Abstract
The ventral urogenital sinus (UGS) of control male mice has two rows of 3–4 prostatic buds at birth, but how androgens regulate ventral bud (VB) number and patterning is unclear. VBs in both sexes appeared to be a mixture of prostatic and urethral buds. UGSs from Tfm male and antiandrogen (flutamide)-exposed mice had small VBs, suggesting that initiation of some VBs is androgen independent. Tfm male mice are widely considered completely androgen insensitive yet their UGSs were 5α-dihydrotestosterone (DHT)-responsive. VBs (6–8) were generally distributed bimodally on the left-right axis at both minimal and normal male androgen signaling. Yet control females and DHT-exposed Tfm males had 13–14 VBs, whose left-right distribution was fairly uniform. These results suggest that VB number and distribution respond biphasically as androgen signaling increases from minimal, and that androgens regulate bud specification. Complete VB agenesis by the selective budding inhibitor 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) required high androgen signaling.
Keywords: urogenital sinus; epithelial budding patterns; prenatal prostate and urethral gland development; bud initiation; testicular feminization (Tfm) mouse; 5α-dihydrotestosterone; 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)
INTRODUCTION
Prostate development begins in fetal mice when ductal progenitors, or buds, emerge from urogenital sinus (UGS) epithelium. Prostatic buds develop in response to androgens, which activate androgen receptors (ARs) in UGS mesenchyme (UGM). This mesenchymal AR activation elicits paracrine signals that act upon UGS epithelium (UGE) to stimulate bud formation (Cunha et al., 1987).
Prostatic buds develop in fetal males in three phases. During specification, developmental signals establish the patterns in which buds will subsequently form. The second phase, initiation, occurs when UGE cell clusters protrude outward, as buds, from the basal UGE cell layer and invade the surrounding UGM. In C57BL/6J male mice, prostatic bud initiation spans a 48 hr window of fetal development (E16.5 to E18.5), occurring first in the anterior and dorsal UGS, then in the lateral and ventral regions. The result of specification and initiation is that prostatic buds form in reproducible numbers in a bilaterally symmetrical pattern. The third and final phase of prenatal bud development is elongation. Postnatally, prostatic buds undergo ductal branching morphogenesis, canalization, and differentiation that ultimately gives rise to anterior, dorsal, lateral, and ventral prostate lobes (Sugimura et al., 1986; Cunha et al., 1987; Lin et al., 2003; Timms, 2008).
Females of many species often have a small amount of ventral prostatic tissue (Price and Williams-Ashman, 1961), and most women have small but actively secreting prostate (Zaviačič et al., 2000). Prostatic buds in female mice are generally confined to the ventral budding area, which is cranial to where the vagina intersects the UGS. These buds do not elongate nearly as much as in males, and prostate development is typically rudimentary (Turner, 1939; Raynaud, 1942a). Nevertheless, UGSs from female mice are capable of male-like prostatic budding, and female mice can develop functional ventral, dorsal, lateral, and anterior prostate lobes, if exposed sufficiently to exogenous androgens (Raynaud, 1938, 1942b; Price and Williams-Ashman, 1961).
In males the UGS also gives rise, via epithelial budding, to two bulbourethral glands and numerous urethral glands. Both develop on the pelvic urethral portion of the UGS, which is caudal to the prostatic urethra (the prostatic budding area). Bulbourethral glands are relatively large structures located near where the pelvic urethra intersects the penile urethra. Female mice also have bulbourethral glands, albeit smaller ones than in males. Urethral glands are present in both sexes in many species (Price and Williams-Ashman, 1961), and in male and androgen-exposed female mice (Hall, 1936; Turner, 1939), but we found no publication that describes urethral glands or buds in control female mice. In male mice there is no clear anatomical boundary between prostatic and urethral buds, and the reported presence of urethral glands in the prostatic area (Sugimura et al., 1985) strongly suggests that the prostatic and urethral budding areas overlap. While most prostatic buds are larger than most urethral buds, prostatic and urethral buds in male mice are similar in size and shape where the prostatic urethra meets the pelvic urethra.
How budding patterns are established in the UGS is not well understood. No reports address how bulbourethral or urethral buds are patterned. With respect to prostatic buds, there is evidence that signaling molecules such as Hoxa-13, Hoxd-13, Noggin, Tgfα, Sox9, and Wnt5a are involved (Podlasek et al., 1997,1999; Warot et al., 1997; Abbott et al., 2003; Cook et al., 2007; Thomsen et al., 2008; Allgeier et al., 2008; Huang et al., 2009). Androgen signaling is essential for prostatic bud development because Tfm male mice, which harbor a spontaneous inactivating AR mutation, have neither prostatic buds nor prostate lobes (Lyon and Hawkes, 1970; Goldstein and Wilson, 1972; Kobayashi, 1984; Mauch et al., 1985). However, it is not clear whether androgen signaling simply provides a permissive environment for prostatic bud formation to occur, or if it plays a more instructive role in determining where and how many buds form.
The ubiquitous environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters prostatic bud patterning in male fetuses. A maternal dose of TCDD on E13.5 can completely prevent ventral prostatic buds from forming in mice, displace dorsal and lateral buds towards the dorsal UGE surface, and reduce dorsolateral bud number. These effects (Lin et al., 2003) do not appear to involve changes in androgen concentrations in the UGS or alterations in responsiveness to androgenic stimulation (Ko et al., 2004b). Instead, they appear to be due to a disruption of dorsoventral patterning (Vezina et al., 2008). The present study focuses on the androgenic regulation of budding patterns in the region of the male UGS where ventral prostatic buds form, and in the corresponding area in females. This is the area most sensitive to TCDD. As shown by the effects of graded androgen exposure on UGS development in females, the ventral prostatic budding region is also the most sensitive to androgens (Greene et al., 1939).
To investigate the role of androgen signaling in ventral bud patterning, we compared bud patterns in fetal mice with three different levels of androgen signaling: (1) wild-type males, in which UGS ARs and circulating androgens are abundant, (2) wild-type females, where UGS ARs are abundant but circulating androgens are much lower than in males (vom Saal, 1989), and (3) Tfm males with a spontaneous, inactivating AR mutation that has been reported to render them incapable of forming prostatic buds (Lasnitzki and Mizuno, 1980; Kobayashi, 1984; Mauch et al., 1985; Takeda et al., 1987). Bud patterns were assessed on the day of birth, approximately 24 hr after all prostatic buds are formed in the normal male UGS. Additional experiments were conducted with UGSs from fetal mice exposed to the potent androgen 5α-dihydrotestosterone (DHT), the AR antagonist flutamide, or the ventral budding inhibitor TCDD. Scanning electron microscopy (SEM) was used to visualize the outermost UGE cell layer (Lin et al., 2003) because this is a highly effective way to detect the presence of small epithelial buds and visualize the pattern in which buds of all sizes were formed.
RESULTS
Terminology
Due to the current inability to identify every ventral bud in most UGSs as either prostatic or urethral, the terminology used in this manuscript needs to be defined. We use the term "ventral buds" to describe all buds in the area where ventral prostatic buds develop in males (and the analogous area in females and Tfm males), regardless of whether these buds are prostatic, urethral, or potentially otherwise. Similarly, "small ventral buds" and "small epithelial buds" refer only to buds that are small in comparison to ventral buds that are clearly prostatic, with no implication (unless otherwise stated) about their function or fate. The small epithelial buds present in UGSs from control females of various species are typically described as prostatic buds (e.g., Raynaud, 1942a; Timms et al., 1999). In describing our own results, "prostatic bud" is used only to refer to buds that ultimately develop into prostatic ducts. In the ventral UGS, these are the large buds in control wild-type males and androgen-exposed females.
Bud Number and Pattern in the Ventral UGS are Sexually Dimorphic and are Associated with the Level of Fetal Androgen Signaling
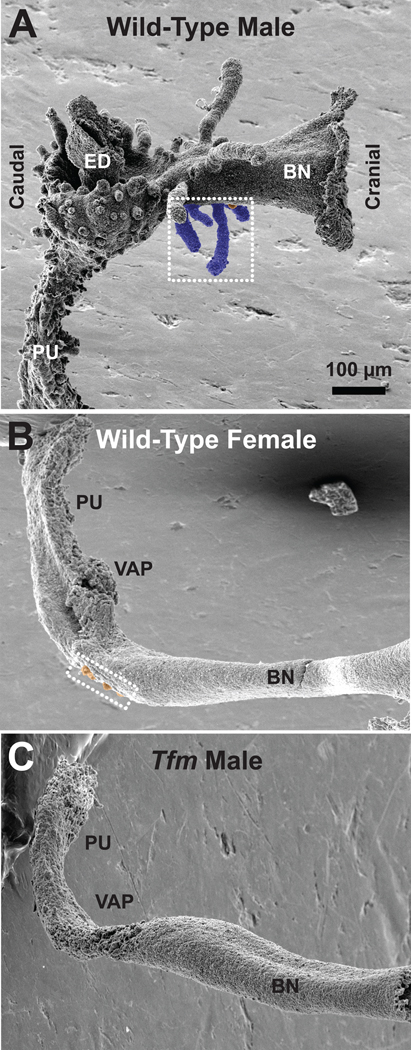
Each image in Fig. 1 is a lateral view of a control UGE, oriented ventral surface down and bladder neck to the right. Wild-type males (Fig. 1A) have ventral, dorsal, lateral, and anterior prostatic buds; ventral prostatic buds have been tinted blue. Prostatic bud diameters and especially lengths vary greatly throughout the UGS, though in prototypical patterns. Male UGSs also have two bulbourethral gland buds (not shown) and numerous urethral buds (all other buds on the pelvic urethra), but urethral bud distribution throughout the rest of the UGS is unclear. UGEs from wild-type females (Fig. 1B) and Tfm males (Fig. 1C) have a markedly different overall shape and a much smaller number of epithelial buds than do wild-type male UGEs. All their buds are short, most are only a few cells in diameter, and most are in a cluster on the ventral surface cranial to the site where the vagina attaches to the UGS. Urethral buds on the pelvic urethra are notably smaller and far fewer in number in females and Tfm males than in wild-type males. In all figures the small ventral buds have been tinted orange. To be identified as a bud, the UGE cell cluster had to be at least 2 cells in diameter (the same as the smaller dorsal prostatic buds in control males) and to protrude visibly above the UGE plane.
Fig. 1.
Scanning electron micrographs of UGS epithelium (UGE) from control wild-type male (A), control wild-type female (B), and control Tfm male (C) mice on the day of birth. All images are lateral views, taken with samples in the same orientation and at the same nominal magnification; the craniocaudal axis is as indicated. The ventral budding region is contained within the white dotted rectangle. Small buds on the ventral UGE surface are tinted orange, while the large ventral prostatic buds found in control males are tinted blue. BN: Bladder neck; ED: ejaculatory duct; PU: pelvic urethra; VAP: vaginal attachment point.
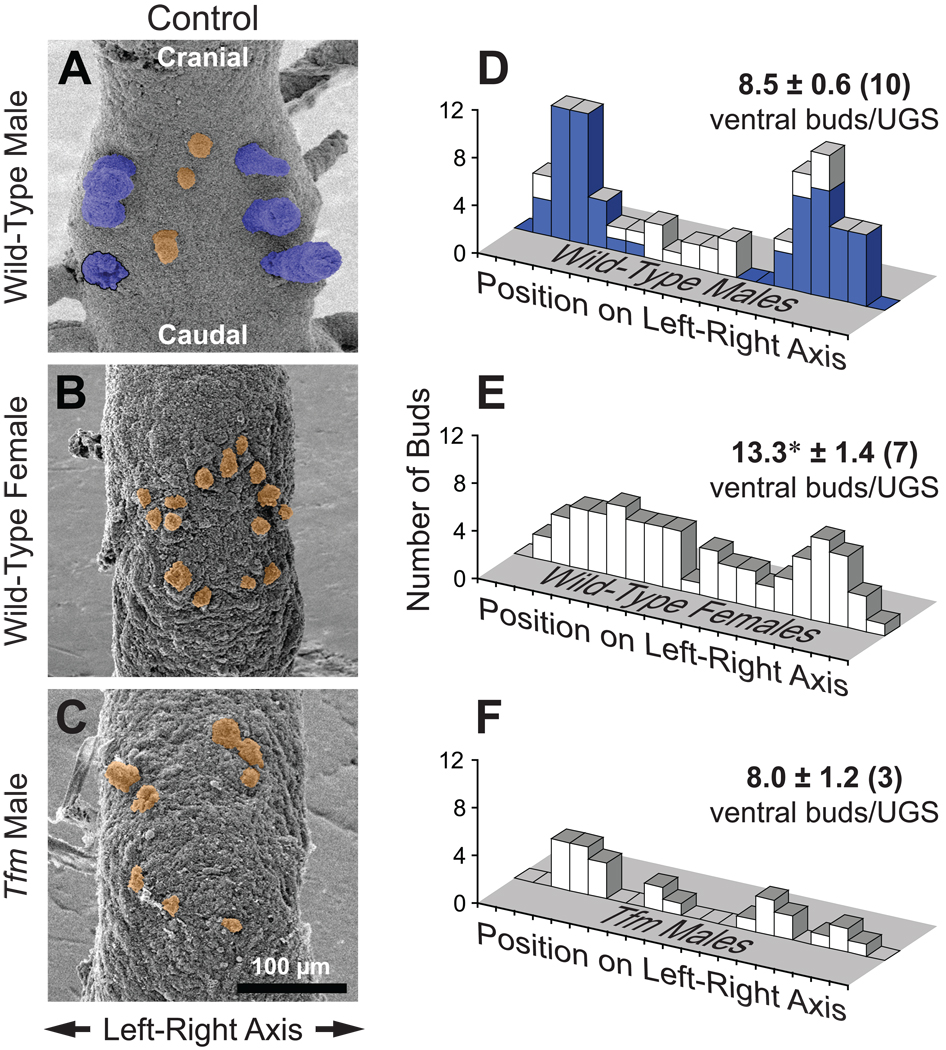
The primary objective of the present study was to better understand the mechanism by which androgens affect fetal prostate development, specifically, their role in regulating ventral bud specification and initiation. Consequently, we began by determining the influence of gender and level of androgen signaling on the number and pattern of buds in the ventral UGS. SEM images of the ventral budding region in wild-type male, wild-type female, and Tfm male UGEs are shown in Fig. 2. In wild-type males (Fig. 2A) the well-developed ventral prostatic buds (tinted blue) were positioned, as expected, in two columns of 3–4 buds apiece located close to and parallel to the left and right sides of the UGE. Surprisingly, additional ventral buds (tinted orange) were found in 7 of the 12 samples examined. These buds were smaller in diameter and shorter than the others and were often located, uncharacteristically for ventral prostatic buds, on or close to the midline. Although not previously reported to be present in the ventral prostatic budding area, these may be urethral buds. Total ventral bud number in wild-type males was 8.5 ± 0.6.
Fig. 2.
The degree of androgen signaling appears to regulate ventral UGS bud number and distribution. A–C: SEM images of ventral UGE from control mice on the day of birth. Samples are from a wild-type male (A), wild-type female (B), and Tfm male (C). Ventral prostatic buds are tinted blue, while small ventral buds are tinted orange. One prostatic bud broke off during sample preparation; its base is outlined in black. All images are ventral views, taken with samples in the same orientation and at the same nominal magnification. D–F: Bud count data and histograms of ventral bud distribution on the left-right axis. Histograms were prepared by plotting the total number of ventral buds, from all replicate samples, present in each of 20 equal segments along the left-right UGE axis. For wild-type males (D), the blue histogram is for ventral prostatic buds and the white histogram is for the markedly smaller ventral buds. Ventral bud counts are shown as mean ± SE, with the number of replicate samples in parentheses. The asterisk indicates a mean bud number significantly different from the wild-type male group (p < 0.05).
UGSs from female mice have long been known to have small ventral buds (Raynaud, 1938, 1942a), and at least some of these are capable of developing into prostatic buds when androgen levels are increased (Raynaud, 1942b), but no previous publication describes their number or the pattern in which they form. We observed 13.3 ± 1.4 small epithelial buds on the ventral UGE surface cranial to where the vagina attaches to the UGS. This is significantly more (by 56%) than the total number of ventral buds in males. Unlike buds in males, these buds generally appeared to be randomly distributed throughout a roughly oval ventral budding area, and there was no detectable relationship between their position and bud size. An example is shown in Fig. 2B.
Previous reports indicate that prostatic buds do not form in Tfm males (Kobayashi, 1984; Mauch et al., 1985). Unexpectedly, we observed that UGSs from Tfm males (Fig. 2C) had small epithelial buds, mostly in the same area in which ventral buds are found in females. The number of buds in the ventral UGE was 8.0 ± 1.2, essentially the same as in wild-type males, although the buds were smaller in diameter and much shorter. The presence of these small, possibly urethral, buds in Tfm males strongly suggests that bud initiation in mice is AR independent for at least some ventral UGS buds.
No one UGS is fully representative of how androgenic status affects the pattern in which ventral buds form. Therefore, scattergrams were prepared by tracing UGE outlines and marking ventral bud positions, then superimposing the tracings from replicate samples. As shown in Suppl. Fig. 1, ventral buds from wild-type males, wild-type females, and Tfm males appeared to be in comparable positions on the craniocaudal axis, while the differences in bud position were on the left-right axis. To better assess the distribution of these buds on the left-right axis, histograms were prepared by plotting the total number of ventral buds per group present in each of 20 equal segments along the left-right UGE axis. As shown in Fig. 2D, control wild-type males displayed the expected bimodal distribution of the large diameter and generally long ventral prostatic buds (blue bars), whereas the small ventral buds (white bars) had a much more uniform left-right distribution. In control females (Fig. 2E), ventral bud position was fairly uniform across the left-right axis; if bimodally distributed, the peaks were much broader than in males. The number of control Tfm male UGSs available was too low to draw any firm conclusions about left-right bud distribution (Fig. 2F). Ventral bud distribution in females passed the Kolmogorov-Smirnov (K-S) test for normality and the Chi-square test for uniform distribution, whereas the buds in wild-type males failed both tests.
The results described above demonstrate for the first time that (1) the well established sexual dimorphisms in UGS development include differences in ventral bud number and pattern, and (2) that UGSs from Tfm males have small epithelial buds. These findings also suggest that androgen signaling regulates the number and position of ventral buds, at least in part, by affecting bud specification rather than bud initiation. To the best of our knowledge, this is also the first demonstration that urethral bud number and size are sexually dimorphic.
Nkx3.1 Expression does not Distinguish Prostatic Buds from Urethral Buds
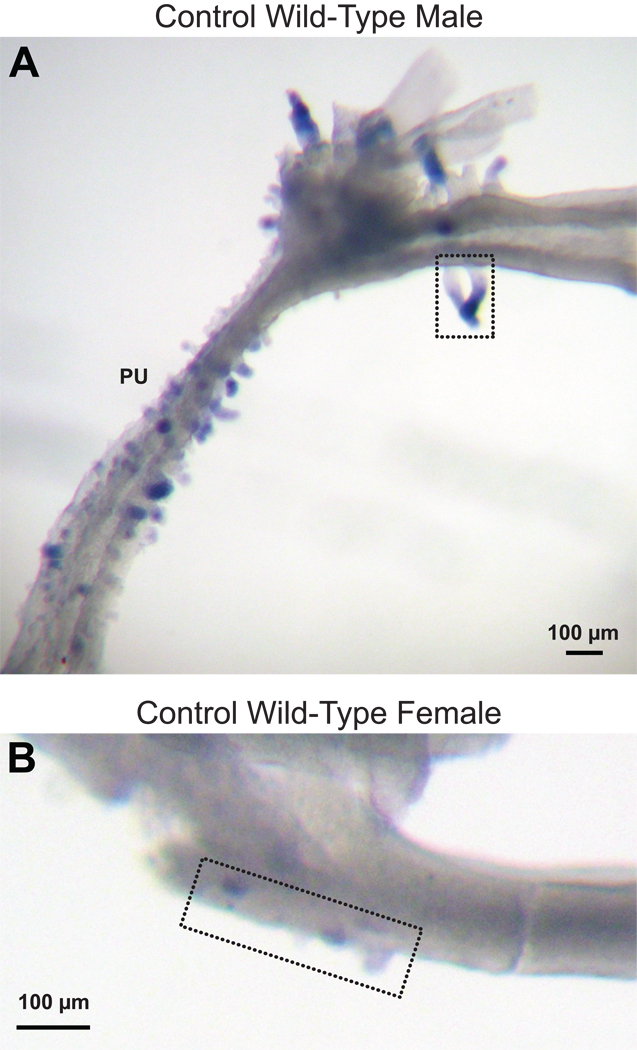
Urethral buds are present on the pelvic urethra in male mice (Hall, 1936; Turner, 1939), but the extent to which they extend into the prostatic urethra is not clear. In an effort to determine if the small ventral buds in wild-type males might be urethral buds (and if ventral buds in females might be a mixture of urethral and prostatic buds), we tested the hypothesis that Nkx3.1 expression can distinguish prostatic buds from urethral buds. Nkx3.1 is the earliest known marker of prostate epithelial development (Bhatia-Gaur et al., 1999). Although one lab has reported that it is expressed in urethral glands and elsewhere (Chen et al., 2005), others describe it as prostate specific (e.g., Bieberich et al., 1996; Bhatia-Guar et al., 1999; Kuslak and Marker, 2007; Thomsen et al., 2008). Therefore, we examined Nkx3.1 expression patterns in UGSs from wild-type males and females. To enhance visibility of small buds, UGM was removed prior to in situ hybridization. As expected, Nkx3.1 expression (blue staining) was seen in ventral, dorsal, lateral, and anterior prostatic buds of male UGEs on the day of birth, but this gene was also expressed in urethral buds present in the pelvic urethra (Fig. 3A). Nkx3.1 expression in female UGEs was far less than in males, but staining was occasionally seen in both the pelvic urethra and the ventral budding area (Fig. 3B). Therefore, Nkx3.1 expression cannot distinguish prostatic from urethral buds in either male or female mice, at least not on the day of birth. Interestingly, Nkx3.1 in males was not visibly expressed in all prostatic and all urethral buds.
Fig. 3.
Nkx3.1 expression does not distinguish prostatic buds from urethral buds. Photomicrographs (lateral views, bladder neck to the right) of UGE from a control wild-type male (A) and a control wild-type female (B) on the day of birth. Nkx3.1 expression (dark blue staining) was determined by in situ hybridization. The ventral budding regions are contained within the black dotted rectangles. PU: pelvic urethra.
Exogenous DHT can Reduce Ventral Bud Number and Induce a Male-like Bimodal Budding Pattern in the Ventral UGS of Female Mice
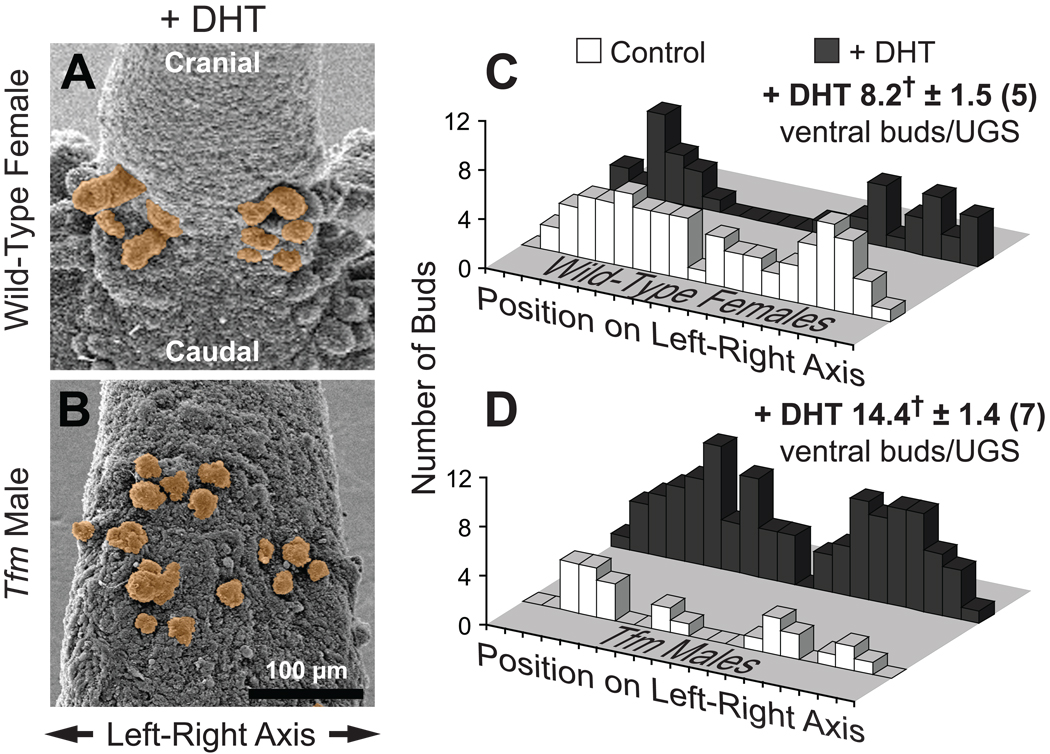
Results described above suggest that the role of androgens in wild-type male mice includes reducing the number of ventral buds and constraining most of them into a sharp bimodal distribution. We therefore tested the hypothesis that the potent AR agonist DHT can induce a sharp bimodal bud distribution in the ventral UGE of female fetuses and reduce ventral bud number. Budding in Tfm males was used as a negative control since these mice typically do not respond to androgens. Dams were implanted on E13.5 (before prostatic budding is initiated) with sustained release pellets containing DHT, and UGEs were examined by SEM on the day of birth. Identical DHT treatment was shown previously to strongly masculinize female fetuses (Lin et al., 2003).
DHT exposure was sufficient for UGEs from DHT-exposed females to resemble UGEs from control males in terms of overall shape, prostatic budding, and urethral budding on the pelvic urethra (results not shown). The remainder of the observations described below refer to the ventral UGS region cranial to the site where the vagina attaches to the UGS. DHT exposure greatly increased the size of some ventral buds and caused ventral bud distribution in females to shift from roughly uniform or very broadly bimodal on the left-right axis to clearly bimodal (Fig. 4A,C). Consequently, left-right bud distribution now failed both the K-S normality test and the Chi-square uniform distribution test. As in males, the largest ventral buds were located at a distance from the midline. In utero DHT exposure also significantly reduced the number of ventral buds per UGS in females, from 13.3 ± 1.4 to 8.2 ± 1.6, a number virtually identical to that seen in control males. These results support the hypothesis that the sexual dimorphism in endogenous androgen concentrations in control fetuses is responsible for males having fewer ventral buds than females and for having a sharp bimodal left-right bud distribution pattern rather than a more uniform distribution.
Fig. 4.
In utero DHT exposure reduces ventral UGS bud number and masculinizes their distribution pattern in wild-type females, while increasing ventral bud number in Tfm males. A,B: SEM images of ventral UGE from DHT-exposed mice on the day of birth. Samples are from a wild-type female (A) and a Tfm male (B). Ventral buds are tinted orange. Both images are ventral views, taken with samples in similar orientations and at the same nominal magnification. C,D: Bud count data and histograms of ventral bud distribution on the left-right axis. Histograms were prepared by plotting the total number of ventral buds, from all replicate samples, present in each of 20 equal segments along the left-right UGE axis. For purposes of comparison, ventral bud distribution histograms from control as well as DHT-exposed mice are shown. Ventral bud numbers for the DHT-exposed groups are shown as mean ± SE, with the number of replicate samples in parentheses. The dagger indicates a mean bud number significantly different from the corresponding control group (p < 0.05).
DHT Induces Bud Formation in Tfm Male UGSs
In utero DHT exposure had no effect on the overall shape of the UGE in Tfm males (results not shown) but it appeared to increase bud length (Fig. 4B; compare with Fig. 2C) in the ventral budding area. DHT exposure significantly increased the number of ventral buds per UGS in Tfm males, from 8.0 ± 1.2 to 14.4 ± 1.4. Ventral bud distribution in DHT-exposed Tfm male UGSs (Fig. 4D) appeared to be broadly bimodal; left-right distribution passed both the K-S test for normality and the Chi-square test for uniform distribution.
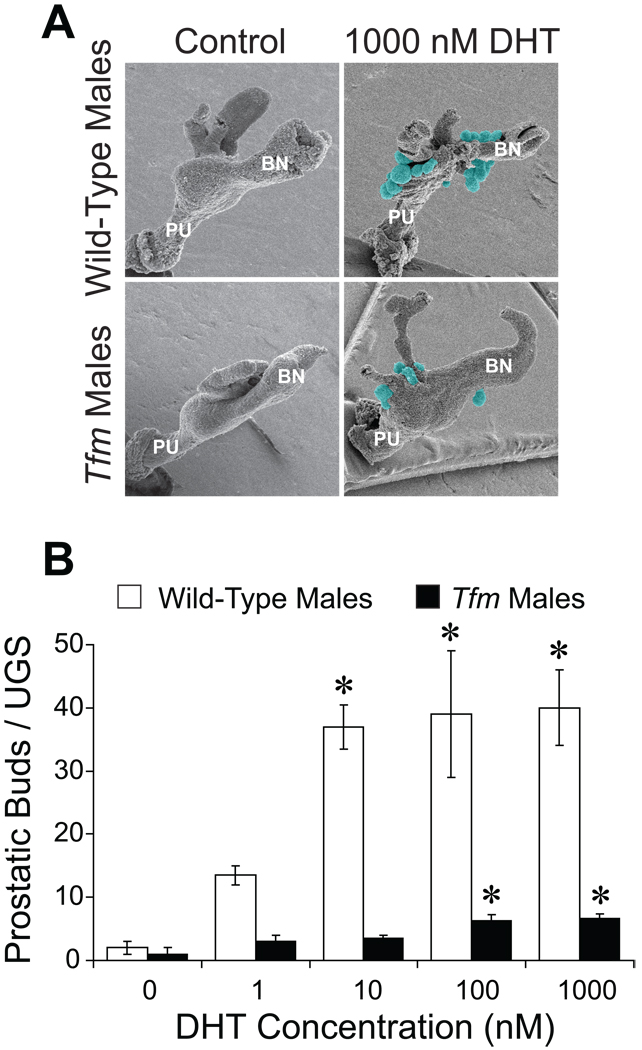
The androgen-dependent increases in Tfm male bud number and possibly length were so unexpected that we sought additional evidence to confirm or refute the finding that Tfm males can respond to DHT. Therefore, UGSs from E14.5 wild-type and Tfm males were cultured in vitro with graded concentrations of DHT. Three days later, UGM was removed, and samples were examined by SEM (Fig. 5A). UGSs incubated with vehicle had few buds. DHT significantly increased total bud number in wild-type UGSs to about 40 and in Tfm male UGSs to about 7 (Fig. 5B). Buds from Tfm UGSs were generally comparable in size to those from wild-type UGSs (Fig. 5A). These in vitro results strongly support the in vivo observations that UGSs from Tfm males are androgen responsive.
Fig. 5.
UGSs from Tfm male mice are DHT responsive. E14.5 UGSs from wild-type males (positive control) and Tfm males were cultured for three days in media containing 0, 1, 10, 100, or 1000 nM DHT. Mesenchyme was then removed and UGE was visualized by SEM. A: Representative samples of wild-type male and Tfm male UGEs after incubation with vehicle (left) or 1000 nM DHT (right). Prostatic buds are highlighted in blue-green. PU: pelvic urethra, BN: bladder neck. B: Prostatic bud counts are presented as the mean ± SE (n ≥ 3). An asterisk indicates that a mean is significantly different from the corresponding control group (p < 0.05).
The Anti-Androgen Flutamide Reduces Ventral UGS Bud Number in Females but not Males and has Little Effect on the Ventral Budding Pattern in Males
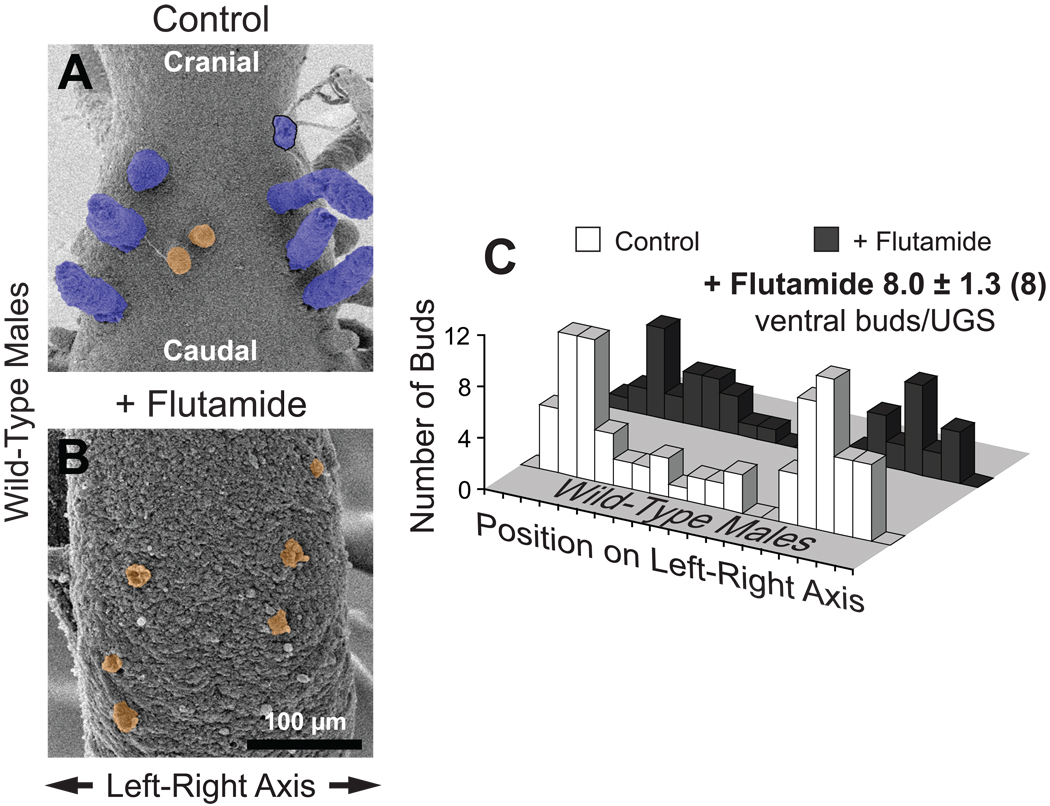
To further test the hypothesis that androgens are necessary for the sharp bimodal ventral bud distribution present in control wild-type males but not females, dams were implanted on E13.5 with sustained release pellets containing the AR antagonist flutamide. Flutamide greatly reduced ventral bud length in wild-type male UGSs (compare Fig. 6A with 6B) but did not significantly change ventral bud number (8.0 ± 1.3 per UGS, versus 8.5 ± 0.6 in control males). Flutamide exposure also had little effect on how ventral buds were distributed on the left-right axis. In both vehicle- and flutamide-exposed wild-type males the distribution was clearly bimodal (Fig. 6C); according to K-S analysis, there is an 85% probability that flutamide did not change the bud distribution pattern.
Fig. 6.
In utero flutamide exposure inhibits bud elongation but has little if any effect on bud specification or initiation in the ventral UGS of wild-type males. A,B: SEM images of ventral UGE from a control (A) and a flutamide-exposed (B) mouse on the day of birth. Ventral buds are tinted orange. One prostatic bud broke off during sample preparation; its base is outlined in black. Both images are ventral views, taken with samples in the same orientation and at the same nominal magnification. C: Bud count data and histograms of ventral bud distribution on the left-right axis. Histograms were prepared by plotting the total number of ventral buds, from all replicate samples, present in each of 20 equal segments along the left-right UGE axis. For purposes of comparison, ventral bud distribution histograms from both control and flutamide-exposed mice are shown. Ventral bud numbers for the flutamide-exposed group are shown as mean ± SE, with the number of replicate samples used to determine bud number in parentheses. Bud distribution was plotted from fewer samples (n = 6) because 2 UGEs were not oriented favorably.
Flutamide significantly reduced ventral bud number in wild-type females by 57% (from 13.3 ± 1.4 to 5.7 ± 1.3) and appeared to reduce bud size (results not shown). Effects on bud distribution were less clear; distribution on the left-right axis was not significantly altered according to the K-S test, but there was no obvious pattern. Small ventral buds were still present even in flutamide-exposed Tfm males (results not shown), and the slight reduction in bud number (to 5.8 ± 0.7) was not statistically significant. Nor did flutamide significantly affect ventral bud distribution on the left-right axis in Tfm males, which was bimodal (results not shown).
The results described above (a) suggest that androgen signaling is not essential for the prototypical male ventral UGS bud specification pattern, and (b) strengthen the hypothesis that the initiation of all ventral buds is not inherently androgen dependent.
DHT Sensitizes Buds in the Ventral UGS to the Budding Inhibitor TCDD
We previously showed that TCDD can prevent ventral prostatic bud formation in male mice, reduce dorsolateral bud number, and reposition dorsal and lateral but not anterior prostatic buds (Lin et al., 2003). TCDD acts during the specification stage of bud formation to affect dorsoventral bud patterning (Vezina et al., 2008). Because androgens and TCDD can each influence bud distribution and ventral bud number, we tested the hypothesis that androgen signaling is required for TCDD to inhibit ventral UGS bud formation.
In utero TCDD exposure (5 µg/kg maternal dose on E15.5) prevented even small buds from forming in the ventral budding area of wild-type males (Fig. 7). In contrast, while TCDD significantly decreased bud number in the ventral budding region of wild-type females (to 4.3 ± 1.0), it did not completely prevent them from forming (Fig. 8A,B). Similarly, TCDD did not fully prevent ventral bud formation in Tfm males (Fig. 8D,E), although bud number (4.9 ± 1.0) tended to be reduced (p = 0.10). TCDD did not detectably alter the distribution of ventral buds in either females or Tfm males (results not shown). These results led us to hypothesize that budding inhibition by TCDD requires a relatively high (i.e., normal male) level of androgen signaling.
Fig. 7.
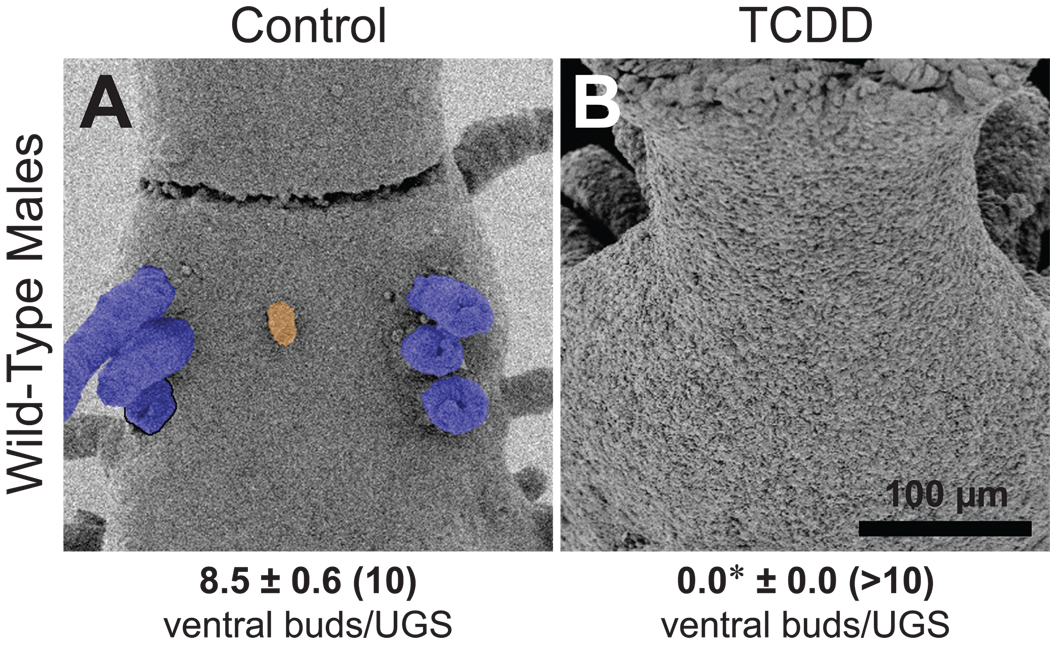
The selective UGS budding inhibitor TCDD prevents buds from forming in the ventral UGS of wild-type males. Dams were dosed orally with vehicle (A) or TCDD (B; 5 µg/kg) on E15.5, and UGSs were collected on the day of birth. SEM images of ventral UGE are shown. Ventral prostatic buds are tinted blue, while the small ventral bud is tinted orange. One prostatic bud broke off during sample preparation; its base is outlined in black. Both images are ventral views, taken with samples in similar orientations (cranial at the top and caudal at the bottom) and at the same nominal magnification. Ventral bud numbers are shown as mean ± SE, with the number of replicate samples in parentheses. The asterisk indicates a mean bud number significantly different from the control group (p < 0.05).
Fig. 8.
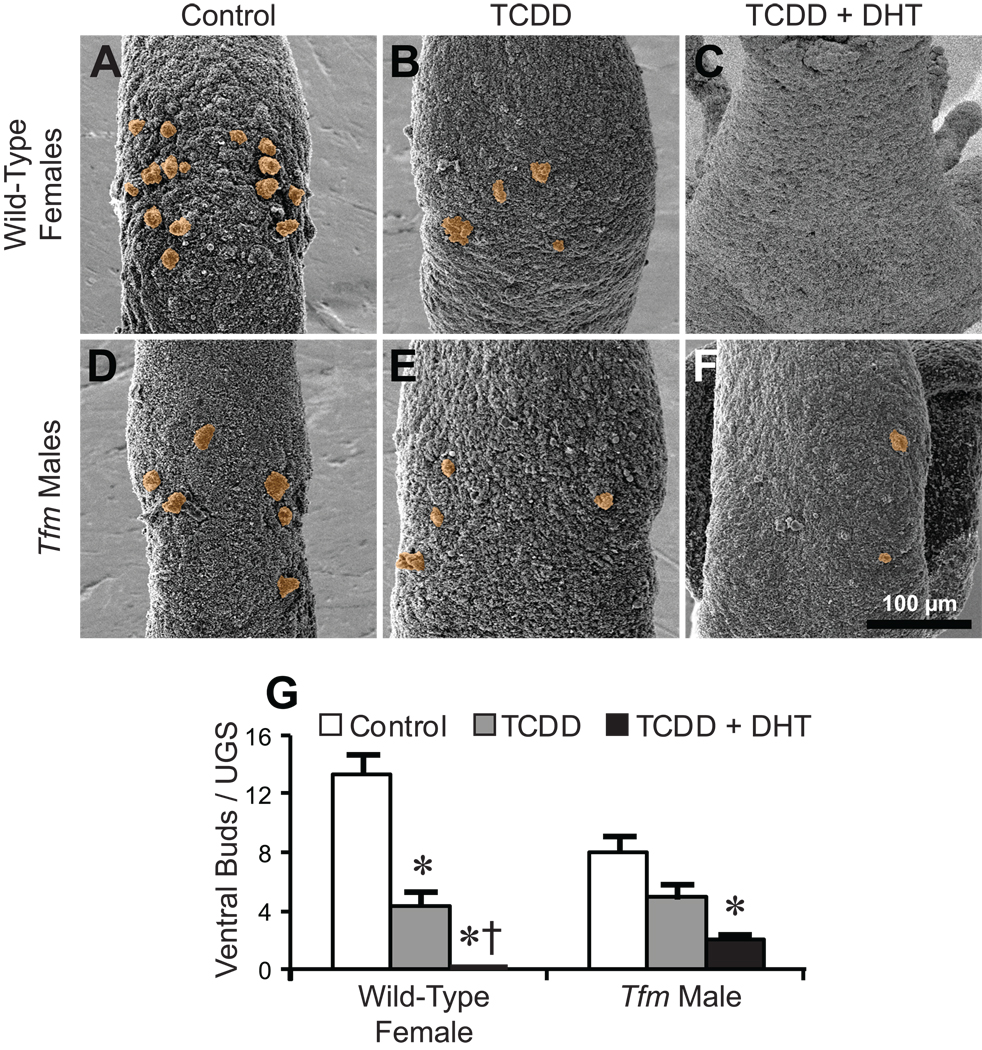
DHT sensitizes the ventral UGS to budding inhibition by TCDD. Some dams were implanted with sustained-release DHT pellets on E13.5, and all dams were dosed orally with vehicle or TCDD (5 µg/kg) on E15.5. UGSs were collected on the day of birth. A–F: SEM images of ventral UGE. Samples are from wild-type females exposed to vehicle/placebo (A), TCDD (B), or DHT + TCDD (C), and from Tfm males exposed to vehicle/placebo (D), TCDD (E), or DHT + TCDD (F). All images are ventral views, taken with samples in the same orientation (cranial at the top and caudal at the bottom) and at the same nominal magnification. Ventral buds are tinted orange. G: Ventral bud counts (mean ± SE). An asterisk indicates a significant difference from the control group, and a dagger indicates a significant difference from the TCDD alone group (p < 0.05). There were 3–7 replicates per group.
To determine whether androgens sensitize ventral buds to TCDD, dams were implanted with sustained release DHT-containing pellets on E13.5, then dosed with 5 µg TCDD/kg on E15.5. While TCDD alone reduced ventral bud number in wild-type females (Fig. 8B), DHT pretreatment plus TCDD completely prevented any of these buds from forming (Fig. 8C; bud counts in Fig. 8G). In Tfm males, DHT pretreatment reduced ventral bud number in the presence of TCDD from 4.9 ± 1.0 per UGS (Fig. 8E) to 2.0 ± 0.4 (Fig. 8F; bud counts in Fig. 8G, p = 0.08). These results support the hypothesis that ventral bud agenesis in TCDD-exposed UGSs requires a substantial degree of androgen signaling.
DISCUSSION
The results described above lead to several new insights into the basic biology of UGS development in the mouse. They also provide the first evidence that the UGS in male Tfm mice is androgen responsive, and reveal that complete inhibition of ventral UGS budding by TCDD requires a high level of androgen signaling. Importantly, they highlight the need to develop methods capable of distinguishing prostatic buds from urethral buds, and they raise new questions about mechanisms of bud specification in the UGS.
New Insights into the Basic Biology of UGS Development
Prostatic buds in male mice develop in characteristic patterns (Sugimura et al., 1986; Timms et al., 1994, 2005; vom Saal et al., 1997; Lin et al., 2003; Timms, 2008). In C57BL/6J males, ventral prostatic buds are present in two rows, with 3 or 4 buds apiece, parallel to the lateral surfaces of the UGE (Lin et al., 2003). These buds are notably absent near the midsagittal plane. The first key finding from this study is that about half the control wild-type males had additional buds in the ventral prostatic budding area. These buds were substantially shorter and narrower than the prostatic buds, their distribution was much more uniform across the left-right axis, and some were located in the midsagittal area. These small buds were similar in size and distribution to the ventral buds in control females that are generally considered to be prostatic, though fewer in number in males. It is doubtful that these small buds are capable of developing into prostatic buds because we saw no increase in the number of large ventral buds when males were exposed prenatally to DHT (Lin et al., 2003; Allgeier, 2007). While no previous publication specifically describes urethral buds or glands as being present in the ventral prostatic budding area, the small buds we observed may well be urethral. Nkx3.1 expression is often said to be prostate specific; if so, its presence or absence should indicate whether the small ventral buds are prostatic or urethral. However, our results confirm the observation of Chen et al. (2005) that Nkx3.1 is expressed in both prostatic buds and in buds that are clearly urethral (i.e., small buds on the pelvic urethra). Regardless of their origin or possible fate, the realization that these buds are present demonstrates that the regulation of ventral epithelial budding in the UGS of male mice is more complex than previously believed.
The second novel observation is that small ventral epithelial buds were present in UGSs from Tfm males, the classic mouse model of complete androgen insensitivity. Prostatic buds have previously been reported to be absent from these mice (Kobayashi, 1984; Mauch et al., 1985), and there appear to be no reports regarding urethral buds. A possible explanation why small buds were seen in UGSs from Tfm male mice in this but not previous studies is that it is easier to recognize that small buds are present from SEM images of the UGE than from UGS serial sections, particularly if one is looking for relatively large prostatic buds rather than for such small projections.
Tfm male mice have a small number of urethral buds in the pelvic urethra (Fig. 1C) but are clearly unable to develop prostate (Lyon and Hawkes, 1970; Goldstein and Wilson, 1972), therefore, the simplest interpretation is that the small ventral buds we observed are urethral rather than prostatic buds. This interpretation holds even though we subsequently discovered that UGSs from Tfm male mice are weakly androgen responsive. Yet there were substantially more small ventral buds in Tfm male mice (as well as in flutamide-exposed wild-type males, flutamide-exposed wild-type females, and flutamide-exposed Tfm males) than in control wild-type males. If all the small ventral buds in each of these groups are urethral buds, their presence would suggest that prostatic bud development inhibits urethral bud development in the ventral UGS, or possibly that buds get re-specified from urethral to prostatic as androgen concentrations increase. Regardless of the identity of the small ventral buds in UGSs with little if any androgen signaling, our findings demonstrate the initiation of at least some ventral UGS buds is either androgen independent or extraordinarily androgen sensitive.
The third discovery is that the number of ventral epithelial buds in control mice is significantly greater in females than in males. This finding, coupled with observations that ventral buds are present when androgen signaling is absent or very low, led to new insights into the regulation of ventral epithelial bud number. Regardless of whether we examined ventral buds from Tfm male UGSs or from flutamide-exposed wild-type male, flutamide-exposed wild-type female, or flutamide-exposed Tfm male UGSs, the mean number of ventral buds when androgenic signaling was absent or extremely weak was in the range of 6 to 8. This was no different from the mean number of ventral buds (about 8) when the degree of androgen signaling was high (i.e., in control wild-type males or in DHT-exposed females). Yet when androgen signaling was low by male standards (i.e., in control females and in DHT-exposed Tfm males), mean ventral bud number was much higher (13 and 14, respectively). These results strongly suggest that ventral UGS bud number responds biphasically to androgenic stimulation: there is a baseline when androgen signaling is absent or very low, additional androgenic stimulation increases the number of ventral buds, and further androgen signaling reduces ventral bud number to about baseline again.
The fourth new observation is that the pattern in which ventral buds develop differs in males and females. Most buds in males conformed to a relatively sharp bimodal distribution on the left-right axis. In contrast, ventral buds in control females were much more uniformly distributed; if bimodal, the peaks were far broader than in males. Although the picture is less clear for ventral bud pattern than for ventral bud number, it similarly appears that the response to androgenic stimulation is biphasic. Bud distribution on the left-right axis was usually bimodal when androgen signaling was absent or very weak (e.g., in flutamide-exposed wild-type males), far more uniform when androgen signaling was low, and sharply bimodal when androgen signaling was high. These observations suggest that androgens act during bud specification to determine where ventral buds will develop.
Control females had more ventral buds than control males, but it did not appear that the pattern in individual females consisted of buds in the standard male pattern (2 rows of 3–4 buds near the lateral UGE surfaces) plus extra buds. Instead, the overall pattern appeared to be different in males and females. Yet when females were exposed to DHT prenatally their ventral budding pattern reverted to one characteristic of males. This sexual dimorphism in ventral bud position raises the possibility that small buds may appear and disappear fairly rapidly in the ventral UGS, such that the cumulative total number of buds that appear during prenatal UGS development would be greater than the number present at any one time. In this scenario, a function of androgens (at concentrations found in control males) would be to cause buds that appear in the proper positions to develop into the much larger ventral prostatic buds, while perhaps facilitating the disappearance of buds (urethral or potentially otherwise) that appear elsewhere.
The UGS in Tfm Male Mice is Androgen Responsive
Due to a spontaneous frame-shift mutation in the X-linked AR gene that encodes a truncated AR protein, Tfm male mice have no male reproductive organs other than testes, and no other male phenotype (Lyon and Hawkes, 1970; Young et al., 1989). Although small biochemical changes in response to testosterone have been reported (Schenkein et al., 1974; Catterall et al., 1986), and although the truncated AR is reported to have weak transcriptional activity (He et al., 1994), other reports indicate that Tfm male mice are completely resistant to androgenic stimulation (Goldstein and Wilson, 1972; He et al., 1991). Tissues from Tfm male mice have been widely used in the belief that they are completely androgen non-responsive.
It has previously been reported that no prostatic buds are present in the UGS of Tfm male mice (Kobayashi, 1984; Mauch et al., 1985), that no prostatic buds form when these UGSs are incubated in vitro with androgens (Laznitzki and Mizuno, 1980; Takeda et al., 1987), that Tfm mice have no prostate (Lyon and Hawkes, 1970), that UGSs from Tfm male mice do not develop into prostate when grown as grafts in control adult males (Cunha and Lung, 1978), and that Tfm mice develop no prostate even when exposed to pharmacological doses of DHT before and after birth (Goldstein and Wilson, 1972). Yet the observation that in utero DHT exposure significantly increased ventral bud number, and follow-up observations that UGSs from Tfm males can form buds as large as in wild-type UGSs when incubated with DHT in vitro, and that bud number is significantly increased by in vitro DHT incubation, provide compelling evidence that the UGS from Tfm male mice is androgen responsive, albeit weakly, after all.
Previous studies showed that buds (which were presumed to be prostatic) could be induced in Tfm male UGSs by epidermal growth factor, keratinocyte growth factor, and transforming growth factor α (Saito and Mizuno, 1995; Mizuno and Saito, 1996), but the present study is the first to find such a response to an androgen. The nature of the buds that formed in each of these studies is unclear, however. In previous in vitro studies where recombined UGE and UGM were studied rather than intact UGS, the damage caused by separation and the developmental delay caused by recombination may have been factors in why bud development was not observed in response to DHT.
The androgen-dependent increase in Tfm male bud number and possibly length raised several possibilities. One is that UGSs are responding to the DHT metabolite 5α-androstane-3β,17β-diol, which binds to estrogen receptor-β (Oliveira et al., 2007). However, low level estrogen exposure, which repatterns prostatic budding, increases bud numbers elsewhere in the UGS but not in the ventral UGS (vom Saal et al., 1997; Timms et al., 2005; Hofkamp et al., 2008), and high-level estrogen exposure reduces prostatic bud numbers throughout the UGS (Raynaud, 1942b; vom Saal et al., 1997). Other possibilities are that truncated ARs in Tfm males may be capable of responding to androgens, that androgens may be acting through a currently unknown AR, or that androgens may be capable of AR-independent actions. Attempts to discriminate among these possibilities were beyond the scope of the current investigation.
Complete Ventral Bud Agenesis by TCDD Requires a High Degree of Androgen Signaling
The ventral region of the UGS is the area most sensitive to both the stimulatory effects of androgens (Greene et al., 1939) and the inhibitory effects of TCDD (Lin et al., 2003). TCDD acts during bud specification to change the pattern in which buds develop on the dorsoventral UGS axis (Vezina et al., 2008), but despite extensive efforts (Lin et al., 2003; Abbott et al., 2003; Ko et al., 2004a,b; Vezina et al., 2007, 2008, 2009; Allgeier et al., 2008, 2009), the mechanisms by which in utero TCDD exposure affects prostatic budding patterns in mice remain unknown.
In wild-type male mice, in utero TCDD exposure prevents all ventral buds from forming. Surprisingly, the same TCDD dose proved incapable of causing complete ventral bud agenesis in either female mice or Tfm male mice. Yet TCDD significantly reduced ventral bud number in DHT-exposed Tfm males, and caused complete ventral bud agenesis in DHT-exposed females. These results demonstrate that TCDD requires a high degree of androgen signaling to completely prevent ventral budding. Although the mechanism by which TCDD re-patterns bud formation in the UGS remains to be determined, it now appears that the mechanism includes a change in androgen-responsive gene expression.
Identification of New Issues in UGS Development
The finding that urethral buds probably extend into the ventral prostatic budding area (or at minimum that some ventral buds are vastly larger than others) raises issues that have not yet been addressed in the UGS development literature. If urethral and prostatic buds are intermingled, does mesenchyme have a mosiac inductive pattern sufficiently detailed to be capable of specifying some buds as prostatic while specifying others a few cells away as urethral? If not, does the same ventral mesenchyme cause individual buds to develop as either prostatic or urethral because of localized epithelial differences? What are the signaling pathways that determine bud fate in areas where prostatic and urethral buds are intermingled? Has the fate of buds already been determined when they first appear in the ventral UGS? Or are they originally bipotential (or temporarily incapable of either prostatic or urethral development) until some subsequent differentiation event occurs? Or are ventral buds urethral by default unless induced to become prostatic? Is bud formation a transient event, whereby buds will rapidly disappear unless stabilized into either a prostatic or urethral bud? Obviously, the development of methods capable of distinguishing buds as prostatic or urethral (or potentially otherwise) will greatly facilitate research on such issues.
In addition to questions about the mechanisms by which UGS buds become prostatic or urethral, the identity of the small ventral buds we observed remains uncertain. The large ventral buds in control wild-type males are prostatic, and it is easy to assume that the small ones are urethral. In contrast, ventral bud lengths and diameters in control females are fairly uniform, and significantly more ventral buds are present than in males. Previous investigators have described the small ventral buds in females as prostatic (e.g., Raynaud, 1942a; Timms et al., 1999), and 6–8 of them are clearly capable of developing as prostatic buds if androgen levels are elevated sufficiently. It seems unreasonable to consider the other 5–7 ventral buds as prostatic because they failed to enlarge when DHT concentrations were elevated. And the fact that they disappeared upon DHT exposure is the opposite of the standard response when females are exposed to exogenous androgens (an increase in total prostatic bud number). But if the extra small buds in control females are urethral, DHT is somehow causing them to disappear from the ventral UGS despite greatly enhancing urethral bud development in the pelvic urethra. In Tfm males, the approximately 8 small ventral buds may well be urethral, but if so, there are far more ventral urethral buds in these mice than in wild-type males. And if the additional buds that form in DHT-exposed Tfm male UGSs are also urethral, DHT increased urethral bud numbers in these animals while seemingly decreasing ventral urethral bud number in females. The extra buds induced by DHT in Tfm male UGSs could potentially be prostatic in nature, yet they are incapable of developing into prostate. We suggest that some ventral buds may be neither prostatic nor urethral. In any case, it appears that prostatic bud development suppresses urethral bud development. Future research in which the location and number of both prostatic ducts and urethral glands in older mice are determined may prove informative about the identities of the buds seen at birth.
In summary, this research into the old issue of how androgen signaling affects UGS development in mice (Raynaud, 1938, 1942b) reveals that some very fundamental issues have not yet been resolved. A great deal remains to be learned about the mechanisms by which androgen signaling regulates the number, position, and fate of buds in the UGS.
EXPERIMENTAL PROCEDURES
Animals and Treatments
B6-Aw-J.Cg-EdaTa-6J+/+ArTfm/J (Tfm) heterozygous female mice and wild-type C57BL/6J male mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and used to establish breeding colonies. Animals were housed in clear plastic micro-isolator cages with corn-cob bedding in 22 ± 1°C rooms with a 24-hr light-dark cycle (lighted 0600 to 1800 hr). Feed (5015 Mouse Diet, PMI Nutrition International, Brentwood, MO) and water were available ad libitum. Timed-pregnant females were generated by housing females overnight with wild-type males. The day after overnight mating was designated E0.5.
For the in vivo experiments, some dams were anesthetized with isoflurane on E13.5, and a placebo, DHT-, or flutamide-releasing pellet (DHT: 15 mg/pellet, 90-day release; flutamide: 35 mg/pellet, 21-day release; Innovative Research of America, Sarasota, FL) was inserted subcutaneously into the interscapular adipose tissue. On E15.5, some dams were dosed orally with TCDD (5 µg/kg, 98% purity, Cambridge Isotopes Laboratories, Andover, MA) or corn oil vehicle (5 ml/kg).
Fetuses were obtained by euthanizing pregnant dams by CO2 overdose. Fetuses and newborn pups were euthanized by decapitation with surgical scissors. Genotyping was performed as described by Gao and Isaacs (1998). All procedures were approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Scanning Electron Microscopy and Bud Counting
Samples were prepared as previously described (Lin et al., 2003). Briefly, UGSs were removed and partially digested in 1% trypsin (Difco, Sparks, MD) at 4°C for 90 min. Enzyme activity was attenuated using 5% fetal bovine serum (Hyclone, Logan UT) and UGM was removed using fine forceps. UGS epithelial samples were fixed in 2.5% glutaraldehyde (Ted Pella, Redding, CA) for 24 hr, dehydrated through a graded series of ethanol, and dried by the critical-point procedure using CO2 prior to being mounted and coated with gold for analysis using a Hitachi S-570 scanning electron microscope (Tokyo, Japan). All images were nominally taken at the same magnification (130×), but because different parts of the samples were different distances from the detector, actual magnifications differ somewhat. To be identified as a bud, the UGE cell cluster had to be at least 2 cells in diameter and protrude visibly above the UGE plane.
In Situ Hybridization
UGE samples were prepared as described above, then fixed overnight at 4°C in 4% paraformaldehyde. In situ hybridization was conducted as previously described (Vezina et al., 2008). Synthesis of the Nkx3.1 probe was previously described (Gray et al., 2004).
In Vitro UGS Organ Culture
E14.5 UGSs were collected individually into calcium- and magnesium-free Hank’s Balanced Salt Solution (HBSS; Sigma-Aldrich, St. Louis, MO) and stored on ice at 4°C while genotyping was performed using tail tissue. Isolated UGSs were placed onto 0.4 µm Millicell-CM filter inserts (Millipore, Billerica, MA) in 6-well plates containing 1 ml media/well and oriented such that one lateral surface of the UGS was in contact with the insert material. UGSs were incubated in Dulbecco’s Modified Eagle’s medium (DMEM)/F12 50/50 (Mediatech, Herndon, VA) supplemented with antibiotic-antimycotic (Mediatech), 2% insulin-transferrin-selenium (Invitrogen, Carlsbad, CA), and 10% charcoal-dextran-stripped fetal bovine serum (Hyclone). Medium also contained 0, 1, 10, 100 or 1000 nM DHT (Sigma-Aldrich) in ethanol vehicle (0.1% v/v). Cultures were maintained for 3 d in a humidified 37°C, 5% CO2/95% air incubator. Culture media were replenished every 48 hr.
For the in vitro experiments, at least four views of each UGS were obtained such that all surfaces of the UGS were visible. Two reviewers independently counted the total number of buds for each sample from these images without knowing what genotype or treatment group the sample was from, and these counts were averaged to give the best estimate of actual bud number for each sample. At least 3 UGSs per treatment were examined in this manner.
Bud Position and Statistical Analysis
Bud counts are shown as mean ± standard error (SE). To prepare bud distribution histograms, the distance between each ventral bud and the left side of the UGE was measured. This distance was divided by the width of the UGE at that position on the craniocaudal axis, in order to normalize bud position data for differences in UGS width within and across samples. Histograms show the ventral bud position data from all replicate samples.
Significant differences between groups were determined by Student's t test, while differences among groups were determined by ANOVA followed by Fisher's least significant difference test. Bud distribution data were subjected to the Kolmogorov-Smirnov (K-S) test for normality and the Chi-square test for uniform distribution. Statistical significance was set at p < 0.05.
Supplementary Material
Scatterplots of ventral budding patterns in UGS epithelium from control wild-type male, wild-type female, and Tfm male mice on the day of birth. Scatterplots were prepared by tracing UGE outlines and marking ventral bud positions, then superimposing tracings from replicate samples. Ventral prostatic bud positions in control wild-type males are represented by blue dots, while all other ventral bud positions are designated by a black dot. All tracings are in the same orientation (cranial at the top and caudal at the bottom) and at the same nominal magnification. Ventral bud counts are shown as mean ± SE, with the number of replicate samples in parentheses.
ACKNOWLEDGMENTS
We thank Dr. Ralph Albrecht and the Biological and Bio-materials Preparation, Imaging, and Characterization Laboratory at the University of Wisconsin for use of the SEM facility, Heather Hardin and Joseph Heintz for obtaining several of the images, and Dr. Sijian Wang of the University of Wisconsin Institute for Clinical and Translational Research for statistical advice.
Grant Information
Grant sponsor: National Institutes of Health. Grant numbers: R37 ES01332, F31 HD049323, F32 ES014284, K01 DK083425, and T32 ES07015.
REFERENCES
- Abbott BD, Lin T-M, Rasmussen NT, Albrecht RM, Schmid JE, Peterson RE. Lack of expression of EGF and TGF-α in the fetal mouse alters formation of prostatic epithelial buds and influences the response to TCDD. Toxicol Sci. 2003;76:427–436. doi: 10.1093/toxsci/kfg238. [DOI] [PubMed] [Google Scholar]
- Allgeier SH. Ph.D. Thesis. University of Wisconsin; 2007. Dioxin and signaling pathways in prostate development. [Google Scholar]
- Allgeier SH, Lin T-M, Vezina CM, Moore RW, Fritz WA, Chiu S-Y, Zhang C, Peterson RE. WNT5A selectively inhibits mouse ventral prostate development. Dev Biol. 2008;324:10–17. doi: 10.1016/j.ydbio.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgeier SH, Vezina CM, Lin T-M, Moore RW, Silverstone AE, Mukai M, Gavalchin J, Cooke PS, Peterson RE. Estrogen signaling is not required for prostatic bud patterning or for its disruption by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2009;239:80–86. doi: 10.1016/j.taap.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia-Gaur R, Donjacour AA, Sciavolino PJ, Kim M, Desai N, Young P, Norton CR, Gridley T, Cardiff RD, Cunha GR, Abate-Shen C, Shen MM. Roles for Nkx3.1 in prostate development and cancer. Genes Dev. 1999;13:966–977. doi: 10.1101/gad.13.8.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieberich CJ, Fujita K, He W-W, Jay G. Prostate-specific and androgen-dependent expression of a novel homeobox gene. J Biol Chem. 1996;271:31779–31782. doi: 10.1074/jbc.271.50.31779. [DOI] [PubMed] [Google Scholar]
- Catterall JF, Watson CS, Kontula KK, Janne OA, Bardin CW. Differential regulation of specific gene expression in mouse kidney by androgens and antiandrogens. Adv Exp Med Biol. 1986;196:213–226. doi: 10.1007/978-1-4684-5101-6_14. [DOI] [PubMed] [Google Scholar]
- Chen H, Mutton LN, Prins GS, Bieberich CJ. Distinct regulatory elements mediate the dynamic expression pattern of Nkx3.1. Dev Dyn. 2005;234:961–973. doi: 10.1002/dvdy.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Vezina CM, Allgeier SH, Shaw A, Yu M, Peterson RE, Bushman W. Noggin is required for normal lobe patterning and ductal budding in the mouse prostate. Dev Biol. 2007;312:217–230. doi: 10.1016/j.ydbio.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Donjacour AA, Cooke PS, Mee S, Bigsby RM, Higgins SJ, Sugimura Y. The endocrinology and developmental biology of the prostate. Endocr Rev. 1987;8:338–362. doi: 10.1210/edrv-8-3-338. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- Gao J, Isaacs JT. Development of an androgen receptor-null model for identifying the initiation site for androgen stimulation of proliferation and suppression of programmed (apoptotic) death of PC-82 human prostate cancer cells. Cancer Res. 1998;58:3299–3306. [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD. Studies on the pathogenesis of the pseudohermaphroditism in the mouse with testicular feminization. J Clin Invest. 1972;51:1647–1658. doi: 10.1172/JCI106966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Fu H, Luo P, Zhao Q, Yu J, Ferrari A, Tenzen T, Yuk D-I, Tsung EF, Cai Z, Alberta JA, Cheng L-P, Liu Y, Stenman JM, Valerius MT, Billings N, Kim HA, Greenberg ME, McMahon AP, Rowitch DH, Stiles CD, Ma Q. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- Greene RR, Burrill MW, Ivy AC. Experimental intersexuality. The effect of antenatal androgens on sexual development of female rats. Am J Anat. 1939;65:415–469. [Google Scholar]
- Hall K. The structure and development of the urethral sinus in the male white mouse, with notes on its occurrence in other rodents. J Anat. 1936;70:413–428. [PMC free article] [PubMed] [Google Scholar]
- He WW, Kumar MV, Tindall DJ. A frame-shift mutation in the androgen receptor gene causes complete androgen insensitivity in the testicular-feminized mouse. Nucleic Acids Res. 1991;19:2373–2378. doi: 10.1093/nar/19.9.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He WW, Lindzey JK, Prescott JL, Kumar MV, Tindall DJ. The androgen receptor in the testicular feminized (Tfm) mouse may be a product of internal translation initiation. Receptor. 1994;4:121–134. [PubMed] [Google Scholar]
- Hofkamp L, Bradley S, Tresguerres J, Lichtensteiger W, Schlumpf M, Timms B. Region-specific growth effects in the developing rat prostate following fetal exposure to estrogenic ultraviolet filters. Environ Health Perspect. 2008;116:867–872. doi: 10.1289/ehp.10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Pu Y, Hu WY, Birch L, Luccio-Camelo D, Yamaguchi T, Prins GS. The role of Wnt5a in prostate gland development. Dev Biol. 2009;328:188–199. doi: 10.1016/j.ydbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K, Moore RW, Peterson RE. Aryl hydrocarbon receptors in urogenital sinus mesenchyme mediate the inhibition of prostatic epithelial bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2004a;196:149–155. doi: 10.1016/j.taap.2003.12.004. [DOI] [PubMed] [Google Scholar]
- Ko K, Theobald HM, Moore RW, Peterson RE. Evidence that inhibited prostatic epithelial bud formation in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed C57BL/6J fetal mice is not due to interruption of androgen signaling in the urogenital sinus. Toxicol Sci. 2004b;79:360–369. doi: 10.1093/toxsci/kfh111. [DOI] [PubMed] [Google Scholar]
- Kobayashi S. Induction of Müllerian duct derivatives in testicular feminized (Tfm) mice by prenatal exposure to diethylstilbestrol. Anat Embryol (Berl) 1984;169:35–39. doi: 10.1007/BF00300584. [DOI] [PubMed] [Google Scholar]
- Kuslak SL, Marker PC. Fibroblast growth factor receptor signaling through MEK-ERK is required for prostate bud induction. Differentiation. 2007;75:638–651. doi: 10.1111/j.1432-0436.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Lasnitzki I, Mizuno T. Prostatic induction: interaction of epithelium and mesenchyme from normal wild-type mice and androgen-insensitive mice with testicular feminization. J Endocrinol. 1980;85:423–428. doi: 10.1677/joe.0.0850423. [DOI] [PubMed] [Google Scholar]
- Lin T-M, Rasmussen NT, Moore RW, Albrecht RM, Peterson RE. Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Sci. 2003;76:171–181. doi: 10.1093/toxsci/kfg218. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- Mauch RB, Thiedemann KU, Drews U. The vagina is formed by downgrowth of Wolffian and Müllerian ducts. Graphical reconstructions from normal and Tfm mouse embryos. Anat Embryol (Berl) 1985;172:75–87. doi: 10.1007/BF00318946. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Saito M. Induction de bourgeons prostatiques dans le sinus urogénital d'embryons de Souris Tfm dépourvu de récepteurs d'androgènes. C R Seances Soc Biol Fil. 1996;190:497–501. [PubMed] [Google Scholar]
- Oliveira AG, Coelho PH, Guedes FD, Mahecha GA, Hess RA, Oliveira CA. 5α-Androstane-3β,17β-diol (3β-diol), an estrogenic metabolite of 5α-dihydrotestosterone, is a potent modulator of estrogen receptor ERβ expression in the ventral prostrate of adult rats. Steroids. 2007;72:914–922. doi: 10.1016/j.steroids.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Podlasek CA, Clemens JQ, Bushman W. Hoxa-13 gene mutation results in abnormal seminal vesicle and prostate development. J Urol. 1999;161:1655–1661. [PubMed] [Google Scholar]
- Podlasek CA, Duboule D, Bushman W. Male accessory sex organ morphogenesis is altered by loss of function of Hoxd-13. Dev Dyn. 1997;208:454–465. doi: 10.1002/(SICI)1097-0177(199704)208:4<454::AID-AJA2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Price D, Williams-Ashman HG. The accessory reproductive glands of mammals. In: Young WC, editor. Sex and Internal Secretions. Third Edition. Baltimore: Waverly Press; 1961. pp. 366–448. [Google Scholar]
- Raynaud A. Intersexualité obtenue expérimentalement chez la souris femelle par action hormonale. Bull Biol Fr Belg. 1938;72:297–354. [Google Scholar]
- Raynaud A. Recherches embryologiques et histologiques sur la différenciation sexuelle normale de la souris. Bull Biol Fr Belg Suppl. 1942a;29:1–114. [Google Scholar]
- Raynaud A. Modification expérimentale de la différentiation sexuelle des embryos de souris par action des hormones androgènes et oestrogènes (primière et deuxième parties) Actualités Sci Indust. 1942b;925:1–264. [Google Scholar]
- vom Saal FS. Sexual differentiation in litter-bearing mammals: influence of sex of adjacent fetuses in utero. J Anim Sci. 1989;67:1824–1840. doi: 10.2527/jas1989.6771824x. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV. Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses. Proc Natl Acad Sci USA. 1997;94:2056–2061. doi: 10.1073/pnas.94.5.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Mizuno T. Le facteur de croissance de l'épiderme (EGF) peut induire des bourgeons prostatiques en l'absence d'androgènes. C R Seances Soc Biol Fil. 1995;189:637–641. [PubMed] [Google Scholar]
- Schenkein I, Levy M, Bueker ED, Wilson JD. Immunological and enzymatic evidence for the absence of an esteroproteolytic enzyme (protease "D") in the submandibular gland of the Tfm mouse. Endocrinology. 1974;94:840–844. doi: 10.1210/endo-94-3-840. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Cunha GR, Donjacour AA. Morphogenesis of ductal networks in the mouse prostate. Biol Reprod. 1986;34:961–971. doi: 10.1095/biolreprod34.5.961. [DOI] [PubMed] [Google Scholar]
- Sugimura Y, Norman JT, Cunha GR, Shannon JM. Regional differences in the inductive activity of the mesenchyme of the embryonic mouse urogenital sinus. Prostate. 1985;7:253–260. [Google Scholar]
- Takeda H, Lasnitzki I, Mizuno T. Change of mosaic pattern by androgens during prostatic bud formation in XTfm/X+ heterozygous female mice. J Endocrinol. 1987;114:131–137. doi: 10.1677/joe.0.1140131. [DOI] [PubMed] [Google Scholar]
- Thomsen MK, Butler CM, Shen MM, Swain A. Sox9 is required for prostate development. Dev Biol. 2008;316:302–311. doi: 10.1016/j.ydbio.2008.01.030. [DOI] [PubMed] [Google Scholar]
- Timms BG. Prostate development: a historical perspective. Differentiation. 2008;76:565–577. doi: 10.1111/j.1432-0436.2008.00278.x. [DOI] [PubMed] [Google Scholar]
- Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS. Estrogenic chemicals in plastic and oral contraceptives disrupt development of the fetal mouse prostate and urethra. Proc Natl Acad Sci USA. 2005;102:7014–7009. doi: 10.1073/pnas.0502544102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms BG, Mohs TJ, Didio LJA. Ductal budding and branching patterns in the developing prostate. J Urol. 1994;151:1427–1432. doi: 10.1016/s0022-5347(17)35273-4. [DOI] [PubMed] [Google Scholar]
- Timms BG, Petersen SL, vom Saal F. Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses. J Urol. 1999;161:1694–1701. [PubMed] [Google Scholar]
- Turner CD. The modification of sexual differentiation in genetic female mice by the prenatal administration of testosterone propionate. J Morphol. 1939;65:353–381. [Google Scholar]
- Vezina CM, Allgeier SH, Moore RW, Lin T-M, Bemis JC, Hardin HA, Gasiewicz TA, Peterson RE. Dioxin causes ventral prostate agenesis by disrupting dorsoventral patterning in developing mouse prostate. Toxicol Sci. 2008;106:488–496. doi: 10.1093/toxsci/kfn183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina CM, Hardin HA, Moore RW, Allgeier SH, Peterson RE. 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits fibroblast growth factor 10-induced prostatic bud formation in mouse urogenital sinus. Toxicol Sci. 2009 doi: 10.1093/toxsci/kfp226. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C, Hicks SM, Moore RW, Peterson RE. TCDD modulates selected developmental signaling pathways during mouse prostate morphogenesis. Organohalogen Compd. 2007;69:629–632. [Google Scholar]
- Warot X, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Gene dosage-dependent effects of the Hoxa-13 and Hoxd-13 mutations on morphogenesis of the terminal parts of the digestive and urogenital tracts. Development. 1997;124:4781–4791. doi: 10.1242/dev.124.23.4781. [DOI] [PubMed] [Google Scholar]
- Young CYF, Johnson MP, Prescott JL, Tindall DJ. The androgen receptor of the testicular-feminized (Tfm) mutant mouse is smaller than the wild-type receptor. Endocrinology. 1989;124:771–775. doi: 10.1210/endo-124-2-771. [DOI] [PubMed] [Google Scholar]
- Zaviačič M, Zajíčková M, Blazeková J, Donárová L, Stvrtina S, Mikulecky M, Zaviačičí T, Holomán K, Breza J. Weight, size, macroanatomy, and histology of the normal prostate in the adult human female: A minireview. J Histotechnol. 2000;23:61–69. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scatterplots of ventral budding patterns in UGS epithelium from control wild-type male, wild-type female, and Tfm male mice on the day of birth. Scatterplots were prepared by tracing UGE outlines and marking ventral bud positions, then superimposing tracings from replicate samples. Ventral prostatic bud positions in control wild-type males are represented by blue dots, while all other ventral bud positions are designated by a black dot. All tracings are in the same orientation (cranial at the top and caudal at the bottom) and at the same nominal magnification. Ventral bud counts are shown as mean ± SE, with the number of replicate samples in parentheses.