Abstract
Organ transplantation represents a unique therapeutic option for irreparable organ dysfunction and rejection of transplants results from a breakdown in operational tolerance. Although endothelial cells (ECs) are the first target in graft rejection following kidney transplantation, their capacity to alloactivate and generate particular T lymphocyte subsets that could intervene in this process remains unknown. By using an experimental model of microvascular endothelium, we demonstrate that, under inflammatory conditions, human ECs induced proliferation of memory CD4+CD45RA− T cells and selectively amplified proinflammatory Th17 and suppressive CD45RA−HLA-DR+FoxP3bright regulatory CD4+ T lymphocytes (Tregs). Although HLA-DR expression on resting microvascular ECs was sufficient to induce proliferation of memory CD4+ T cells, Treg amplification was dependent on the interaction with CD54, highly expressed only under inflammatory conditions. Moreover, expansion of Th17 cells was dependent on IL-6 and STAT-3, and inhibition of either specifically impaired Th17, without altering Treg expansion. Collectively these data reveal that the HLA-DR+ ECs regulate the local inflammatory allogeneic response, promoting either an IL-6/STAT-3–dependent Th17 response or a contact-CD54–dependent regulatory response according to the cytokine environment. Finally, these data open therapeutic perspectives in human organ transplantation based on targeting the IL-6/STAT-3 pathway and/or promoting CD54 dependent Treg proliferation.
Keywords: immune regulation, MHC class II
The allogeneic response to organ transplantation was initially considered to be predominantly mediated by the Th1 population whereas the Th2 population was believed to oppose Th1 cell activation, thus favoring graft survival (1). Recent studies have identified additional CD4+ T cell subsets including IL-17–producing CD4+ T cells (Th17) and CD4+FoxP3+ regulatory T cells (Tregs) (2). A unique role for Th1 cells was excluded by using genetically deficient mice and T lymphocyte transfer experiments in MHC class II mismatched organ transplantation models (3, 4). Th2 cytokines have been implicated in acute graft rejection (5), and involvement of Th17 was shown in cardiac and lung allograft rejection (6, 7). In the absence of a Th1 alloresponse, Th17 mediates a violent proinflammatory response, resulting in accelerated allograft rejection (6). The Th1/Th2 paradigm is thus obsolete in models of organ transplantation. Recent studies have revealed reciprocal control of differentiation of proinflammatory Th17 and tolerogenic induced Treg subsets in mouse models (8–10) and in human studies (11–13). Whether this equilibrium between Treg and Th17 subsets occurs in organ transplantation and, if so, can be skewed in one or other direction, is unknown.
In the context of organ transplantation, human endothelial cells (ECs) express MHC class I and class II molecules, and may therefore act both as stimulators of and targets for alloimmune responses (14). ECs stimulate memory CD4+ T cell production of IFN-γ, thereby promoting a CD4+ Th1 profile (15). EC allostimulation of CD4+ T cells requires expression of HLA-DR and the costimulatory molecule LFA-3 independently of CD80 and CD86 (16, 17). ECs therefore have the potential to activate memory CD4+ T cells but their ability to orient the alloimmune response toward induced Treg-mediated tolerance or a proinflammatory effector response remains unknown. In MHC class II fully mismatched mouse models, vascular ECs activate and induce programmed death ligand 1 (PD-L1)–dependent Tregs, which inhibit in vitro and in vivo proliferation of alloreactive CD4+ T cells (18).
Human ECs express LFA-3 and PD-L1 (19) and we therefore investigated their ability to allostimulate CD4+ T cells and to generate regulatory or effector T cell populations. To address this question, we have studied the capacity of human microvascular ECs to stimulate HLA-DR–typed CD4+ T cells from allogeneic donors. The results reveal that human endothelium simultaneously generates proinflammatory Th17 and functional HLA-DR+ Tregs under inflammatory conditions. These data lead to the suggestion that allograft endothelium participates in controlling CD4+ T lineage plasticity and can thus induce a regulatory or proinflammatory response, depending on the local cytokine microenvironment.
Results
HLA-DR Expression on Resting ECs Is Necessary and Sufficient to Induce CD4+ T Cell Alloproliferation.
The microvascular EC line HMEC-1 (20) constitutively expressed the costimulatory molecule LFA-3 whereas expression of HLA-DR was induced by activation with IFN-γ [activated ECs (aECs); Fig. 1A]. In contrast with previous studies, ECs did not express the adhesion molecule CD54 in steady-state conditions (20), although it was strongly expressed after activation with IFN-γ (Fig. 1A). Constitutive expression of the costimulatory molecules LFA-3 and PD-L1 and the adhesion molecule VE-cadherin was unchanged by IFN-γ treatment (i.e., aECs; Fig. 1A and Fig. S1A). To understand the role of HLA-DR in the absence of inflammatory stimuli, ECs were transduced with a lentiviral HLA-DR construct leading to constitutive surface expression of HLA-DR (ECsαβ) comparable to that of aECs. LFA-3 and VE-cadherin expression remained similar to that observed in resting ECs (Fig. 1A). CD54 expression was induced in ECsαβ activated with IFN-γ (aECsαβ) to a similar level as that observed in aECs (Fig. 1A).
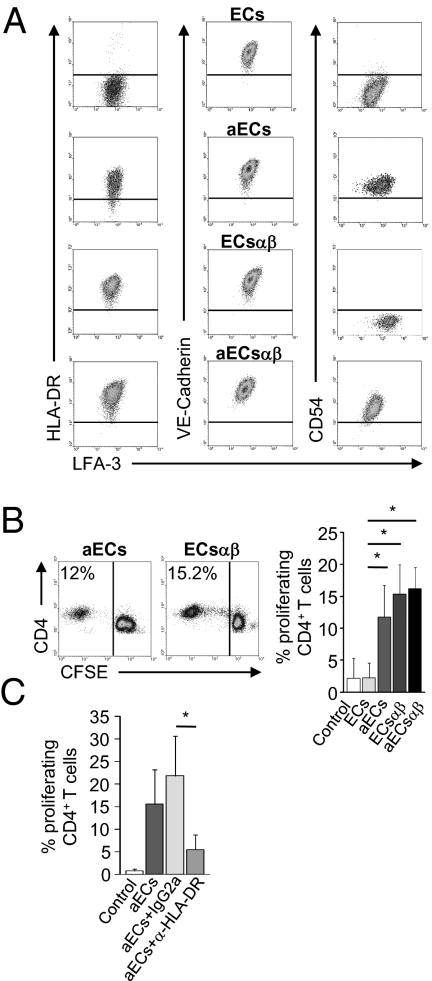
Fig. 1.
HLA-DR expression on resting microvascular ECs is necessary and sufficient to induce proliferation of allogeneic CD4+ T cells. (A) Expression of HLA-DR, LFA-3, VE-cadherin, and CD54 was determined by flow cytometry in resting ECs, in ECs transduced with a HLA-DRαβ lentivirus construct (ECsαβ), and in ECs or ECsαβ activated with IFN-γ (aECs or aECsαβ). (B) The mean percentage of proliferating allogeneic CD4+ T cells cultured with resting ECs and aECs (n = 11) or ECsαβ (n = 4) is shown. Representative proliferation of CFSE-labeled CD4+ T cells cultured with aECs (Left) and ECsαβ (Middle) is shown. (C) aECs were pretreated with a HLA-DR blocking antibody or an isotype control and cocultured with CFSE-labeled CD4+ T cells (n = 4). Comparisons were made by using the nonparametric Mann–Whitney U test (*P < 0.05).
We next tested the ability of ECs and ECsαβ, activated with IFN-γ or not, to induce alloproliferation of CD4+ T cells. Fig. 1B shows that resting ECs failed to induce proliferation whereas resting ECsαβ constitutively expressing HLA-DR induced CD4+ T cell proliferation on day 7 (15.35 ± 4.56%), as did aECs (11.72 ± 4.16%) and aECsαβ (16.15 ± 3.32%). Proliferation is allorecognition-dependent as an HLA-DR–blocking antibody inhibits T cell proliferation by more than 70% (5.47 ± 3.31% at day 7 vs. 21.88 ± 8.81% with isotype IgG2a; P = 0.02; Fig. 1C).
HLA-DR expression by resting ECs is therefore necessary and sufficient to induce CD4+ T cell alloproliferation.
Coculture of CD4+ T Cells with aECs Results in Selective Expansion of Treg and Th17 Populations.
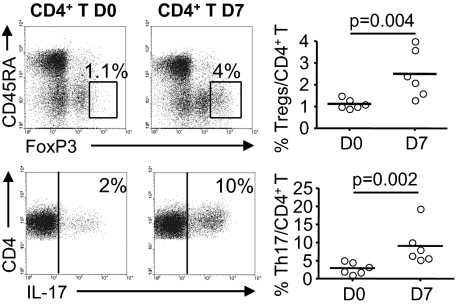
We next determined whether aECs generated particular CD4+ T lymphocyte subsets. We compared the proportion of CD45RA−FoxP3bright cells (i.e., Tregs) among CD4+ T cells on days 0 and 7 of culture. The percentage of Tregs was significantly increased (2.5 ± 1.08% of CD4+ T at day 7 vs. 1.13 ± 0.22% at day 0; P = 0.004; Fig. 2). In contrast, the proportion of CD4+IFN-γ+IL-2+ Th1 or CD4+IL-4+IL-10+ Th2 subsets was unaltered after 7 d of coculture with aECs. We also observed a marked increase in the proportion of CD4+IL-17+ cells (9.06 ± 5.31% at day 7 vs. 2.91 ± 1.6% at day 0; P = 0.002; Fig. 2).
Fig. 2.
Induction of regulatory and proinflammatory CD4+ T cell subsets by ECs. FoxP3 expression (Upper) and IL-17 production (Lower) were analyzed in CD4+ T cells on days 0 and 7 (Left, Middle) of culture with aECs. Representative cytofluorometric analysis of one allogeneic donor is shown. The CD4+FoxP3bright gate distinguished CD4+FoxP3bright from total CD4+CD45RA+FoxP3+ cells. The percentage of CD4+FoxP3bright cells (Tregs, Upper) and of CD4+IL-17+ cells (Th17, Lower) is shown. Horizontal lines (Right) show mean levels. Comparisons were made by using the Mann–Whitney U test.
Microvascular ECs therefore generate proinflammatory Th17 and regulatory CD4+ T cell populations under inflammatory conditions.
Expansion of Tregs by Allogeneic ECs Requires Cell–Cell Contact and CD54 Engagement.
As it has been shown that CD45RA−FoxP3bright Tregs are highly proliferative (21), we analyzed proliferation of carboxyfluorescein succinimidyl ester (CFSE)-labeled CD45RA−FoxP3− and FoxP3low (memory CD4+ T cells) and CD45RA−FoxP3bright cells (i.e., Tregs) after coculture with aECs. After 7 d, we observed extensive proliferation of Tregs, which was significantly higher than that of memory T cells (63.69 ± 23.3% vs. 19 ± 7.43%; P = 0.001; Fig. 3A; a representative cytofluorometric analysis of one allogeneic donor is shown in Fig. S2), whereas naive CD45RA+ T cells did not proliferate, as previously reported (22). This alloproliferative profile is specific to ECs, as professional antigen presenting cells induced equivalent proliferation of memory and Treg cells (Fig. 3A and Fig. S2). Moreover, the Treg response was observed only under inflammatory conditions, as resting ECsαβ induced comparable proliferation of memory and Treg cells [36 ± 11.34% vs. 30.67 ± 11.4%; P value not significant (NS)] and IFN-γ activation of ECsαβ restored the selective proliferation of Tregs (78.62 ± 10.13% vs. 22.45 ± 5.56% of memory T cells; P = 0.02).
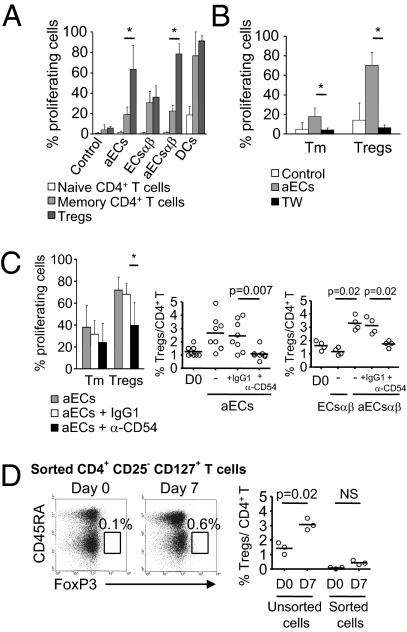
Fig. 3.
EC expression of CD54 is necessary for the proliferation and the expansion of Tregs. (A) Allogeneic CFSE-labeled CD4+ T cells were cultured with aECs, ECsαβ, aECsαβ, and dendritic cells. The mean ± SEM percentage of proliferation within the regulatory (CD45RA−FoxP3bright, Tregs), memory (CD45RA−FoxP3− or low, Tm), and naive (CD45RA+FoxP3−) CD4+ T lymphocytes was determined on day 7 (n = 6). CD4+ T cell autoproliferation (control) is shown. (B) aECs were cultured in the lower chamber of 96-well transwell (TW) plates and CFSE-labeled responder CD4+ T cells were added to the upper chamber. The mean ± SEM proliferation of Tm and Tregs is depicted (n = 4). (C) aECs were pretreated with α-CD54 or IgG1 isotype and cocultured with CFSE-labeled CD4+ T cells. The mean percentage ± SEM of Tm and Treg proliferation is shown (n = 8). The percentage of Tregs (Right) within CD4+ T cells cultured with aECs and aECsαβ pretreated or not with α-CD54 or IgG1 and with ECsαβ was analyzed on day 7. The control shows the percentage of Tregs on day 0. Horizontal lines represent mean levels for ECs (n = 8) and for ECsαβ (n = 4). (D) FoxP3+ expression in CD4+CD25−CD127+ T cells sorted from unprimed CD4+ T cells is shown on day 0 and after 7 d of culture with aECs. Representative analysis of one allogeneic donor (Left, Middle) is shown. Controls were unsorted CD4+ T cells from the same donors on days 0 and 7. Horizontal lines (Right) represent the mean levels (n = 3). Comparisons were made by using the Mann–Whitney U test (*P < 0.05).
We next examined whether aEC-induced alloproliferation of Tregs required soluble or contact-dependent inducible factors. Fig. 3B shows that contact inhibition in the presence of transwells abrogated Treg alloproliferation.
Based on the IFN-γ–induced expression of CD54 on aECsαβ, we examined the role of CD54 in Treg proliferation and/or expansion. CD54 blockade selectively inhibited Treg proliferation (39.88 ± 21.12% with α-CD54 vs. 68.12 ± 10.02% with IgG1; P = 0.02; Fig. 3C) without modifying proliferation of the memory subset (23.95 ± 17.5% with α-CD54 vs. 31.5 ± 12.8% with IgG1; P value NS; Fig. 3C). Moreover, inhibition of proliferation was associated with inhibition of Treg expansion in CD4+ T cell-aEC cocultures (1.07 ± 0.42% with α-CD54 vs. 2.45 ± 1.15% with IgG1; P = 0.007; Fig. 3C). Similarly, CD54-expressing aECsαβ induced Treg expansion, unlike resting ECsαβ, which do not express CD54 (3.3 ± 0.53% vs. 1.17 ± 0.25%; P = 0.02; Fig. 3C), and CD54 blocking inhibited expansion (1.75 ± 0.25% with α-CD54 vs. 3.12 ± 0.62% with IgG1; P = 0.02; Fig. 3C).
Finally, coculture of CD4+FoxP3− with aECs did not generate CD4+CD45RA−FoxP3bright cells (Fig. 3D), thus excluding conversion as the mechanism of expansion in this model.
Collectively, these data indicate that Treg expansion by aECs is a result of their alloproliferation and requires CD4+ T cell interaction with CD54-expressing aECs.
IL-6 and STAT-3 Phosphorylation Are Selectively Required for Th17 Expansion.
We next investigated the origin of the expansion of the Th17 population. IL-6 secretion by ECs was high and was increased threefold by EC activation (Fig. 4A). We also identified significant phosphorylation of STAT-3 (P-STAT-3) in proliferating CD4+ T cells after 7 d of culture with aECs (20.27 ± 10.65% vs. 0.3 ± 0.11% in nonproliferating CD4+ T cells; P = 0.002; Fig. 4B).
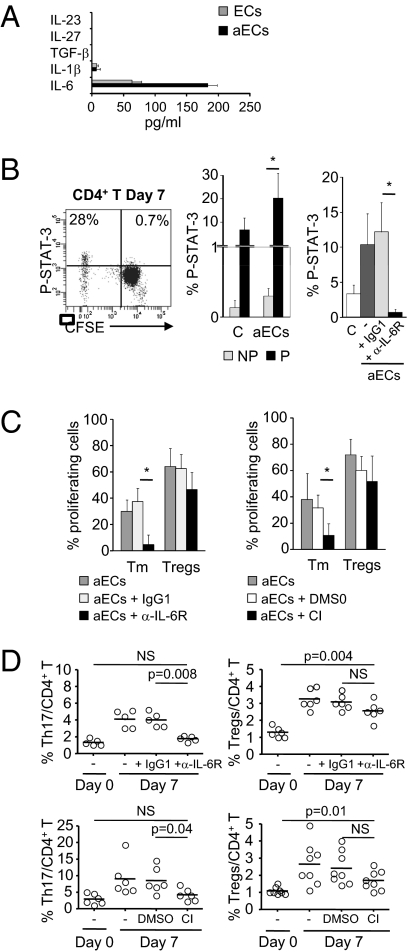
Fig. 4.
IL-6 and STAT-3 activation is required for Th17 expansion without altering regulatory T cell expansion. (A) Cytokine detection in supernatants of resting ECs and aECs after 3 d of culture (n = 2). (B) STAT-3 phosphorylation (P-STAT-3) was analyzed by flow cytometry in CFSE-labeled CD4+ T cells at day 7 of culture with aECs or alone (control, C). A representative scatterplot of one allogeneic donor is shown (Left). The mean ± SEM of P-STAT-3 in proliferating (P) or nonproliferating (NP) cells (Middle) and in total CD4+ T cells in presence or absence of an IL-6R Ab or the isotype IgG1 (Right) is shown (n = 6). (C) The mean ± SEM proliferation of Tm and Tregs cultured with aECs in presence or absence of IL-6R Ab, IgG1 (Left; n = 6 donors), cucurbitacin I (CI), or DMSO (Right; n =8 donors) was analyzed on day 7. (D) The percentage of CD4+IL17+ (Left) and CD4+CD45RA−FoxP3bright (Right) cells on day 7 with IL-6R Ab (Upper) or with CI or DMSO (Lower) is shown. Horizontal lines indicate the mean of each group. The groups were compared by using the Mann–Whitney U test.
The role of IL-6 and P-STAT-3 in Th17 amplification was determined by coculturing aECs with allogeneic CD4+ T cells in the presence of an IL-6R blocking antibody or a P-STAT-3 inhibitor (cucurbitacin I) directed against Janus kinases (23).
First, IL-6R blockade inhibited STAT-3 phosphorylation in CD4+ T cells cocultured with aECs (0.68 ± 0.43% with α-IL-6R at day 7 vs. 12.17 ± 4.17% with IgG1; P = 0.002; Fig. 4B).
Second, memory T cell proliferation after coculture with aECs was selectively inhibited by IL-6R mAb (5.58 ± 7.17% vs. 37.3 ± 10.34% with IgG1; P = 0.002; Fig. 4C) and by cucurbitacin I (10.7 ± 9% vs. 31.6 ± 9.84% with DMSO; P = 0.002), whereas Treg proliferation was unchanged by IL-6R mAb (46.5 ± 13.11% vs. 62.67 ± 10.69% with IgG1; P value NS) or cucurbitacin I (51.71 ± 19.67% vs. 60.22 ± 10.67% with DMSO; P value NS).
Finally, blocking IL-6R inhibited CD4+IL-17+ cell expansion (1.76 ± 0.32% with α-IL-6R vs. 4 ± 0.86% with IgG1; P = 0.008; Fig. 4D) without altering the proportion of Tregs (2.56 ± 0.55% of CD4+ T cells vs. 3.08 ± 0.45% with IgG1; P value NS), which remained significantly higher than on day 0 (P = 0.004; Fig. 4D). Similarly, cucurbitacin I selectively inhibited CD4+IL-17+ expansion (4.23 ± 1.87% vs. 8.53 ± 3.9%; P = 0.04; Fig. 4D) but not Treg expansion (1.71 ± 0.56% vs. 2.4 ± 0.9%; P value NS).
Expansion of Th17 cells by aEC is thus IL-6/P-STAT-3–dependent and implicates memory CD4+ T cell proliferation.
Allogeneic CD4+CD45RA−FoxP3bright T Cell Population Expanded by EC Interaction Have Phenotypic and Functional Characteristics of Tregs.
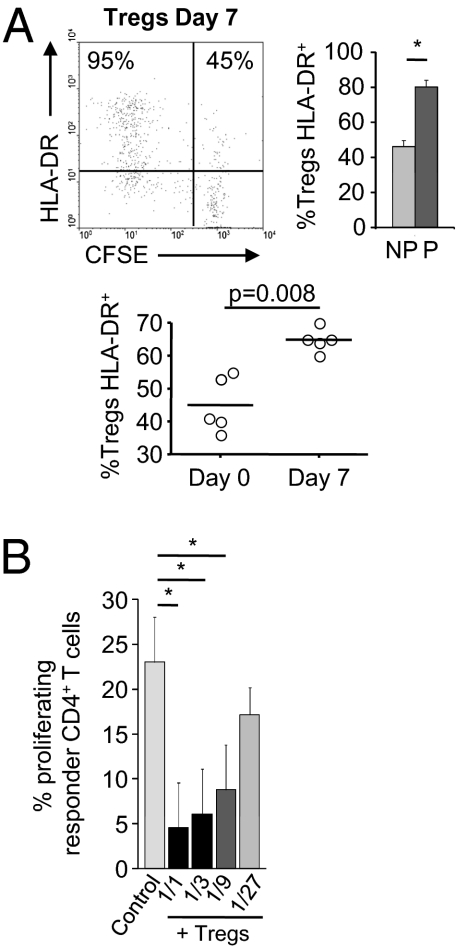
HLA-DR expression on CD4+CD25bright T cells identifies mature, functionally distinct Tregs involved in contact-dependent in vitro suppression (24). We therefore examined HLA-DR expression on Tregs expanded by EC allostimulation. As shown in Fig. 5A, Treg proliferation is associated with increased HLA-DR expression (80.2 ± 9.45% of proliferating Tregs vs. 46.28 ± 8.62% of nonproliferating Tregs; P = 0.002), leading to an enlarged proportion of HLA-DR–expressing Tregs (64.8 ± 3.5% vs. 45 ± 8.45%; P = 0.008; Fig. 5A). These data provide indirect evidence that the CD4+CD45RA−FoxP3bright population are functional regulatory cells.
Fig. 5.
Allospecific FoxP3brightCD4+ T cells generated by interaction with aECs have Treg characteristics and function. (A) HLA-DR expression was analyzed on CFSE-labeled Tregs after a 7-d culture with aECs. Representative analysis of one allogeneic donor (Left) is shown. The mean ± SEM of HLA-DR+ cells in proliferating (P) versus nonproliferating (NP) FoxP3brightCD4+ population (Top, Right; n = 5) is shown. The percentage of HLA-DR+ in the total FoxP3brightCD4+ population on days 0 and 7 of culture with aECs is shown (Bottom; n = 5). Comparisons were made by using the nonparametric Mann–Whitney U test. (B) CD127lowCD25bright T cells sorted from CD4+ T cells primed with allogeneic aECs for 7 d were added to a second allogeneic coculture of fresh autologous CFSE-labeled CD4+ T cells with aECs. Controls included allogeneic cocultures without addition of suppressor cells (control). Responder CD4+ T cell proliferation was measured on day 7 (mean ± SEM, n = 3, paired t test).
Because phenotypic markers are an imperfect gauge of Treg function, we evaluated expanded Treg function. CD4+CD25brightCD127low cells were sorted from CD4+ T cells harvested after 7 d of culture with aECs and added to a second allogeneic coculture composed of aECs and autologous responder CD4+ T cells. These experiments revealed a dose-dependent inhibition of CD4+ T cell proliferation by CD4+CD25bright T cells with more than 80% inhibition at a suppressor-to-responder ratio of 1:1 (mean, 80.6%; range, 88.7–76.2%; P < 0.05; Fig. 5B) and as much as 60% inhibition at a ratio of 1:9 (mean, 67.3%; range, 47.3–68.4%; P < 0.05). aEC-expanded Tregs therefore significantly suppress alloproliferation of autologous CD4+ T cells.
In addition, ECαβ- or aECαβ-activated Tregs equally suppress autologous CD4+ T cell proliferation induced by ECsαβ or aECsαβ, indicating that CD54 is not required for the suppressive function of Tregs induced by ECs (Fig. S3).
Primary Microvascular ECs Induced Selective Expansion of Tregs and Th17 Under Inflammatory Conditions.
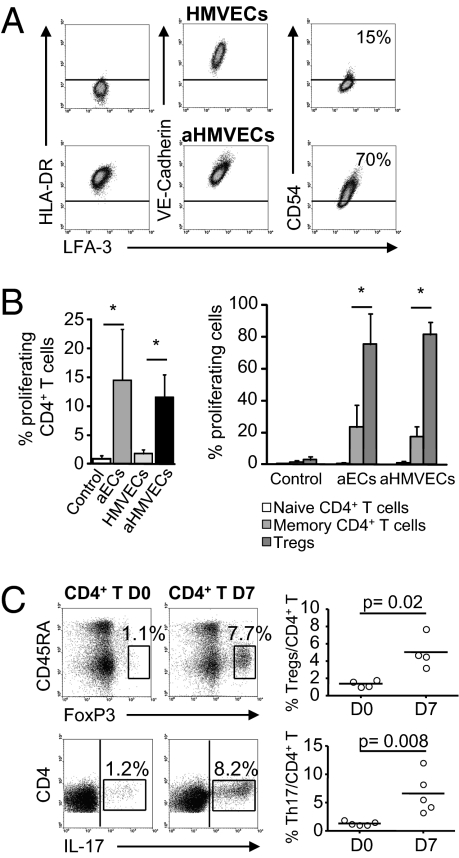
To further understand the ability of human microvascular ECs in expanding Th17 and Tregs under inflammatory conditions, we studied primary human dermal microvascular ECs (HMVECs). Similarly to HMEC-1, resting primary ECs constitutively expressed the costimulatory molecule LFA-3 and expression of HLA-DR and CD54 was strongly up-regulated by IFN-γ (Fig. 6A).
Fig. 6.
Activated HMVECs induced the selective expansion of Th17 and Tregs. (A) Expression of HLA-DR, LFA-3, VE-cadherin, and CD54 was determined in resting and aHMVECs. (B) Allogeneic CFSE-labeled CD4+ T cells were cultured with aECs or HMVECs activated or not for 3 d with IFN-γ. The mean (± SEM) percentage of CD4+ T cell proliferation (Left; n = 4) and within the naive, memory, and Treg cells (Right; n = 4) was determined on day 7. Autoproliferation is shown (control). (C) Expression of CD45RA−FoxP3bright (Upper) and production of IL-17 (Lower) were analyzed in CD4+ T cells on days 0 and 7 (Left, Middle) of culture with aHMVECs. Representative analysis of one allogeneic donor is shown. Comparisons were made by using the Mann–Whitney U test.
Activated HMVECs (aHMVECs) induced alloproliferation of CD4+ T cells and selective proliferation of Tregs after 7 d of coculture (81.75 ± 7.54% of Tregs vs. 17.55 ± 6.31% of memory T cells; P = 0.02; Fig. 6B).
Alloactivation was associated with the expansion of CD4+IL-17+ (6.62 ± 3.54% at day 7 vs. 1.32 ± 0.38% at day 0; P = 0.008; Fig. 6C) and CD4+CD45RA−FoxP3bright cells (5.02 ± 1.9% at day 7 vs. 1.37 ± 0.38% at day 0; P = 0.02; Fig. 6C).
These results suggest that the expansion of proinflammatory and regulatory T cell subsets is a general property of human microvascular ECs.
Discussion
This study provides evidence for human EC expansion of proinflammatory and regulatory T cell subsets. In our experimental setting, Tregs and Th17 were increased in proliferating CD4+ T lymphocytes cocultured with allogeneic ECs. The model is relevant to human organ transplantation for two reasons. First, many previous studies examined the role of ECs from large vessels (namely human umbilical vein ECs) in T cell activation (16, 19); however, it is the microvasculature and not the macrovasculature that is implicated in the immune response that leads to allograft rejection. We therefore concentrated on microvascular ECs. Second, MHC class II and costimulatory molecule-expressing murine ECs cannot induce alloproliferation of CD4+ T lymphocytes (25). Results from studies of murine microvascular endothelium are therefore difficult to extrapolate to human organ transplantation.
Human EC activation of memory CD4+ T cells, resulting in IL-2 secretion and proliferation (15, 22), is dependent on HLA-DR and LFA-3 expression (16, 22). This study reports that HLA-DR expression in resting human ECs is necessary and sufficient for CD4+ T cell alloproliferation whereas selective proliferation and expansion of Tregs occurs only in response to IFN-γ aECs. HLA-DR expression in ECs is thus insufficient to induce selective proliferation and expansion of Tregs.
Experiments with transwells revealed that Treg proliferation is contact-dependent, and a unique mechanism of Treg expansion dependent on CD54 expression by ECs was uncovered by the following observations: lack of increase of Tregs by ECs expressing HLA-DR but not CD54 (ECsαβ), Treg amplification by HLA-DR and CD54-expressing ECs (i.e., aECs and aECsαβ), and inhibition of Treg amplification by CD54 blocking. Mechanisms of Treg expansion include proliferation of existing Tregs and “education” of CD4+FoxP3− T cells (26). We suggest that Treg expansion results from proliferation in this model, as (i) blocking Treg proliferation with α-CD54 inhibited expansion and (ii) coculture of CD4+FoxP3− with aECs did not generate CD4+CD45RA−FoxP3bright cells. CD45RA−FoxP3bright Tregs are highly proliferative, but their capacity for self-renewal is limited by their rapid apoptosis and they seem to be mainly derived from CD45RA+FoxP3+ naive Tregs (21). It is probable that Treg expansion in our model results from activation, proliferation, and conversion of naive Tregs into memory CD4+CD45RA−FoxP3bright Tregs.
CD54 interacts with LFA-1 on CD4+ T cells and enhances TCR engagement with MHC II-peptide ligand (27). Given the known impairment of IL-2 production by CD4+ T cells in LFA-1–deficient mice (28), defective Treg proliferation in the absence of CD54 may be caused by inadequate TCR activation and IL-2 production. CD54 has also been implicated in conversion of Th17 to a Treg phenotype by mesenchymal stem cells (29). Thus, CD54 should be considered as an important protein for Treg development under certain conditions of inflammation.
Although PD-L1 has been involved in EC-mediated increased Treg suppression (30), PD-L1 expression was unaltered by IFN-γ activation in our model and blocking PD-L1 expression did not alter the expansion of Tregs (Fig. S1B).
HLA-DR expression on CD4+CD25brightFoxP3bright cells defines a mature population of functional Tregs (24). In our model, Treg expression of HLA-DR correlated with proliferation in response to aECs, suggesting that allostimulation promotes expansion of allospecific Tregs, and we have shown that their functional activity targeted the CD4+ T cell population that had initially alloproliferated in response to the same ECs.
The present study identifies a signaling profile for T cells alloactivated by IL-6–secreting ECs. IL-6 induced P-STAT-3 in proliferating cells, and inhibition of either IL-6R or P-STAT-3 disrupted memory T cell proliferation. Results of P-STAT-3 inhibition or IL-6R blockade indicate that aEC induction of Th17 cells is IL-6/P-STAT-3–dependent and implicates memory CD4+ T cell proliferation. Two IL-6–mediated mechanisms have been proposed: IL-6 conversion of Tregs into Th17 cells (10, 13) and IL-6 activation of STAT-3, which is required for memory T cell proliferation and survival (31). Our results are consistent with the latter and lead to the suggestion that EC production of IL-6 in inflammatory conditions activates STAT-3 in memory T cells, thereby promoting proliferation of preformed Th17 cells. We have identified IL-6 as a key cytokine in human Th17 amplification within the context of memory T cell proliferation.
One limitation of our study was the variability in the frequencies of Tregs and Th17 induced by aECs between different donors. This variability is most likely a result of different degrees of CD4+ T allostimulation induced in the different donors by the same HLA-DR molecule. Indeed, Nadazdin et al. (32) reported that the frequency of allospecific memory T cells varied widely depending on the nature of the responder/stimulator MHC combination tested, even when fully MHC-mismatched combinations were studied. This suggests that certain MHC gene disparities are poorly immunogenic in selected responder/stimulator combinations.
Our data concord with the current concept of CD4+ T regulatory and proinflammatory lymphocyte plasticity (10). Plasticity has been demonstrated in the tumor microenvironment (33), and our data extend this finding to the environment of the allogeneic endothelium under inflammatory conditions such as those observed following organ transplantation.
Extrapolation of these data leads to the proposal that ECs of the engrafted organ orient graft-infiltrating T lymphocyte populations toward a proinflammatory or regulatory function and thus play a cardinal role in graft survival. We propose that, whereas cytokine secretion would enhance the local proinflammatory Th17 lymphocytes, a counterbalance would be provided by contact-dependent expansion of regulatory T lymphocytes.
Finally, these data point to unique therapeutic targets in transplantation such as increasing expression of CD54 in ECs or inhibiting the IL-6/P-STAT-3 pathway to favor a regulatory rather than an inflammatory graft-infiltrating population under inflammatory conditions.
Methods
Cell Culture and Reagents.
HMEC-1 was provided by A. Kesikli (University of Regensburg, Germany) and used between passages eight and 15 (33). Primary ECs (i.e., HDMECs; Lonza) were used between passages three and six (34, 35).
Lentiviral Constructs and HMEC-1 Transduction.
The HLA-DR α and β cDNAs were amplified by PCR using the oligonucleotides 5′-AAAGGATCCATGGCCATAAGTGGAGTC-3′ and 5′-AAACATATGTTACAGAGGCCCCCTGCG-3′ for HLA-DRα and 5′-AAAGGATCCATG GTGTGTCTGAAGCTC-3′ and 5′AAACATATGTCAGCTCACGAGTCCTGT-3′ for HLA-DRβ. PCR products were digested with BamHI and Nde I and cloned into the pWPXL lentiviral vector (Addgene) digested with BamHI and Nde I. HLADRβ1*11.02 lentiviral particles were produced by transfecting HEK293T cells by the calcium phosphate method with pWPXL-HLA-DR (α or β), packaging plasmid psPAX2, and envelope plasmid pMD2.G (Addgene), and harvesting supernatants after 48 h.
Blood Samples.
Peripheral blood mononuclear cells (PBMCs) were collected from healthy donors fully mismatched at the HLA-DR locus, with regard to the HMEC-1 line. Informed consent was obtained in accordance with the Declaration of Helsinki.
Phenotypic Analysis and Cell Sorting by Flow Cytometry.
LFA-3-FITC, HLA-DR-PE, PD-L1, VE-cadherin or CD45RA-PC-7, CD54 or CD4-PB, and P-STAT-3-APC (S727, clone 49) (36) (BD Biosciences) antibodies were used. Intranuclear FoxP3 was detected according to the manufacturer's instructions (e-Bioscience). Intracellular cytokines IFN-γ, TNF-α or IL-4-FITC, IL-10-PE, IL2-APC (BD Biosciences), and IL-17–Alexa Fluor 647 (e-Bioscience) were detected in 50 ng/mL phorbol myristate acetate and 1 μM ionomycin-treated PBMCs or CD4+ T cells (5 h) in the presence of Golgi-Stop (BD Biosciences).
Selected CD4+ T cells (Miltenyi Biotec) were stained with CD4-FITC, CD127-PE, and CD25-APC (BD Biosciences) before sorting on a FACSAria device (BD Biosciences).
Allostimulation Assays.
CFSE-labeled PBMCs or CD4+ T cells (2.5 μM; Molecular Probes/Invitrogen) were stimulated with irradiated (20 Gy) ECs (1:1) for 7 d in RPMI-10% human AB serum (Biowest). ECs were activated with IFN-γ (100 UI/mL; R&D Systems) as indicated. Use of transwell inserts (4.26-mm diameter, 1-μm pore size; VWR) or pretreatment of HMEC-1 with CD54 (10 μg/mL; R&D Systems), PD-L1 (5 μg/mL; e-Bioscience), HLA-DR (10 μg/mL; clone L243) or mouse IgG antibodies before coculture are indicated. Cucurbitacin I (50 nM; Tocris Bioscience) or anti-IL-6R (10 μg/mL; R&D Systems) was added on day 0 of coculture.
Cytokine Detection by ELISA.
Cytokines were measured in culture supernatants by using IL-1β, IL-6, TGF- β (BD Biosciences), IL-23 (Abcam), and IL-27 (R&D Systems) kits.
Alloantigen Specific Suppression Assays.
CD4+CD25brightCD127low Tregs were cytofluorographically sorted from CD4+ T cells, primed by allogeneic aECs, and then added to a second coculture containing fresh autologous CD4+ T cells and new allogeneic aECs at the indicated ratios.
Statistical Analysis.
Data are expressed as mean ± SEM. In analysis of variables deviating from a normal distribution, Mann–Whitney U tests were used. The paired Student t test was used for pairwise comparisons. The program used was Statistica 6 (Statsoft).
Acknowledgments
Financial support was received from Agence de la Biomédecine, Fondation pour la Recherche sur le Cerveau, and Fondation pour la Recherche Médicale. C.T. is supported by Institut National de la Santé et de la Recherche Médicale. J.B. is supported by a fellowship from the Commissariat à l'Energie Atomique.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011811108/-/DCSupplemental.
References
- 1.Waaga AM, et al. Regulatory functions of self-restricted MHC class II allopeptide-specific Th2 clones in vivo. J Clin Invest. 2001;107:909–916. doi: 10.1172/JCI11427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Konieczny BT, et al. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–2064. [PubMed] [Google Scholar]
- 4.Zhou P, et al. Role of STAT4 and STAT6 signaling in allograft rejection and CTLA4-Ig-mediated tolerance. J Immunol. 2000;165:5580–5587. doi: 10.4049/jimmunol.165.10.5580. [DOI] [PubMed] [Google Scholar]
- 5.Nickerson P, et al. Prolonged islet allograft acceptance in the absence of interleukin 4 expression. Transpl Immunol. 1996;4:81–85. doi: 10.1016/s0966-3274(96)80043-8. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X, et al. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burlingham WJ, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lochner M, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med. 2008;205:1381–1393. doi: 10.1084/jem.20080034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beriou G, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113:4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayyoub M, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc Natl Acad Sci USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Lamki RS, Bradley JR, Pober JS. Endothelial cells in allograft rejection. Transplantation. 2008;86:1340–1348. doi: 10.1097/TP.0b013e3181891d8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiao SL, et al. Human effector memory CD4+ T cells directly recognize allogeneic endothelial cells in vitro and in vivo. J Immunol. 2007;179:4397–4404. doi: 10.4049/jimmunol.179.7.4397. [DOI] [PubMed] [Google Scholar]
- 16.Marelli-Berg FM, et al. Major histocompatibility complex class II-expressing endothelial cells induce allospecific nonresponsiveness in naive T cells. J Exp Med. 1996;183:1603–1612. doi: 10.1084/jem.183.4.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pober JS, et al. Interactions of T lymphocytes with human vascular endothelial cells: Role of endothelial cells surface antigens. Immunobiology. 1984;168:483–494. doi: 10.1016/s0171-2985(84)80132-1. [DOI] [PubMed] [Google Scholar]
- 18.Krupnick AS, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175:6265–6270. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 19.LaGier AJ, Pober JS. Immune accessory functions of human endothelial cells are modulated by overexpression of B7-H1 (PDL1) Hum Immunol. 2006;67:568–578. doi: 10.1016/j.humimm.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Ades EW, et al. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- 21.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Hughes CC, Savage CO, Pober JS. Endothelial cells augment T cell interleukin 2 production by a contact-dependent mechanism involving CD2/LFA-3 interaction. J Exp Med. 1990;171:1453–1467. doi: 10.1084/jem.171.5.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi X, Franko B, Frantz C, Amin HM, Lai R. JSI-124 (cucurbitacin I) inhibits Janus kinase-3/signal transducer and activator of transcription-3 signalling, downregulates nucleophosmin-anaplastic lymphoma kinase (ALK), and induces apoptosis in ALK-positive anaplastic large cell lymphoma cells. Br J Haematol. 2006;135:26–32. doi: 10.1111/j.1365-2141.2006.06259.x. [DOI] [PubMed] [Google Scholar]
- 24.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 25.Kreisel D, et al. Vascular endothelium does not activate CD4+ direct allorecognition in graft rejection. J Immunol. 2004;173:3027–3034. doi: 10.4049/jimmunol.173.5.3027. [DOI] [PubMed] [Google Scholar]
- 26.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann MF, et al. Distinct roles for LFA-1 and CD28 during activation of naive T cells: Adhesion versus costimulation. Immunity. 1997;7:549–557. doi: 10.1016/s1074-7613(00)80376-3. [DOI] [PubMed] [Google Scholar]
- 28.Kandula S, Abraham C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J Immunol. 2004;173:4443–4451. doi: 10.4049/jimmunol.173.7.4443. [DOI] [PubMed] [Google Scholar]
- 29.Ghannam S, Pène J, Torcy-Moquet G, Jorgensen C, Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 30.Bedke T, Pretsch L, Karakhanova S, Enk AH, Mahnke K. Endothelial cells augment the suppressive function of CD4+ CD25+ Foxp3+ regulatory T cells: Involvement of programmed death-1 and IL-10. J Immunol. 2010;184:5562–5570. doi: 10.4049/jimmunol.0902458. [DOI] [PubMed] [Google Scholar]
- 31.Durant L, et al. Diverse targets of the transcription factor STAT3 contribute to T cell pathogenicity and homeostasis. Immunity. 2010;32:605–615. doi: 10.1016/j.immuni.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nadazdin O, et al. Phenotype, distribution and alloreactive properties of memory T cells from cynomolgus monkeys. Am J Transplant. 2010;10:1375–1384. doi: 10.1111/j.1600-6143.2010.03119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leveque L, et al. Interleukin 2-mediated conversion of ovarian cancer-associated CD4+ regulatory T cells into proinflammatory interleukin 17-producing helper T cells. J Immunother. 2009;32:101–108. doi: 10.1097/CJI.0b013e318195b59e. [DOI] [PubMed] [Google Scholar]
- 34.Eissner G, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: Protective effect of defibrotide. Blood. 2002;100:334–340. doi: 10.1182/blood.v100.1.334. [DOI] [PubMed] [Google Scholar]
- 35.Shiao SL, McNiff JM, Pober JS. Memory T cells and their costimulators in human allograft injury. J Immunol. 2005;175:4886–4896. doi: 10.4049/jimmunol.175.8.4886. [DOI] [PubMed] [Google Scholar]
- 36.Lu SX, et al. STAT-3 and ERK 1/2 phosphorylation are critical for T-cell alloactivation and graft-versus-host disease. Blood. 2008;112:5254–5258. doi: 10.1182/blood-2008-03-147322. [DOI] [PMC free article] [PubMed] [Google Scholar]