Abstract
Chronic endoplasmic reticulum (ER) stress was recently revealed to affect hypothalamic neuroendocrine pathways that regulate feeding and body weight. However, it remains unexplored whether brain ER stress could use a neural route to rapidly cause the peripheral disorders that underlie the development of type 2 diabetes (T2D) and the metabolic syndrome. Using a pharmacologic model that delivered ER stress inducer thapsigargin into the brain, this study demonstrated that a short-term brain ER stress over 3 d was sufficient to induce glucose intolerance, systemic and hepatic insulin resistance, and blood pressure (BP) increase. The collection of these changes was accompanied by elevated sympathetic tone and prevented by sympathetic suppression. Molecular studies revealed that acute induction of metabolic disorders via brain ER stress was abrogated by NF-κB inhibition in the hypothalamus. Therapeutic experiments further revealed that acute inhibition of brain ER stress with tauroursodeoxycholic acid (TUDCA) partially reversed obesity-associated metabolic and blood pressure disorders. In conclusion, ER stress in the brain represents a mediator of the sympathetic disorders that underlie the development of insulin resistance syndrome and T2D.
During the recent two decades, the epidemic of type 2 diabetes (T2D) has reached an explosive scale in the United States and many other developed countries. The risk factors for the development of T2D include a group of prognostic disorders known collectively as insulin resistance syndrome, which manifests frequently in the form of glucose intolerance, insulin resistance, dyslipidemia, and blood pressure (BP) increase in association with aging and obesity. As broadly documented in the literature (1–7), all of these disorders are characterized by the existence of stress and inflammatory molecules in the circulation and various tissues. Although it is still poorly understood how all these pathophysiological changes are etiologically connected, a variety of intracellular stresses have been proposed as primary pathogenic factors (8, 9). These advances have included the recent understanding on endoplasmic reticulum (ER) stress (10–12), a set of intracellular molecular responses when the ER fails to adapt to various physiological or pathological conditions that challenge the normal functions of ER. Under diabetes-prone environmental changes, induction of ER stress was reported to occur in insulin-secreting pancreatic β-cells (13–15) and various insulin-responsive peripheral tissues (16–18), which together cause the compromised regulation of glucose homeostasis by insulin. Most recently, chronic ER stress was revealed to occur in the hypothalamus under conditions of nutritional excess and cause hypothalamic hormonal (leptin and insulin) defects that promote feeding and weight gain (19, 20). Such effects of chronic brain ER stress are predicted to incur long-term pathological changes that contribute to T2D in a manner which is secondary to weight gain and obesity (19, 20).
The central nervous system (CNS), in particular, the comprised hypothalamus, provides key regulations on various metabolic processes of the body. Recent research has elucidated multiple endocrine pathways that mediate hypothalamic control of feeding and body weight (21–23). On the other hand, the CNS, including the hypothalamus, critically controls the autonomic nervous system outflow, which has acute effects on physiology, such as metabolism (24). The neural pathways of the CNS may explain the recent observations that pharmacologic manipulation of the hypothalamus can rapidly alter glucose metabolism in the peripheral tissues without the involvement of feeding or body weight change (25–29). Thus, the development of T2D conceivably involves a neural mechanism that alters peripheral metabolism in a body weight (obesity)-independent manner; however, investigation of such mediators in the CNS still remains insufficient. Notably, ER stress has been known as an inducer of various pathological changes not only in peripheral metabolic tissues (10–12) but also in the brain (30). Very recently, ER stress in the hypothalamus was shown to promote appetite and weight gain to contribute to obesity-associated diseases (19, 20). In light of other research that has revealed the action of the hypothalamus in acute regulation of peripheral metabolism (25–29), the present study investigated whether brain ER stress could have an acute, neural effect on peripheral physiology to direct the development of T2D and the metabolic syndrome.
Results
Pharmacologic Induction of Brain ER Stress in Mice.
Development of ER stress involves multiple steps of intracellular molecular pathways, including a pathway directed by inositol-requiring enzyme 1 (IRE-1) and X-box–binding protein 1 (XBP-1), a pathway directed by activating transcription factor 6 (ATF-6), and a pathway directed by protein kinase R-like ER kinase (PERK) and eukaryotic translation initiation factor 2α subunit (eIF2α). Because of the complexity of ER stress cascades, genetic methods that chronically created ER stress in animals often introduced confounding pathophysiological changes (31, 32). Alternatively, pharmacologic strategies are useful to acutely induce ER stress in cultured cells and animals. Thapsigargin (TG), a classical ER stress-inducing chemical, has been extensively used to rapidly induce tissue ER stress through peripheral injection (such as i.p.) in different animal species, including rodents (33–35). In this research, we performed intrabrain injection of TG via cannula preimplanted into the ventral third ventricle, which is anatomically adjacent to the hypothalamus. Normal C57BL/6 mice received a single injection of TG (1.0 μg), and the hypothalamus and other brain regions were harvested at 0, 2, 4, and 8 h postinjection for Western blot analysis of two ER stress indicators, phosphorylated IRE-1α and phosphorylated PERK. Data revealed that TG increased hypothalamic phosphorylation levels of both IRE-1α and PERK between 2 and 4 h postinjection, whereas these effects diminished at 8 h postinjection (Fig. S1). ER stress induction in other brain regions ranged from modest to negligible levels. In sum, TG injection via the ventral third ventricle can induce a short-term ER stress in the brain and offers a pharmacologic model to study the roles of short-term ER stress that are generated in the brain.
Acute Induction of Systemic Insulin Resistance by Brain ER Stress.
Recent literature has reported that hypothalamic ER stress can interfere with the feeding-inhibitory action of leptin (19, 20). Herein, we explored whether short-term brain ER stress induced by TG could affect feeding and body weight of mice. Through preimplanted brain cannula, normal chow-fed C57BL/6 mice received 3 consecutive d of TG injections at the dose of 0, 0.3, 0.6, or 1.0 μg/d. Food intake levels of injected mice were monitored at 2, 4, 16, and 24 h postinjection on each day. Compared with vehicle injection, only the highest dose of TG (1.0 μg) resulted in promotion of food intake between 2 and 4 h postinjection (Fig. S2A). The ∼16- to 24-h food intake was, however, comparable between TG- and vehicle-treated mice, which was consistent with the time course of ER stress induction by the chemical injection (Fig. S1). In line with the 24-h feeding, the protocol of 3-d TG treatment did not significantly affect body weight of the mice (Fig. S2B). Also, adiposity was similar between TG- and vehicle-treated mice, assessed with MRI scanning (fat mass: 3.8 ± 0.3 g vs. 3.0 ± 0.5 g, P = 0.3, and lean mass: 21.2 ± 0.5 g vs. 20.9 ± 1.2 g, P = 0.8, in vehicle- vs. TG-treated mice). Thus, the previously proposed influence of brain ER stress on feeding and body weight imbalance (19, 20) should require accumulative effects of chronic brain ER stress, which was not offered by the acute, short-term brain ER stress in this experiment. In recognition of recent literature that demonstrated that the brain control of body weight and systemic glucose metabolism is dissectible (25–29), we examined whether short-term ER stress in the brain could rapidly affect peripheral glucose metabolism. Mice that received 3 d of TG treatment at various doses were subjected to glucose tolerance test (GTT). As shown in Fig. 1 A and B, there was a significant induction of glucose intolerance in the mice that received 3 d of TG injections at the dose of 0.6 or 1.0 μg/d. Insulin tolerance test (ITT) was also performed, and data revealed that TG treatment at the dose of 0.6 or 1.0 μg/d significantly blunted the hypoglycemic effect of insulin injection (Fig. 1 C and D). In addition, we measured blood insulin levels of the mice and found that TG treatment (0.6 or 1.0 μg/d) significantly increased fasting insulin levels (Fig. 1E). Together, these data suggest that brain ER stress acutely causes systemic insulin resistance and glucose intolerance.
Fig. 1.
Short-term brain ER stress causes systemic insulin resistance. (A–E) C57BL/6 mice received daily third-ventricle injections of TG at the doses of 0.3, 0.6, or 1.0 μg/d for 3 consecutive d. Vehicle injection was used as negative control and is labeled as 0 in the bar graphs. Mice were fasted overnight before receiving the final injection on day 3 and were examined at 2 h postinjection for GTT (A and B), ITT (C and D), or plasma insulin levels (E). Area under the curve (AUC) data for GTT (B) and ITT (D) were calculated. ITT data are presented as the percentages of time-course blood glucose levels over the baseline level (C) and the percentage changes of AUC over the control (vehicle injection) group (D). *P < 0.05; **P < 0.01; n = 8–9 mice per group. (F–H) Mice injected with TG (1.0 μg/d) or vehicle for 3 consecutive d were anesthetized and challenged with insulin (INS) (5.0 units/kg) or saline for 3 min via the inferior vena cava. Liver samples were rapidly harvested and analyzed for insulin signaling with immunoprecipitation (IP) and Western blots. (G) Tyrosine phosphorylation (p-Tyr) of IRβ and IRS2, the binding of p85 to IRS2, and phosphorylated Akt (p-Akt) were quantitatively normalized by the total protein levels (Total) of IRβ, IRS2, p85, and Akt, respectively. β-actin (β-act) was used as an internal control. (H) Liver tissues were harvested from mice that received 3-d TG vs. vehicle treatment and analyzed for mRNA levels of gluconeogenic enzymes pepck and g6pase with real-time RT-PCR. *P < 0.05; **P < 0.01; n = 4–6 mice per group; AU, arbitrary unit. (Error bars reflect mean ± SEM.)
Brain ER Stress Impairs Hepatic Insulin Signaling.
The neural connection between the hypothalamus and the liver plays an important role in the CNS control of systemic glucose homeostasis (24, 26). Suggested by this knowledge, we tested whether short-term TG treatment could be sufficient to affect hepatic insulin signaling. In the experiment, mice received 3 d of TG injections via third-ventricle cannula and were subjected to an i.v. injection of insulin, and liver tissues were collected for Western blot analysis of insulin signaling cascade at various molecular levels. As shown in Fig. 1 F and G, TG injection (1.0 μg/d) significantly impaired insulin-induced tyrosine phosphorylation of insulin receptor (IR) and IR substrate 2 (IRS2) in the liver. Because of the reduced tyrosine phosphorylation, the recruitment of PI3-kinase regulatory subunit p85 by IRS2 significantly decreased (Fig. 1 F and G). As a result, Akt phosphorylation, which represents a key functional step downstream of PI3-kinase, was poorly induced by insulin (Fig. 1 F and G). Furthermore, because hepatic insulin signaling is known to suppress gene expression of gluconeogenic enzymes, we tested whether the induction of hepatic insulin resistance by brain ER stress could be accompanied by elevated gene expression of gluconeogenic enzymes, including phosphoenolpyruvate carboxykinase (pepck) and glucose-6-phosphatase (g6pase). Using real-time RT-PCR, we observed that mRNA levels of pepck and g6pase significantly increased in mice treated with TG compared with the control mice (Fig. 1H). Altogether, these findings suggest that brain ER stress can mediate systemic insulin resistance by inhibiting hepatic insulin signaling, and this effect is dissociable from obesity.
Brain ER Stress Elevates BP Levels.
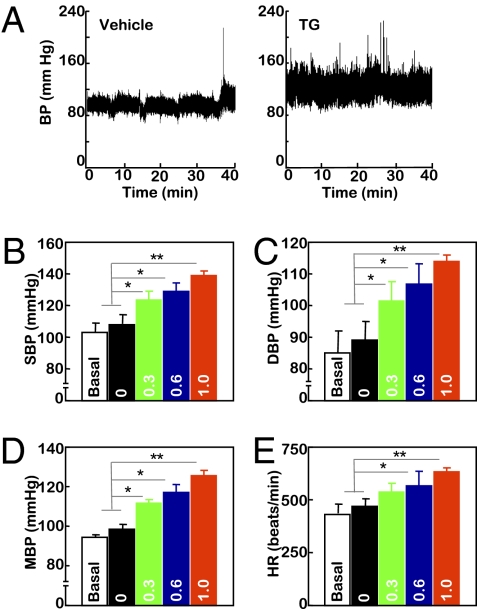
BP increase represents another component of insulin resistance syndrome. To more extensively examine the role of brain ER stress in insulin resistance syndrome, the experimental model established in Fig. 1 was used to test whether brain ER stress could acutely affect BP. Normal C57BL/6 mice received third-ventricle cannulation followed by radio-transmitter probe implantation for continuous BP monitoring. After postsurgery recovery, mice were adapted to third-ventricle injection via preimplanted cannula until the BP levels were not affected by the injection procedure per se. Subsequently, mice received daily injections of TG at the dose of 0, 0.3, 0.6, or 1.0 μg/d for 3 consecutive d, and BP monitoring continued throughout the 3-d treatment period. As shown in Fig. 2 A–E, TG treatment increased BP levels of the mice in a dose-dependent manner. When the injection dose increased to 1.0 μg/d, there was a profound increase of BP, with systolic, diastolic, and mean BP values elevated by 29.84, 24.77, and 27.46 mmHg, respectively. Such effects tended to be detected on day 2 but not on day 1. In summary, short-term induction of brain ER stress in mice was sufficient to induce BP elevation, another pathological aspect of the insulin resistance syndrome that underlies T2D.
Fig. 2.
Short-term brain ER stress elevates BP levels in mice. Mice with third-ventricle cannulation were implanted with BP radio transmitter in the carotid artery for continuous telemetric monitoring of BP and heart rates (HR). TG at the doses of 0.3, 0.6, or 1.0 μg/d or control vehicle (labeled as 0 in the bar graphs) was injected for 3 consecutive d. BP and HR of mice were obtained before the 3-d treatment period to obtain the baseline levels (labeled as Basal) and continuously monitored during the 3-d treatment period. (A) Representative profiles of BP in response to 1.0 μg of TG (Right) vs. vehicle (Left) injection. (B–E) Data represent average BP and HR values over a 2-h period after the final injection on day 3. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP. *P < 0.05; **P < 0.01; n = 4 mice per group. (Error bars reflect mean ± SEM.)
Brain ER Stress Up-Regulates the Sympathetic Tone in the Periphery.
The induction of both glucose intolerance and BP elevation by short-term brain ER stress may suggest a rapid neural modulation of the involved physiology. Hence, we decided to test whether the metabolic effects of short-term brain ER stress could be driven by a shift of the sympathetic nervous system activity toward sympathetic excitation. C57BL/6 mice received an intraventral third-ventricle injection of TG (0.3 μg/d) via preimplanted cannula for 3 consecutive d, and at 2 h after the last TG injection, the spontaneous renal sympathetic nerve activities were recorded. It was observed that the TG treatment led to a significantly higher mean frequency of firing rate (mean ± SD: 144.0 ± 15.4 spikes per s) compared with vehicle-treated controls (mean ± SD: 129.0 ± 15.9 spikes per s) (P < 0.001; Fig. 3 A and B). Also, the spike amplitude was significantly larger in TG-treated animals (mean ± SD: 46.7 ± 26.8 μV) than controls (mean ± SD: 29.0 ± 17.4 μV) (P < 0.001; Fig. 3 A and C). In addition to this electrophysiological study, we measured the plasma levels of norepinephrine, which is a known biochemical indicator of the sympathetic activity. As shown in Fig. 3D, the 3-d TG treatment increased plasma norepinephrine levels by nearly threefold. Thus, both electrophysiological and biochemical data suggest that brain ER stress up-regulates the peripheral sympathetic tone, which may represent an underlying basis for the metabolic and BP-raising effects of short-term brain ER stress.
Fig. 3.
Sympathetic activation in mice with short-term brain ER stress. (A–D) Mice received daily injections of TG (0.3 μg/d) or vehicle for 3 consecutive d. (A–C) At 2 h after the final injection on day 3, animals were anesthetized, and RSNA was recorded (A). n = 3–4 mice per group. The frequency of firing (B) and the spike amplitude (C) were both significantly different between TG- and vehicle-treated groups (P < 0.001). (D) Plasma norepinephrine (NE) concentrations of mice after 3-d TG (1.0 μg) or vehicle treatment. *P < 0.05; n = 7 mice per group. (E and F) C57BL/6 mice received daily third-ventricle injections of TG vs. vehicle (Veh) for 3 consecutive d, and the final injection was provided to overnight-fasted mice in combination with i.p. injection of prazosin (Prz, 1 mg/kg). At 2 h postinjection, mice were subjected to GTT (E) or blood sampling to measure plasma insulin concentrations (F). Data presented for GTT are the AUC values. NS, not significant; n = 8–9 mice per group. (G–I) Mice preimplanted with third-ventricle cannula and artery BP telemetric probes received daily third-ventricle injections of TG vs. vehicle (Veh) for 3 consecutive d, and the final injection was provided in combination with (+) or without (−) i.p. injection of prazosin (Prz, 1 mg/kg). Data represent average BP values over a 2-h period after the final injection. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP. *P < 0.05; n = 4 mice per group. (Error bars reflect mean ± SEM.)
Adrenergic Blocker Reverses the Systemic Effects of Brain ER Stress.
We subsequently examined whether sympathetic up-regulation indeed represents a mechanism for the metabolic and BP-raising effects of brain ER stress. In the literature, the α-adrenergic blocker prazosin has been used to assess the sympathetic controls of glucose metabolism (36) and BP (37). Thus, we tested if this chemical could abrogate the induction of glucose intolerance and BP elevation by brain ER stress. C57BL/6 mice received third-ventricle injections of TG (1.0 μg/d) for 3 consecutive d, and prazosin (1 mg/kg, i.p.) was administrated before the final TG injection on day 3. Mice were subjected to GTT using the same protocol in Fig. 1 A and B. Data revealed that prazosin completely prevented TG from causing glucose intolerance (Fig. 3E). Consistently, in the presence of prazosin, TG failed to increase the plasma insulin levels in the mice (Fig. 3F). Both sets of data confirmed that prazosin indeed abrogated the effect of TG treatment in causing systemic insulin resistance. In addition, telemetric BP monitoring revealed that prazosin significantly attenuated the hypertensive effect of TG treatment (Fig. 3 G–I). In sum, sympathetic up-regulation in the periphery underlies the metabolic and BP-raising effects of brain ER stress.
Activation of Hypothalamic NF-κB by Brain ER Stress.
Numerous reports have demonstrated that the induction and pathological actions of ER stress are associated with inflammation (10, 11, 38). Our recent work has linked ER stress to inflammatory nuclear transcription factor NF-κB in the hypothalamus and proposed this link as a potential basis for neural inflammation that is associated with obesity-induced T2D (19). In this study, we were interested in profiling how NF-κB in the hypothalamus might react to the pharmacologic induction of ER stress. Normal C57BL/6 mice received a single dose of TG injection (1.0 μg) via preimplanted third-ventricle cannula, and hypothalami were harvested at 0, 2, 4, and 8 h after TG injection for Western blot analysis of NF-κB signaling. As shown in Fig. 4 A and B, phosphorylation levels of NF-κB subunit RelA significantly increased at 2 and 4 h after TG injection. Given that IκBα phosphorylation and its subsequent degradation are required for the activation of the classical NF-κB pathway, we also examined the phosphorylation and protein levels of IκBα. It was revealed that IκBα phosphorylation increased while its total protein levels decreased in the hypothalamus during the time course of 2–4 h after TG injection (Fig. 4 A and B). The effects of TG injection on NF-κB signaling substantially decreased at 8 h after TG injection (Fig. 4 A and B), which correlated with the time course of brain ER stress after TG injection (Fig. S1). Thus, brain ER stress induced by TG activated NF-κB in the hypothalamus. Because a single injection of TG led to hypothalamic NF-κB activation, NF-κB might be mechanistically involved in the disease action of short-term brain ER stress.
Fig. 4.
ER stress activates hypothalamic NF-κB to affect peripheral physiology. (A and B) C57BL/6 mice received a single injection of TG (1.0 μg) via preimplanted cannula. Hypothalami were harvested for Western blot analysis of NF-κB signaling at the indicated time points after TG injection. Phosphorylated RelA (p-RelA), phosphorylated IκBα (p-IκBα), and total protein levels of IκBα were measured to reflect NF-κB activities. Vehicle injection was used as the negative control (labeled as 0 h). β-actin (β-act) was used as an internal control. (B) p-RelA and p-IκBα levels were normalized by the total protein levels of RelA and β-actin, respectively. *P < 0.05; **P < 0.01; n = 4–6 mice per group; AU, arbitrary unit. (C) Mice received intra-arcuate injections of adenoviruses expressing either HA-tagged DNIκBα or GFP. At 1 wk postinjection, hypothalamic sections were immunostained for GFP (Upper) and HA (Lower) to verify the site-specific gene delivery. Nuclear staining with DAPI (blue) labels all of the cells in the sections and is merged with GFP (green) or HA (red) staining. Arc, arcuate nucleus; 3V, third ventricle. (Scale bar = 50 μm.) (D and E) Intra-Arc DNIκBα or GFP adenovirus-injected mice received daily injections of TG (1 μg/d) or vehicle (Veh) via third-ventricle cannula for 3 consecutive d. At 2 h after the final injection, mice were examined for GTT. (E) AUC data for GTT. *P < 0.05; n = 6–10 mice per group. (F) DNIκBα or GFP adenovirus-injected mice were implanted with third ventricle cannula and intra arterial telemetric BP probe. BP levels were monitored daily for 3 d before and after TG vs. vehicle injection. Data presented are average mean BP (MBP) values over a 2-h period after the final injection on day 3. *P < 0.05; n = 4 mice per group. (Error bars reflect mean ± SEM.)
NF-κB Inhibition Reverses the Systemic Effects of Brain ER Stress.
To examine whether hypothalamic NF-κB could mediate the disease outcomes of brain ER stress, we used our established viral injection approach (19) to generate mice with virus-mediated NF-κB inhibition in the hypothalamic arcuate nucleus. Dominant-negative IκBα (DNIκBα), which has been established as NF-κB–specific inhibitor (39, 40), was used to inhibit the NF-κB activity. Normal C57BL/6 mice received intra-arcuate injections of adenoviruses expressing HA-tagged DNIκBα or GFP, immediately followed by third-ventricle implantation of cannula. As verified in Fig. 4C, virus-mediated exogenous gene expression of HA-tagged DNIκBα or GFP was induced specifically in the arcuate nucleus. We further used Western blot analysis to evaluate hypothalamic ER stress and NF-κB activities of these mice in response to third-ventricle TG injection. In GFP adenovirus-injected control mice, TG injection markedly induced hypothalamic ER stress and NF-κB activation (Fig. S3). The profound induction of ER stress and NF-κB activation was primarily attributed to the pharmacologic effect of TG because the nonspecific effect caused by adenoviral injection procedure per se was small (Fig. S3). However, arcuate delivery of DNIκBα prevented the up-regulation of hypothalamic NF-κB by TG (Fig. S3). GFP vs. DNIκBα adenovirus-injected mice received 3-d treatment of TG (1.0 μg/d) or vehicle via preimplanted third-ventricle cannula as described in Fig. 1 and were subsequently subjected to GTT. TG treatment impaired glucose tolerance in GFP adenovirus-injected mice (Fig. 4 D and E), which was similar to the observation in Fig. 1 A and B. In contrast, DNIκBα expression in the arcuate nucleus prevented the induction of glucose intolerance by TG treatment (Fig. 4 D and E). These protective effects were unrelated to the body weight of these mice (Fig. S4). Additional experiments using telemetry showed that TG significantly increased BP levels in GFP adenovirus-injected mice but not in DNIκBα adenovirus-injected mice (Fig. 4F). These results together suggested that hypothalamic NF-κB worked as a mediator of the peripheral effects of short-term brain ER stress.
Inhibition of Brain ER Stress Acutely Reverses Insulin Resistance Syndrome.
Finally, we explored whether acute suppression of brain ER stress could rapidly reverse obesity-associated glucose and BP disorders, given that obesity induced through chronic high-fat diet (HFD) feeding was accompanied by the induction of brain/hypothalamic ER stress (Fig. S5). In the experiment, C57BL/6 mice with HFD-induced obesity received two separate third-ventricle injections of the ER stress inhibitor TUDCA (1 μg) or vehicle, given at the beginning and the end of an overnight fasting, respectively. At 2 h after the second injection, mice were subjected to metabolic profiling. As predicted, HFD-fed mice displayed glucose intolerance, insulin insensitivity, and hyperinsulinemia (Fig. 5 A–E). However, TUDCA treatment significantly, although partially, reversed these metabolic disorders (Fig. 5 C–G). Molecular analysis further demonstrated that TUDCA treatment partially improved hepatic insulin signaling (Fig. 5 F and G) and significantly reduced the mRNA levels of hepatic gluconeogenic enzymes (Fig. 5H) in HFD-fed mice. Subgroups of the mice were subjected to telemetry, and data revealed that TUDCA treatment significantly decreased systolic, diastolic, and mean BP levels of HFD-fed mice (Fig. 5 I–K). Notably, because TUDCA treatment was provided only during an overnight fasting period in these experiments, the involvement of food intake was excluded. Also, the duration of the overnight period was too short to cause body weight changes between TUCDA-injected and vehicle-injected mice. In this context, the therapeutic effects of TUDCA further consolidated the finding that brain ER stress can rapidly induce neural dysregulation to mediate the development of T2D and related diseases.
Fig. 5.
Acute reversal of obesity-related disorders by inhibiting brain ER stress. Male C57BL/6 mice were maintained on normal chow (Chw) or HFD for 5 mo, since 2 mo of age, and then implanted with third-ventricle cannula. After 2 wk of postoperative recovery, mice received third-ventricle injection of 1.0 μg of TUDCA (TU) or vehicle (Veh) on the night of day 1, followed by overnight fasting and a second injection of TUDCA or vehicle in the morning of day 2. (A–E) Mice at 2 h postinjection on day 2 were separately subjected to GTT (A and B), ITT (C and D), and plasma insulin measurement (E). AUC data of GTT (B) and ITT (D) are presented. ITT data are presented as the percentages of time-course blood glucose over the baseline levels (C) and the percentages of AUC values of treatment groups over the control group (D). *P < 0.05; **P < 0.01; n = 8–9 mice per group. (F and G) Mice at 2 h postinjection on day 2 were anesthetized and challenged with insulin (INS; 5.0 units/kg) or saline for 3 min via abdominal aorta injection. Liver samples were rapidly harvested and analyzed for insulin signaling with immunoprecipitation (IP) and Western blotting. (G) Phosphorylated IRβ (p-IRβ), phosphorylated IRS2 (p-IRS2), binding of p85 to IRS2, and phosphorylated Akt (p-Akt) were quantitatively normalized by the total protein levels (Total) of IRβ, IRS2, p85, and Akt, respectively. β-actin (β-act) was used as an internal control. **P < 0.01; n = 4–6 mice per group; AU, arbitrary unit. (H) Liver tissues were harvested from chow-fed versus HFD-fed mice after 2-d TUDCA or vehicle treatment and analyzed for mRNA levels of pepck and g6pase. *P < 0.05; n = 4–6 mice per group; AU, arbitrary unit. (I–K) Mice were continuously monitored for BP and heart rates (HR) via preimplanted artery BP telemetric probes. Data presented represent average BP values over a 2-h period after TUDCA or vehicle injection on day 2. SBP, systolic BP; DBP, diastolic BP; MBP, mean BP. *P < 0.05; **P < 0.01; n = 4 mice per group. (Error bars reflect mean ± SEM.)
Discussion
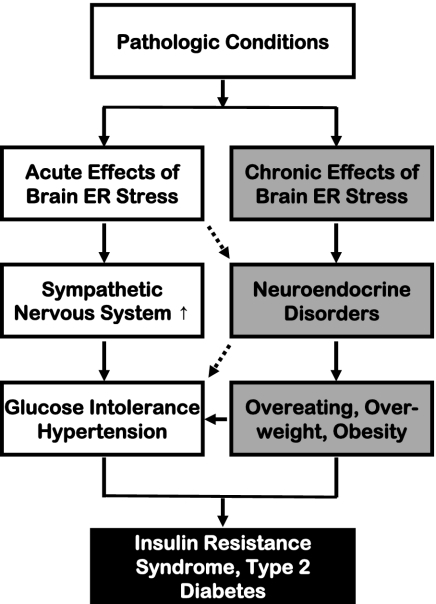
ER stress can be induced by aberrant changes in various intracellular processes, such as protein folding/modification, redox balance, energy consumption, and Ca2+ regulation. The pathogenic roles of chronic ER stress have been identified in neurodegenerative diseases that are characterized by excessive intracellular accumulation and aggregation of misfolded proteins, as seen in Alzheimer's disease, Parkinson disease, Huntington disease, and amyotrophic lateral sclerosis (30, 41, 42). Unexpectedly, recent studies using several genetic models of ER stress, including PERK knockout mice (32), eIF2α knock-in mice (31), and XBP-1 knockout mice (17), revealed that these mice manifested diabetes or prediabetes symptoms that were related to defects in insulin secretion and actions caused by local ER stress. Classically, neurological diseases and metabolic diseases (such as T2D) involve distinct biological/physiological disciplines, but recent attention to the epidemic coexistence of neurodegenerative diseases and T2D has challenged this traditional view (43). Along with this knowledge, the CNS was recently recognized as a critical regulator of not only feeding/body weight but also peripheral insulin–glucose homeostasis (25–29). Using pharmacologic approaches, the current study has revealed that short-term brain ER stress up-regulates the sympathetic pathway to rapidly cause several key components of the so-called metabolic syndrome, including glucose intolerance, insulin resistance, and BP increase. Compared with genetic ER stress models, our pharmacologic approach dissected the physiological outcomes of brain ER stress without the impact of chronic feeding or body weight changes. Hence, this study reveals a paradigm by which brain ER stress uses the sympathetic route to acutely and directly mediate a cluster of peripheral metabolic disorders associated with T2D and related problems (Fig. 6). This finding complements the recent research which has proposed that chronic brain ER stress causes neuroendocrine dysfunctions (such as central leptin and insulin resistance) to mediate eating and body weight disorders (19, 20).
Fig. 6.
The models of acute versus chronic brain ER stress in obesity/T2D syndrome. Pathological conditions associated with obesity and T2D induce both acute and chronic ER stress in the brain. Acute brain ER stress rapidly up-regulates the sympathetic nervous system activity to cause peripheral insulin resistance, glucose intolerance, and related BP dysregulation. Chronic brain ER stress, on the other hand, disrupts the hypothalamic neuroendocrine functions to cause eating disorder, overweight, and obesity. These neural and neuroendocrine processes are physiologically interconnected and are affecting each other at multiple levels; the combined effects of these two processes represent a significant CNS basis for body weight-independent and body weight-dependent development of T2D and related problems.
Although the pharmacologic approach that delivered ER stress modulators via the brain ventricle should have broad effects in various brain regions, the involvement of the hypothalamus deserves attention. Indeed, the pharmacologic induction of ER stress was prominent in the hypothalamus, and the disease effects of brain ER stress were substantially reversible by hypothalamic NF-κB inhibition. Further, the ER stress–NF-κB connection revealed by the acute models in this study agrees with the knowledge that NF-κB can work as a downstream player of chronic ER stress (19, 44). All these understandings together can highlight the susceptibility of the hypothalamus to stress and inflammation. Nevertheless, it remains an important topic regarding whether and how the neural mechanisms of T2D and related disease require ER stress in other brain regions, in particular the regions that closely interact with the sympathetic nervous system. Answering these questions would call for further investigations.
Materials and Methods
Animal Manipulations and Analyses.
Adult male C57BL/6 mice (Jackson Laboratory) were implanted with a guide cannula into the third ventricle following the method described previously (19). TG and TUDCA were injected at a volume of 2 μL through the preimplanted cannula. Prazosin was injected intraperitonially. Intra-arcuate injections of Ad5-CMV–driven DNIκBα and Ad5-CMV–driven GFP were performed according to the technique established in our previous research (19). GTT, ITT, and measurements of body weight and food intake were performed at the indicated time points. Plasma insulin and norepinephrine were measured by ELISA. Transcardial perfusion, brain sectioning, immunostaining, and imaging were performed according to the previously established protocol (19). For details see SI Materials and Methods.
Telemetric Measurement of BP.
BP of conscious mice was recorded with a radiotelemetry monitoring system (Data Sciences International) by preimplanting a pressure sensor in the carotid artery following standard procedures (45). For radiotelemetric probe (model TA11PA-C10; DSI) implantation, mice were anesthetized, and the left common carotid artery was separated. After ligating one end of the artery (right below the carotid bifurcation) with 4-0 suture (Ethicon) and occluding the distal end with a microclip, a small incision was made near the proximal ligated end, and the pressure transmission catheter was guided into the artery and secured optimally in place with sutures. The radio telemetric transmitter attached to the catheter was passed s.c. and inserted into a s.c. pocket formed by a blunt dissection in the right flank. Mice were allowed 1–2 wk for postsurgical recovery. BP levels were continuously recorded at a sampling rate of 2,000 Hz over a 300-s segment duration.
Measurement of Renal Sympathetic Nerve Activity (RSNA).
Left renal nerves were isolated in anesthetized mice and RSNA was recorded by pure iridium microelectrode. Amplified, filtered and digitized signals were fed into a PowerLab data acquisition system and LabChart 7 software (AD Instruments) for acquisition and data analysis. For details, see SI Materials and Methods.
Immunoprecipitation, Western Blotting, and Real-Time RT-PCR.
Liver tissues collected from anesthetized mice with 3-min insulin (5 units/kg) or vehicle stimulation via inferior vena cava, were used for immunoprecipitation and Western blotting following the method previously described (19, 40). For Real-time RT-PCR, cDNA synthesized from total RNA was PCR amplified and quantified with SYBR Green PCR MasterMix (Applied Biosystems). For details, see SI Materials and Methods.
Statistical Analyses.
Student's t tests were used for comparisons involving only two groups. ANOVA and appropriate post hoc analyses were used for comparisons involving more than two groups. Data were presented as mean ± SEM. P < 0.05 was considered significant.
Acknowledgments
This study was supported by National Institutes of Health Grants R01 DK078750 and R01 AG031774 and American Diabetes Association Junior Faculty Award 1-07-JF-09 (all to D.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006875108/-/DCSupplemental.
References
- 1.Ruan H, Lodish HF. Regulation of insulin sensitivity by adipose tissue-derived hormones and inflammatory cytokines. Curr Opin Lipidol. 2004;15:297–302. doi: 10.1097/00041433-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 3.Lehrke M, Lazar MA. Inflamed about obesity. Nat Med. 2004;10:126–127. doi: 10.1038/nm0204-126. [DOI] [PubMed] [Google Scholar]
- 4.Shoelson SE, Goldfine AB. Getting away from glucose: Fanning the flames of obesity-induced inflammation. Nat Med. 2009;15:373–374. doi: 10.1038/nm0409-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cai D. NFκB-mediated metabolic inflammation in peripheral tissues versus central nervous system. Cell Cycle. 2009;8:2542–2548. doi: 10.4161/cc.8.16.9386. [DOI] [PubMed] [Google Scholar]
- 6.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: Modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petersen KF, Shulman GI. Etiology of insulin resistance. Am J Med. 2006;119(5 Suppl 1):S10–S16. doi: 10.1016/j.amjmed.2006.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cifuentes ME, Pagano PJ. Targeting reactive oxygen species in hypertension. Curr Opin Nephrol Hypertens. 2006;15:179–186. doi: 10.1097/01.mnh.0000214776.19233.68. [DOI] [PubMed] [Google Scholar]
- 9.Frisard M, Ravussin E. Energy metabolism and oxidative stress: Impact on the metabolic syndrome and the aging process. Endocrine. 2006;29:27–32. doi: 10.1385/ENDO:29:1:27. [DOI] [PubMed] [Google Scholar]
- 10.Scheuner D, Kaufman RJ. The unfolded protein response: A pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glimcher LH, Lee AH. From sugar to fat: How the transcription factor XBP1 regulates hepatic lipogenesis. Ann N Y Acad Sci. 2009;1173(Suppl 1):E2–E9. doi: 10.1111/j.1749-6632.2009.04956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Back SH, et al. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metab. 2009;10:13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang CJ, et al. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated β-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 15.Sachdeva MM, et al. Pdx1 (MODY4) regulates pancreatic β cell susceptibility to ER stress. Proc Natl Acad Sci USA. 2009;106:19090–19095. doi: 10.1073/pnas.0904849106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ozcan U, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozcan U, et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Vera L, Fischer WH, Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460:534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozcan L, et al. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- 22.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 23.Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 24.Klieverik LP, et al. Thyroid hormone modulates glucose production via a sympathetic pathway from the hypothalamic paraventricular nucleus to the liver. Proc Natl Acad Sci USA. 2009;106:5966–5971. doi: 10.1073/pnas.0805355106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pocai A, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- 26.Lam TK, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 27.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 28.Morton GJ. Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol. 2007;583:437–443. doi: 10.1113/jphysiol.2007.135590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gelling RW, et al. Insulin action in the brain contributes to glucose lowering during insulin treatment of diabetes. Cell Metab. 2006;3:67–73. doi: 10.1016/j.cmet.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 30.Hetz C, et al. XBP-1 deficiency in the nervous system protects against amyotrophic lateral sclerosis by increasing autophagy. Genes Dev. 2009;23:2294–2306. doi: 10.1101/gad.1830709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scheuner D, et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 32.Harding HP, et al. Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell. 2001;7:1153–1163. doi: 10.1016/s1097-2765(01)00264-7. [DOI] [PubMed] [Google Scholar]
- 33.Chae HJ, et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15:355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 34.Timmins JM, et al. Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest. 2009;119:2925–2941. doi: 10.1172/JCI38857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yan W, et al. Control of PERK eIF2α kinase activity by the endoplasmic reticulum stress-induced molecular chaperone P58IPK. Proc Natl Acad Sci USA. 2002;99:15920–15925. doi: 10.1073/pnas.252341799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ross H, Moser M. Prazosin, diuretics, and glucose intolerance. Ann Intern Med. 1993;119:859–860. doi: 10.7326/0003-4819-119-8-199310150-00017. [DOI] [PubMed] [Google Scholar]
- 37.Khoury AF, Kaplan NM. α-Blocker therapy of hypertension: An unfulfilled promise. JAMA. 1991;266:394–398. [PubMed] [Google Scholar]
- 38.Martinon F, Chen X, Lee AH, Glimcher LH. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nat Immunol. 2010;11:411–418. doi: 10.1038/ni.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai D, et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell. 2004;119:285–298. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 40.Cai D, et al. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakagawa T, et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-β. Nature. 2000;403:98–103. doi: 10.1038/47513. [DOI] [PubMed] [Google Scholar]
- 42.Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 43.Janson J, et al. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–481. doi: 10.2337/diabetes.53.2.474. [DOI] [PubMed] [Google Scholar]
- 44.Ma Y, Hendershot LM. ER chaperone functions during normal and stress conditions. J Chem Neuroanat. 2004;28:51–65. doi: 10.1016/j.jchemneu.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Farah VM, Joaquim LF, Bernatova I, Morris M. Acute and chronic stress influence blood pressure variability in mice. Physiol Behav. 2004;83:135–142. doi: 10.1016/j.physbeh.2004.08.004. [DOI] [PubMed] [Google Scholar]