654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loyola A, Almouzni G. Histone chaperones, a supporting role in the limelight. Biochim Biophys Acta. 2004;1677:3–11. doi: 10.1016/j.bbaexp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58:15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- 15.Kleczkowska HE, Marra G, Lettieri T, Jiricny J. hMSH3 and hMSH6 interact with PCNA and colocalize with it to replication foci. Genes Dev. 2001;15:724–736. doi: 10.1101/gad.191201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoek M, Stillman B. Chromatin assembly factor 1 is essential and couples chromatin assembly to DNA replication in vivo. Proc Natl Acad Sci USA. 2003;100:12183–12188. doi: 10.1073/pnas.1635158100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moggs JG, et al. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol Cell Biol. 2000;20:1206–1218. doi: 10.1128/mcb.20.4.1206-1218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaufman PD, Kobayashi R, Kessler N, Stillman B. The p150 and p60 subunits of chromatin assembly factor I: a molecular link between newly synthesized histones and DNA replication. Cell. 1995;81:1105–1114. doi: 10.1016/s0092-8674(05)80015-7. [DOI] [PubMed] [Google Scholar]
- 19.Shibahara K, Verreault A, Stillman B. The N-terminal domains of histones H3 and H4 are not necessary for chromatin assembly factor-1- mediated nucleosome assembly onto replicated DNA in vitro. Proc Natl Acad Sci USA. 2000;97:7766–7771. doi: 10.1073/pnas.97.14.7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trojan J, et al. Functional analysis of hMLH1 variants and HNPCC-related mutations using a human expression system. Gastroenterology. 2002;122:211–219. doi: 10.1053/gast.2002.30296. [DOI] [PubMed] [Google Scholar]
- 21.Dong F, van Holde KE. Nucleosome positioning is determined by the (H3–H4)2 tetramer. Proc Natl Acad Sci USA. 1991;88:10596–10600. doi: 10.1073/pnas.88.23.10596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shibahara K, Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96:575–585. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- 23.Genschel J, Bazemore LR, Modrich P. Human exonuclease I is required for 5′ and 3′ mismatch repair. J Biol Chem. 2002;277:13302–13311. doi: 10.1074/jbc.M111854200. [DOI] [PubMed] [Google Scholar]
- 24.Lucchini R, Wellinger RE, Sogo JM. Nucleosome positioning at the replication fork. EMBO J. 2001;20:7294–7302. doi: 10.1093/emboj/20.24.7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128:721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 26.Carruthers LM, Tse C, Walker KP, Hansen JC. Assembly of defined nucleosomal and chromatin arrays from pure components. Methods Enzymol. 1999;304:19–35. doi: 10.1016/s0076-6879(99)04004-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaufman PD, Kobayashi R, Stillman B. Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev. 1997;11:345–357. doi: 10.1101/gad.11.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Myung K, Pennaneach V, Kats ES, Kolodner RD. Saccharomyces cerevisiae chromatin-assembly factors that act during DNA replication function in the maintenance of genome stability. Proc Natl Acad Sci USA. 2003;100:6640–6645. doi: 10.1073/pnas.1232239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadyrov FA, et al. Saccharomyces cerevisiae MutLalpha is a mismatch repair endonuclease. J Biol Chem. 2007;282:37181–37190. doi: 10.1074/jbc.M707617200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blackwell LJ, Martik D, Bjornson KP, Bjornson ES, Modrich P. Nucleotide-promoted release of hMutSa from heteroduplex DNA is consistent with an ATP-dependent translocation mechanism. J Biol Chem. 1998;273:32055–32062. doi: 10.1074/jbc.273.48.32055. [DOI] [PubMed] [Google Scholar]
- 31.Warren JJ, et al. Structure of the human MutSalpha DNA lesion recognition complex. Mol Cell. 2007;26:579–592. doi: 10.1016/j.molcel.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 32.Li F, Tian L, Gu L, Li GM. Evidence that nucleosomes inhibit mismatch repair in eukaryotic cells. J Biol Chem. 2009;284:33056–33061. doi: 10.1074/jbc.M109.049874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gorman J, Plys AJ, Visnapuu ML, Alani E, Greene EC. Visualizing one-dimensional diffusion of eukaryotic DNA repair factors along a chromatin lattice. Nat Struct Mol Biol. 2010;17:932–938. doi: 10.1038/nsmb.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Javaid S, et al. Nucleosome remodeling by hMSH2-hMSH6. Mol Cell. 2009;36:1086–1094. doi: 10.1016/j.molcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dzantiev L, et al. A defined human system that supports bidirectional mismatch-provoked excision. Mol Cell. 2004;15:31–41. doi: 10.1016/j.molcel.2004.06.016. [DOI] [PubMed] [Google Scholar]