Abstract
The function of the liver is well-preserved during the aging process, although some evidence suggests that liver regeneration might be impaired with advanced age. We observed a decreased ability of the liver to restore normal volume after partial hepatectomy in elderly mice, and we identified a pathway that rescued regeneration and was triggered by serotonin. 2,5-dimethoxy-4-iodoamphetamine (DOI), a serotonin receptor agonist, reversed the age-related pseudocapillarization of old liver and improved hepatosinusoidal blood flow. After hepatectomy, the open fenestrae were associated with a restored attachment of platelets to endothelium and the initiation of a normal regenerative response, including the up-regulation of essential growth mediators and serotonin receptors. In turn, hepatocyte proliferation recovered along with regain of liver volume and animal survival. DOI operates through the release of VEGF, and its effects could be blocked with anti-VEGF antibodies both in vitro and in vivo. These results suggest that pseudocapillarization in the aged acts as a barrier to liver regeneration. DOI breaks this restraint through an endothelium-dependent mechanism driven by VEGF. This pathway highlights a target for reversing the age-associated decline in the capacity of the liver to regenerate.
Keywords: aging liver, fenestrations, sinusoidal microperfusion
The most significant limiting factor for survival after liver surgery and transplantation of a partial graft is the ability of the remnant liver to regenerate (1–3), as documented in human (4, 5) and several animal models (6–8). Although in young patients, small remnant livers (up to 25% of the normal size) can regenerate fully within a few weeks, this process is impaired in the diseased (9, 10) and possibly, older (11) livers.
Conventional histological examination of the liver hardly differs between young and old individuals, although a few features have been identified. For example, the size of the liver and the sinusoidal flow decrease with age, akin to the energy stores (glycogen and ATP in hepatocytes) (12–16). It is well-known that there is an age-associated decline in the clearance of a number of drugs (17–19). We have recently shown that protective strategies during liver surgery, such as ischemic preconditioning, are lost in patients older than 65 y of age (14, 20, 21).
Impaired hepatocyte proliferation was also documented in old mice after major hepatectomy, but the impact on animal survival and underlying mechanisms was not evaluated (22). Finally, a study from Japan in recipients of partial grafts showed a lower liver volume 1 wk after transplantation in patients receiving a graft from donors older than 50 y of age (23). Advanced age is also associated with ultrastructural changes in the hepatic sinusoid, called pseudocapillarization, which includes the defenestration (reduction of porosity) and thickening of the sinusoidal endothelium (24–26). Similar findings for the defenestration and capillarization of the hepatic sinusoidal endothelium have been observed in the early stage of cirrhosis, both in human (27) and animal models (28, 29).
In previous studies, we identified platelet-derived serotonin [5-hydroxytryptamine (5-HT)] and 5-hydroxytryptamine receptor 2 (HTR2) receptors as potent initiators of liver regeneration after major hepatectomy (3) as well as orthotopic partial liver graft transplantation (OLT) in young mice (30). The use of the HTR2 receptor agonist 2,5-dimethoxy-4-iodoamphetamine (DOI) rescued hepatocyte proliferation and animal survival after the transplantation of small, otherwise nonviable grafts. 5-HT has proteiform mitogenic effects in many biological systems, including an impact on tumor growth (31, 32), angiogenesis (32–34), and production of oxidative stress (35, 36).
In the current study, we investigated the impact of age on liver regeneration and animal survival in a well-established model of major hepatectomy in mice. We further searched for pathways that affect the capacity of old livers to regenerate.
Results
Does Age Affect Animal Survival and Liver Regeneration After Major Hepatectomy?
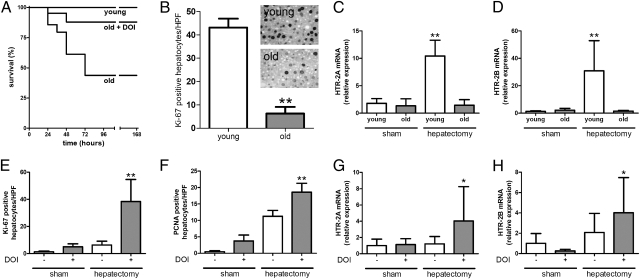
We first examined the impact of age on animal survival and liver regeneration after 70% hepatectomy. Although all young animals survived after liver resection, 52% (11/21) of the 2-y-old mice died within 4 d of surgery (Fig. 1A). We then tested whether this high mortality rate in old animals correlated with a failure of the liver to regenerate. We assessed established markers of hepatocyte proliferation [proliferation marker Ki-67 and proliferating cell nuclear antigen (PCNA)] 48 h after hepatectomy as well as the mitotic index at 4 d as performed in previous studies (3). Quantification of both markers disclosed a dramatic decrease in cell proliferation in older animals (Fig. 1B and Fig. S1), and the mitotic index was significantly decreased (12.1 ± 1.7 vs. 5.7 ± 1.4 mitosis/high power field in young vs. old mice, respectively, P < 0.001). No fibrosis or cirrhosis was observed on histology, and animals with steatosis were excluded.
Fig. 1.
Impaired survival and liver regeneration in old mice after 70% partial hepatectomy is reversed by DOI, a specific serotonin receptor 2 agonist. (A) Survival of young (age = 7–8 wk) and old (age = 2 y) mice after partial hepatectomy. P < 0.001 in young vs. old animals. DOI is a specific serotonin receptor 2 agonist that improved survival in old animals (P = 0.02 old vs. old + DOI; n = 21 in each group). (B) Liver regeneration assessed by quantification of immunostainings for proliferation marker Ki-67 on liver specimens 48 h after partial hepatectomy in young and old animals (n = 5). **P < 0.008 vs. young. (C and D) Gene expression of HTR2A and HTR2B in liver tissue 48 h after sham operation or partial hepatectomy in young and old mice. (C) Relative mRNA levels of HTR2A normalized to sham-operated animals (n = 5). **P < 0.008 vs. old after hepatectomy. (D) Relative mRNA levels of HTR2B normalized to sham-operated animals (n = 5). **P < 0.008 vs. old after hepatectomy. (E and F) Quantification of immunostainings for Ki-67 and PCNA 48 h after sham operation or partial hepatectomy in old animals. Both groups were treated either with or without DOI before and after hepatectomy as described in Materials and Methods. (E) Quantification of Ki-67–positive hepatocytes (n = 5). **P < 0.001 vs. sham and hepatectomy without DOI. (F) Quantification of PCNA-positive hepatocytes (n = 5). **P < 0.001 vs. sham and hepatectomy without DOI. Means ± SD are shown. (G and H) Gene expression of HTR2A and HTR2B in liver tissue 48 h after sham operation or partial hepatectomy with and without DOI treatment in old mice. (G) Relative mRNA levels of HTR2A normalized to sham-operated animals (n = 5). *P < 0.05 vs. sham with and without DOI. (H) Relative mRNA levels of HTR2B normalized to sham-operated animals (n = 5). *P < 0.05 vs. sham with DOI.
These initial experiments showed a significant impact of age on animal survival after major hepatectomy, a finding associated with a failure of liver regeneration in older animals.
Does DOI, a Serotonin Receptor Agonist, Improve Regeneration in the Older Liver?
In previous studies, we found that platelet-derived serotonin mediates liver regeneration after major hepatectomy (3) and rescues liver regeneration and animal survival in a mouse model of partial OLT (30). These observations were associated with an induction of Htr2 receptor expression after hepatectomy.
As expected, we detected Htr2 receptor up-regulation in young mice 48 h after hepatectomy. In old mice, this up-regulation was absent, likely reflecting their regenerative impairment (Fig. 1 C and D). To test whether the serotonin system is involved in the deficient regeneration, we pretreated old animals with DOI, a potent agonist of HTR2. DOI increased the weight of the liver remnant 48 h after hepatectomy (0.74 ± 0.09 g in pretreated animals vs. 0.57 ± 0.06 g in vehicle-treated animals, P = 0.017) and significantly improved hepatocyte proliferation (Fig. 1 E and F). Likewise, DOI converted a survival of only 48% (10/21) to 86% (18/21) in hepatectomized old mice (Fig. 1A).
We next examined whether the rescuing effects of DOI can be associated with alterations in Htr2 receptor expression. Pretreatment with DOI restored the up-regulation of Htr2 in old mice. However, DOI treatment alone (in the absence of hepatectomy) had no effect on Htr2 expression in old animals at any time point tested (Fig. 1 G and H), indicating that receptor up-regulation is related to the regenerative process but not to the principal defect affected by DOI. Furthermore, no expression differences between young and old were noted after sham operation. Thus, we examined whether old animals display decreased levels of serotonin. Measurement of platelet serotonin contents in young and old animals revealed similar levels in both groups [young (1,670 ± 1,141 ng/mL) vs. old (1,932 ± 751 ng/mL), P = 0.93].
Together, these results indicate that exogenous activation of the serotonin system by DOI restores the deficient regeneration of old livers. However, the deficiency ameliorated by DOI is not associated with alterations in hepatic serotonin receptor expression or the endogenous serotonin levels in old mice.
Does DOI Affect Structural Changes in the Sinusoid Lining?
In a next step, we examined whether the deficiency associated with impaired regenerative capacity may involve pseudocapillarization of the sinusoidal lining. Pseudocapillarization, consisting of thickening of the sinusoidal endothelial cells (SECs) and loss of its porous sieve mimicking capillaries, is a feature of the old liver (24, 25, 37–42) and may affect liver regeneration (for example, by preventing growth factors to reach hepatocytes).
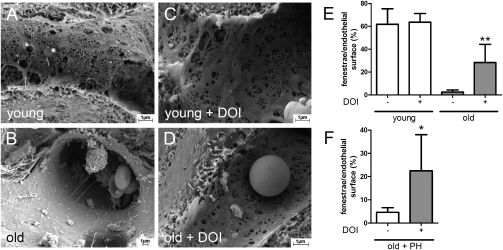
Using scanning EM, we evaluated the sinusoidal lining in native young and old animals before and after hepatectomy. As shown in Fig. 2 A and B, old animals displayed characteristic changes of pseudocapillarization with loss of fenestration, whereas young animals disclosed a thin sinusoidal lining containing many fenestrae. The use of DOI had no significant impact in young animals but increased the number of fenestrae in old animals (Fig. 2 C–E). DOI treatment not only increased the number of fenestrae after (Fig. 2F) but also before partial hepatectomy, suggesting that the correction of pseudocapillarization enables a normal regenerative response.
Fig. 2.
Loss of endothelial fenestrae in old mice is reversed by DOI administration. (A and B) Representative scanning EM micrographs of young and old animals showing a decreased number of fenestrae in old animals. (C and D) DOI administration for 2 d every 12 h opens fenestrae in old animals. (E) Quantification of area of fenestrae per total endothelial surface area in young and old mice with and without DOI treatment before sham operation (n = 5). **P = 0.007 vs. old without DOI. (F) Number of fenestrae 48 h after partial hepatectomy with and without DOI pretreatment in old animals (n = 5). *P = 0.034 vs. without DOI.
Does Opening of the Fenestrae Enhance Perfusion and Platelet Adhesion?
The presence of pseudocapillarization may disturb endothelium-dependent processes required for normal liver regeneration, such as sinusoidal blood flow and platelet adhesion to sinusoids after resection injury.
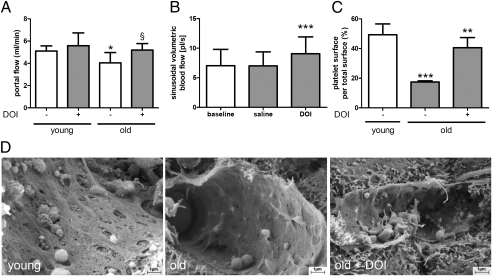
Hepatic perfusion is reduced in the elderly (43). Likewise, we observed significantly lower portal flow at baseline in old compared with young mice. DOI improved the portal flow (Fig. 3A). In addition, DOI also increased microperfusion in old livers (Fig. 3B and Fig. S2). Furthermore, deficient platelet adhesion was evident on scanning EM in old livers after hepatectomy and was improved by DOI pretreatment (Fig. 3 C and D).
Fig. 3.
DOI improves blood flow and enhances platelet adhesion on endothelial cells. (A) Portal blood flow measurements in young and old mice treated with and without DOI for 48 h (n = 4). *P < 0.041 vs. young; §P = 0.081 vs. without DOI. (B) Microcirculatory blood flow in sinusoids assessed in old mice treated for 48 h with and without DOI (n = 4). ***P < 0.01 vs. baseline and saline. (C) Quantification of platelet surface per total endothelial surface in young and old mice 48 h after partial hepatectomy. Old animals were pretreated with or without DOI (n = 5). ***P < 0.001 vs. young without DOI; **P < 0.001 vs. old without DOI. (D) Photographs of endothelial cells after partial hepatectomy pretreated with and without DOI in old animals.
Therefore, we conclude that DOI reverses pseudocapillarization and associated endothelium-dependent deficiencies.
Which DOI-Dependent Pathway Mediates Opening of Fenestrae and Hepatocyte Proliferation?
We focused our subsequent investigations on the mechanisms through which DOI mediates the opening of fenestrae in old mice. We measured the effects of DOI on gene expression of putative mediators of liver regeneration.
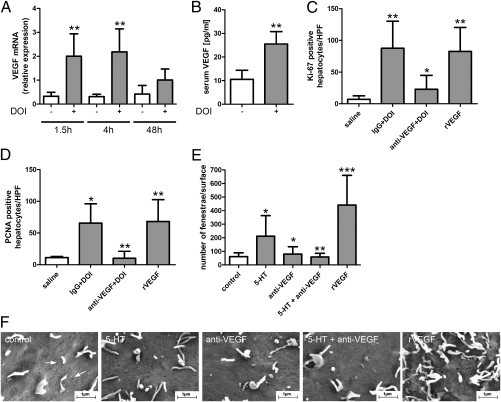
TNF-α was not significantly affected (fold induction = 1.52 ± 1.99 vs. 3.35 ± 2.71, P = 0.222, n = 5), whereas DOI increased Il6 48 h posthepatectomy (2.23 ± 3.24 vs. 25.88 ± 27.46, P = 0.03, n = 5) but not earlier. Hepatocyte growth factor (HGF) elevation was observed as early as 1.5 h after hepatectomy and persisted at 48 h (Fig. S3). However, the strongest DOI-mediated increase restricted to earlier times after hepatectomy was noted for VEGF (Fig. 4A). Thus, we examined whether DOI can up-regulate VEGF directly (i.e., without hepatectomy). Indeed, serum VEGF levels were raised by DOI treatment alone (Fig. 4B), suggesting that VEGF may mediate the ameliorating effects of DOI on fenestration.
Fig. 4.
VEGF mediates DOI-induced opening of fenestrae and liver regeneration. (A) Gene expression of VEGF in old animals treated with (gray bars) and without (white bars) DOI (n = 5). **P < 0.008 vs. animals without DOI. (B) Serum VEGF levels in old mice after treatment with and without DOI for 48 h (n = 4). **P = 0.007. (C and D) Assessment of liver regeneration 48 h after partial hepatectomy in old mice in the presence of unspecific IgG or anti-VEGF antibodies followed by DOI injection. Administration of recombinant VEGF alone followed by partial hepatectomy also enhanced liver regeneration. (C) Quantification of Ki-67 immunostainings (n = 5). **P < 0.001 vs. control; *P < 0.05 vs. IgG + DOI. (D) Quantification of PCNA immunostainings (n = 5). **P < 0.001 anti-VEGF + DOI vs. IgG + DOI and recombinant VEGF vs. control; *P < 0.05 vs. control. (E and F) Quantification and photographs of fenestrae (white arrows) in the endothelial cell line SK HEP-1 incubated with 5-HT, anti-VEGF antibodies alone, anti-VEGF antibodies plus 5-HT, or recombinant VEGF. *P < 0.05 vs. control or 5-HT; **P < 0.001 vs. 5-HT; ***P < 0.001 vs. control.
To test whether VEGF acts downstream of DOI, we subjected old mice to anti-VEGF antibodies before DOI treatment and hepatectomy and evaluated markers of hepatocyte proliferation (Fig. 4 C and D). DOI improved liver regeneration in the presence of nonspecific IgG antibodies in old animals, whereas the application of anti-VEGF antibodies blunted the proliferative effect of DOI. Furthermore, the administration of exogenous recombinant VEGF also enhanced regeneration to the levels of DOI-treated animals (Fig. 4 C and D).
VEGF is a potent factor regulating the number and size of fenestrae in isolated liver sinusoidal endothelial cells, and its signaling is required for the formation of fenestrae in vivo (44). We, therefore, tested whether serotonin is able to regulate fenestrae through VEGF on SK HEP-1 sinusoidal cells (45) in vitro. After 50 min of 5-HT treatment, the number of fenestrae increased from 61 ± 29 to 211 ± 152/μm2. Anti-VEGF antibodies blocked this 5-HT–induced effect, whereas exogenous VEGF alone had a similar effect as 5-HT treatment alone (Fig. 4 E and F). Similar results were also observed after incubation with 5-HT for only 10 min, suggesting a rather fast process.
Taken together, this set of experiments indicates that DOI mediates liver regeneration in old mice through a VEGF-dependent pathway, resulting in the opening of fenestrae in liver sinusoids.
Discussion
Life expectancy has increased worldwide and especially in the Western world, which has lead to an increased interest in research focusing on age-related changes. In the current study, we show that impaired liver regeneration after major tissue loss in the old can be restored through a pathway involving the serotonin system, VEGF, and the opening of the liver SEC fenestrae. These findings open doors for developing innovative strategies to ameliorate surgery and transplantation in the aging population.
A few investigators have looked at the impact of age on the regenerative ability of the liver. Both animal models (12, 22, 46, 47) and clinical (23) observations have indicated a significant impairment in liver regeneration in relation to age. For example, a dramatic decrease in BrdU incorporation has been described in the liver of 12-mo-old mice compared with 2-mo-old animals subjected to a 70% hepatectomy (22). The best evidence in humans arises from the assessment of the liver volume as a surrogate of regeneration in a small series of living donor liver transplantations (LDLT). Grafts from living donors older than 50 y of age disclosed a graft volume per standard liver volume ratio (GV/SLV) of 65% by day 7, whereas grafts from donors younger than 30 y of age had a significantly higher GV/SLV of 82%. Here, we could not only confirm that older animals display impaired liver regeneration assessed by several markers of hepatocyte proliferation, but we could document impaired animal survival after 70% hepatectomy in old compared with young mice, a finding associated with the failure to regenerate.
Our focus then moved to the mechanisms responsible for the failure of liver regeneration in older animals. Based on our previous findings that platelets containing serotonin (5-HT) mediate liver regeneration in young mice (3) and that DOI, an agonist of HTR2, rescues liver regeneration and survival in a model of partial OLT (30), we subsequently tested the effect of DOI in an older population of mice. The dramatic effects of DOI in rescuing animal survival and liver regeneration after liver resection in this group led us to speculate on the existence of a novel age-dependent pathway for regeneration. Serotonin receptor expression in the liver, which is up-regulated in response to hepatectomy in young mice, was blunted in the old, suggesting that the regenerative process was not properly initiated. Notably, blood levels of serotonin were not different in old vs. young mice. Likewise, serotonin receptor levels were similar in sham-operated mice regardless of age, and DOI had no effect on receptor expression before hepatectomy. Together, these findings implied that the age-associated deficiency leading to impaired liver regeneration in the aged must be of another kind.
The older liver is morphologically characterized by changes in the sinusoids, including the thickening of the sinusoid lining and the loss of fenestrae, a phenomenon known as pseudocapillarization. Another intriguing feature related to age is the loss of the hepatic clearance of a number of drugs (48), which has been partially explained by the inability of the drug to pass the sinusoid lining, preventing hepatic metabolism and clearance. These two features of age led us to generate the hypothesis that platelet-derived serotonin and possibly even platelets themselves may not reach their targets, such as hepatocytes, to initiate the process of liver regeneration. This concept was supported by the reduced adhesion of platelets to SEC in old compared with young mice after hepatectomy.
Indeed, we discovered concomitant changes in the sinusoidal lining, with a dramatic opening of the fenestrae in response to DOI. Importantly, the opening of fenestration occurred before hepatectomy and was accompanied by an improvement of the reduced hepatic blood flow in the old. This suggested that pseudocapillarization is, at least in part, responsible for the blunted liver regeneration in old mice. Moreover, DOI-mediated opening of fenestrae was associated with a restored platelet–SEC adhesion and a rise in essential hepatic growth mediators after hepatectomy. These observations are consistent with our hypothesis, proposing that pseudocapillarization acts as a barrier to the proper initiation of liver regeneration.
With the knowledge that VEGF is a potent relaxant of the sinusoidal lining, we tested the possibility that DOI may trigger release of VEGF. Both assaying VEGF and blocking VEGF in DOI-treated animals and cell culture confirm this novel pathway. Similar observations in podocytes of the kidney that release VEGF within 1 h when stimulated with serotonin (49) suggest a highly conserved pathway shared by several cell types. Intriguingly, reduced availability of VEGF has recently been shown to accelerate age-associated damage of the kidney (50). Given that DOI directly increases systemic VEGF, our findings might have broader implications for the protection from age-related deficiencies. A figure summarizing this new concept is proposed in Fig. 5.
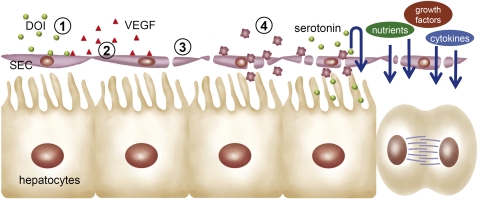
Fig. 5.
Schematic illustration of the DOI-mediated pathway for the enhancement of liver regeneration. Exogenous DOI (1) stimulates endothelial cells to express and release VEGF (2), which binds to endothelial Vegf receptors and opens fenestrae, eventually improving hepatic perfusion (3). The open fenestrae enable the adhesion of platelets to sinusoids after injury. The activated platelets release endogenous serotonin to initiate a regenerative response. The improved porosity and blood flow increase the availability of additional factors and nutrients required for the initiation and completion of liver regeneration (4).
In conclusion, we could show that liver regeneration is impaired in old mice because of a deficiency in the fenestration of hepatic sinusoids. Exogenous DOI can ameliorate this defect through a pathway that involves VEGF, which, in turn, regulates the opening of endothelial fenestrae, improving microcirculation and enabling a normal regenerative response after injury. Therefore, the age-associated changes of fenestrae can be reversed by agonists of serotonin receptors or the addition of VEGF, which restores the capacity of old livers to regenerate.
Materials and Methods
Animals.
Young (7- to 8-wk-old) male C57BL/6J mice were purchased from Harlan, and 24-mo-old C57BL/6J mice purchased from National Institutes of Health. All animals were kept in the animal facility of the University Hospital Zurich with access to standardized chow and water ad libitum. All procedures were approved by the Veterinary Office of the Canton Zurich and performed between 8:00 AM and 12:00 AM in compliance with institutional animal care guidelines. Group size was n = 5 in each study group unless otherwise indicated.
Animal Procedures.
Procedures were performed under isoflurane/O2 anesthesia. Buprenorphin (0.1 mg/kg body weight) was injected i.p. during anesthesia and repeated s.c. 12 h later. After a midline laparotomy, the liver was freed from its ligaments. Sham-operated animals were closed again with a double running suture. The model of 70% partial hepatectomy was performed as described previously (3, 51) according to the standard method described by Higgins and Anderson (52).
The serotonin agonist DOI (1 mg/kg i.p., Sigma-Aldrich), anti-mouse VEGF antibodies (50 μg; R&D Systems), and nonselective IgG antibodies (50 μg; R&D Systems) were injected two times per day starting 48 h before hepatectomy; 9 μg murine recombinant VEGF (PeproTech) were injected i.p. 24 h before the liver resection, and 1 μg was injected i.p. 30 min before the liver resection.
Histological Examination.
Fixation of tissue and immunohistochemistry were performed as described (32).
Preparation of Liver Tissue for Scanning EM.
Samples were cut into 1-mm3 blocks and fixed in 1.5% glutaraldehyde/0.12 M sodium cacodylate buffer. After fixation, blocks were submerged in 1% osmium tetroxide, dehydrated in ethanol, and embedded in Epon. Semithin (1 μm) sections were cut and stained with 1% toluidine blue solution. For scanning EM, dehydrated blocks were dried with hexamethyldisilazane, and they were subsequently broken in liquid nitrogen mounted on stubs and sputter-coated with a thin layer of 20 nm gold. Morphometric analysis was performed on randomly acquired digitalized scanning EM images at 30,000× magnification. For each experimental variable, 10 images in the periportal and pericentral zones (regions up to 100 μm in diameter) were randomly selected and analyzed at a magnification of 30,000×. Three animals were tested at each time point. All experiments were repeated three times.
Scanning and Transmission EM of SK Hep1 Cells.
After cells in the culture were 90% confluent, they were fixed with 2.5% glutaraldehyde in 0.1 mol/L sodium cacodylate buffer with 1% sucrose. Cells on cover slips were exposed to osmium (1% OsO4/0.1 mol/L sodium cacodylate buffer), dehydrated in an ethanol gradient to 100%, and incubated for 2 min in hexamethyl-disilazane. Coverslips were then mounted on stubs, sputter-coated with platinum, and examined using a JEOL 6380 scanning electron microscope. Fenestration numbers were counted manually by an investigator blinded to the identity of the slide using a Carl Zeiss AxioVision LE 4.8 program (Carl Zeiss).
Hepatic Microcirculation.
Hepatic microcirculation was studied using sidestream dark-field (SDF) imaging technology that emits green polarized light (wavelength = 548 nm), which illuminates hepatic sinusoids. Serial measurements were analyzed as described previously (53) to obtain sinusoidal volumetric blood flow (picoliter per second).
Quantitative Real-Time PCR.
RNA extraction and real-time PCR were performed as described (32). TaqMan gene expression assays (Applied Biosystems) for VEGF (Mm00437304_m1), HGF (Mm01135190_m1), IL-6 (Mm00446190_m1), TNF-α (Mm 00443258_m1), Htr2a (Mm00555764_m1), and Htr2b (Mm00434123_m1) were used to quantify the mRNA expression of the respective genes.
Cell Culture.
SK Hep-1 cells were obtained from ATCC and maintained in DMEM without FCS but supplemented with antibiotics (penicillin and streptomycin). They were plated on IbidiTreat microdishes (35 mm low; Ibidi). Cells were treated with recombinant VEGF (100 ng/mL; Calbiochem), DOI (100 μg/mL), anti-VEGF antibodies (10 μg/mL; Avastin), or a combination of these substances. All experiments were performed in triplicate.
Statistics.
Ordinal variables were compared with the Mann–Whitney test. A statistically significant difference was considered when P < 0.05. Error bars represent SD. Statistical analyses were performed using the software package GraphPad 5.0 and SPSS 18.0.
Acknowledgments
We thank Udo Ungethüm and Martha Bain for excellent technical support. We are grateful to Ashraf El-Badry for help with intravital microscopy. This work was supported by Swiss National Science Foundation Grant 3200B0-109906 (to P.-A.C.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012531108/-/DCSupplemental.
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Clavien PA, et al. What is critical for liver surgery and partial liver transplantation: Size or quality? Hepatology. 2010;52:715–729. doi: 10.1002/hep.23713. [DOI] [PubMed] [Google Scholar]
- 3.Lesurtel M, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312:104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 4.Schmucker DL. Age-related changes in liver structure and function: Implications for disease? Exp Gerontol. 2005;40:650–659. doi: 10.1016/j.exger.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 5.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43(2 Suppl 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 6.Timchenko NA. Aging and liver regeneration. Trends Endocrinol Metab. 2009;20:171–176. doi: 10.1016/j.tem.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Mackey S, Singh P, Darlington GJ. Making the liver young again. Hepatology. 2003;38:1349–1352. doi: 10.1016/j.hep.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Tian Y, et al. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci USA. 2006;103:4598–4603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taub R. Liver regeneration in health and disease. Clin Lab Med. 1996;16:341–360. [PubMed] [Google Scholar]
- 10.Kaita KD, Pettigrew N, Minuk GY. Hepatic regeneration in humans with various liver disease as assessed by Ki-67 staining of formalin-fixed paraffin-embedded liver tissue. Liver. 1997;17:13–16. doi: 10.1111/j.1600-0676.1997.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 11.Clavien PA, Petrowsky H, DeOliveira ML, Graf R. Strategies for safer liver surgery and partial liver transplantation. N Engl J Med. 2007;356:1545–1559. doi: 10.1056/NEJMra065156. [DOI] [PubMed] [Google Scholar]
- 12.Jin J, Wang GL, Shi X, Darlington GJ, Timchenko NA. The age-associated decline of glycogen synthase kinase 3beta plays a critical role in the inhibition of liver regeneration. Mol Cell Biol. 2009;29:3867–3880. doi: 10.1128/MCB.00456-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerrieri F, Capozza G, Kalous M, Papa S. Age-related changes of mitochondrial F0F1 ATP synthase. Ann N Y Acad Sci. 1992;671:395–402. doi: 10.1111/j.1749-6632.1992.tb43813.x. [DOI] [PubMed] [Google Scholar]
- 14.Selzner M, Selzner N, Jochum W, Graf R, Clavien PA. Increased ischemic injury in old mouse liver: An ATP-dependent mechanism. Liver Transpl. 2007;13:382–390. doi: 10.1002/lt.21100. [DOI] [PubMed] [Google Scholar]
- 15.Woodhouse KW, Wynne HA. Age-related changes in liver size and hepatic blood flow. The influence on drug metabolism in the elderly. Clin Pharmacokinet. 1988;15:287–294. doi: 10.2165/00003088-198815050-00002. [DOI] [PubMed] [Google Scholar]
- 16.Le Couteur DG, et al. Pseudocapillarization and associated energy limitation in the aged rat liver. Hepatology. 2001;33:537–543. doi: 10.1053/jhep.2001.22754. [DOI] [PubMed] [Google Scholar]
- 17.Woodhouse K, Wynne HA. Age-related changes in hepatic function. Implications for drug therapy. Drugs Aging. 1992;2:243–255. doi: 10.2165/00002512-199202030-00007. [DOI] [PubMed] [Google Scholar]
- 18.Woodhouse K. Drugs and the liver. Part III: Ageing of the liver and the metabolism of drugs. Biopharm Drug Dispos. 1992;13:311–320. doi: 10.1002/bdd.2510130502. [DOI] [PubMed] [Google Scholar]
- 19.McLean AJ, Le Couteur DG. Aging biology and geriatric clinical pharmacology. Pharmacol Rev. 2004;56:163–184. doi: 10.1124/pr.56.2.4. [DOI] [PubMed] [Google Scholar]
- 20.Clavien PA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–850. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrowsky H, et al. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921–928. doi: 10.1097/01.sla.0000246834.07130.5d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krupczak-Hollis K, Wang X, Dennewitz MB, Costa RH. Growth hormone stimulates proliferation of old-aged regenerating liver through forkhead box m1b. Hepatology. 2003;38:1552–1562. doi: 10.1016/j.hep.2003.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Ikegami T, et al. The impact of donor age on living donor liver transplantation. Transplantation. 2000;70:1703–1707. doi: 10.1097/00007890-200012270-00007. [DOI] [PubMed] [Google Scholar]
- 24.Hilmer SN, et al. Age-related changes in the hepatic sinusoidal endothelium impede lipoprotein transfer in the rat. Hepatology. 2005;42:1349–1354. doi: 10.1002/hep.20937. [DOI] [PubMed] [Google Scholar]
- 25.Le Couteur DG, Fraser R, Cogger VC, McLean AJ. Hepatic pseudocapillarisation and atherosclerosis in ageing. Lancet. 2002;359:1612–1615. doi: 10.1016/S0140-6736(02)08524-0. [DOI] [PubMed] [Google Scholar]
- 26.Le Couteur DG, et al. Age-related changes in the liver sinusoidal endothelium: A mechanism for dyslipidemia. Ann N Y Acad Sci. 2007;1114:79–87. doi: 10.1196/annals.1396.003. [DOI] [PubMed] [Google Scholar]
- 27.Jansen PL. Liver disease in the elderly. Best Pract Res Clin Gastroenterol. 2002;16:149–158. doi: 10.1053/bega.2002.0271. [DOI] [PubMed] [Google Scholar]
- 28.Dobbs BR, Rogers GW, Xing HY, Fraser R. Endotoxin-induced defenestration of the hepatic sinusoidal endothelium: A factor in the pathogenesis of cirrhosis? Liver. 1994;14:230–233. doi: 10.1111/j.1600-0676.1994.tb00080.x. [DOI] [PubMed] [Google Scholar]
- 29.Mori T, et al. Defenestration of the sinusoidal endothelial cell in a rat model of cirrhosis. Hepatology. 1993;17:891–897. [PubMed] [Google Scholar]
- 30.Tian Y, et al. Activation of serotonin receptor-2B rescues small-for-size liver graft failure in mice. Hepatology. 2011;53:253–262. doi: 10.1002/hep.23960. [DOI] [PubMed] [Google Scholar]
- 31.Soll C, et al. Serotonin promotes tumor growth in human hepatocellular cancer. Hepatology. 2010;51:1244–1254. doi: 10.1002/hep.23441. [DOI] [PubMed] [Google Scholar]
- 32.Nocito A, et al. Serotonin regulates macrophage-mediated angiogenesis in a mouse model of colon cancer allografts. Cancer Res. 2008;68:5152–5158. doi: 10.1158/0008-5472.CAN-08-0202. [DOI] [PubMed] [Google Scholar]
- 33.Jackson LN, et al. Development and characterization of a novel in vivo model of carcinoid syndrome. Clin Cancer Res. 2009;15:2747–2755. doi: 10.1158/1078-0432.CCR-08-2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ho-Tin-Noé B, Goerge T, Cifuni SM, Duerschmied D, Wagner DD. Platelet granule secretion continuously prevents intratumor hemorrhage. Cancer Res. 2008;68:6851–6858. doi: 10.1158/0008-5472.CAN-08-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nocito A, et al. Serotonin mediates oxidative stress and mitochondrial toxicity in a murine model of nonalcoholic steatohepatitis. Gastroenterology. 2007;133:608–618. doi: 10.1053/j.gastro.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 36.García JJ, et al. N-acetylserotonin suppresses hepatic microsomal membrane rigidity associated with lipid peroxidation. Eur J Pharmacol. 2001;428:169–175. doi: 10.1016/s0014-2999(01)01342-5. [DOI] [PubMed] [Google Scholar]
- 37.Ito Y, et al. Age-related changes in the hepatic microcirculation in mice. Exp Gerontol. 2007;42:789–797. doi: 10.1016/j.exger.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wisse E, De Zanger RB, Charels K, Van Der Smissen P, McCuskey RS. The liver sieve: Considerations concerning the structure and function of endothelial fenestrae, the sinusoidal wall and the space of Disse. Hepatology. 1985;5:683–692. doi: 10.1002/hep.1840050427. [DOI] [PubMed] [Google Scholar]
- 39.McLean AJ, et al. Age-related pseudocapillarization of the human liver. J Pathol. 2003;200:112–117. doi: 10.1002/path.1328. [DOI] [PubMed] [Google Scholar]
- 40.Fraser R, Dobbs BR, Rogers GW. Lipoproteins and the liver sieve: The role of the fenestrated sinusoidal endothelium in lipoprotein metabolism, atherosclerosis, and cirrhosis. Hepatology. 1995;21:863–874. [PubMed] [Google Scholar]
- 41.Schaffner F, Poper H. Capillarization of hepatic sinusoids in man. Gastroenterology. 1963;44:239–242. [PubMed] [Google Scholar]
- 42.Cogger VC, et al. Hepatic sinusoidal pseudocapillarization with aging in the non-human primate. Exp Gerontol. 2003;38:1101–1107. doi: 10.1016/j.exger.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 43.Zeeh J, Platt D. The aging liver: Structural and functional changes and their consequences for drug treatment in old age. Gerontology. 2002;48:121–127. doi: 10.1159/000052829. [DOI] [PubMed] [Google Scholar]
- 44.Funyu J, Mochida S, Inao M, Matsui A, Fujiwara K. VEGF can act as vascular permeability factor in the hepatic sinusoids through upregulation of porosity of endothelial cells. Biochem Biophys Res Commun. 2001;280:481–485. doi: 10.1006/bbrc.2000.4148. [DOI] [PubMed] [Google Scholar]
- 45.Heffelfinger SC, Hawkins HH, Barrish J, Taylor L, Darlington GJ. SK HEP-1: A human cell line of endothelial origin. In Vitro Cell Dev Biol. 1992;28A:136–142. doi: 10.1007/BF02631017. [DOI] [PubMed] [Google Scholar]
- 46.Iakova P, Awad SS, Timchenko NA. Aging reduces proliferative capacities of liver by switching pathways of C/EBPalpha growth arrest. Cell. 2003;113:495–506. doi: 10.1016/s0092-8674(03)00318-0. [DOI] [PubMed] [Google Scholar]
- 47.Wang GL, et al. Growth hormone corrects proliferation and transcription of phosphoenolpyruvate carboxykinase in livers of old mice via elimination of CCAAT/enhancer-binding protein alpha-Brm complex. J Biol Chem. 2007;282:1468–1478. doi: 10.1074/jbc.M608226200. [DOI] [PubMed] [Google Scholar]
- 48.Corsonello A, Pedone C, Incalzi RA. Age-related pharmacokinetic and pharmacodynamic changes and related risk of adverse drug reactions. Curr Med Chem. 2010;17:571–584. doi: 10.2174/092986710790416326. [DOI] [PubMed] [Google Scholar]
- 49.Xu J, et al. Characterization of a putative intrarenal serotonergic system. Am J Physiol Renal Physiol. 2007;293:F1468–F1475. doi: 10.1152/ajprenal.00246.2007. [DOI] [PubMed] [Google Scholar]
- 50.Yamaji M, et al. VEGF-A loss in the haematopoietic and endothelial lineages exacerbates age-induced renal changes. Microvasc Res. 2010;80:372–383. doi: 10.1016/j.mvr.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 51.Selzner N, et al. ICAM-1 triggers liver regeneration through leukocyte recruitment and Kupffer cell-dependent release of TNF-alpha/IL-6 in mice. Gastroenterology. 2003;124:692–700. doi: 10.1053/gast.2003.50098. [DOI] [PubMed] [Google Scholar]
- 52.Higgins GM, Anderson RM. Experimental pathology of the liver. I. Restoration of the liver of the white rat following partial surgical removal. Arch Pathol (Chic) 1931;12:186–202. [Google Scholar]
- 53.Furrer K, et al. Selective portal vein embolization and ligation trigger different regenerative responses in the rat liver. Hepatology. 2008;47:1615–1623. doi: 10.1002/hep.22164. [DOI] [PubMed] [Google Scholar]