Abstract
The interplay of proliferation and differentiation is essential for normal development and organogenesis. Geminin is a cell cycle regulator which controls licensing of origins for DNA replication, safeguarding genomic stability. Geminin has also been shown to regulate cellular decisions of self-renewal versus commitment of neuronal progenitor cells. We discuss here our recent analysis of mice with conditional inactivation of the Geminin gene in the immune system. Our data indicate that Geminin is not indispensable for every cell division: in the absence of Geminin, development of progenitor T-cells appears largely unaffected. In contrast, rapid cell divisions, taking place in vitro upon TCR receptor activation or in vivo during homeostatic proliferation, are defective.
Key words: Geminin, Cdt1, stem cells, licensing, self-renewal, differentiation, cell cycle duration
Geminin Regulates Proliferation and Differentiation
The development of multicellular organisms requires the generation of numerous specialized cells organized in a structure necessary for function. The acquisition of specialized function takes place progressively. During embryogenesis, tissue stem and progenitor cells are undergoing several rounds of division before they adopt a non-dividing differentiated phenotype. Therefore self-renewal and differentiation of stem/progenitor cells should be coordinated to meet criteria set by the function and the size constraints of the developing organ. During development, diffusible factors and membrane receptors influence the specification and differentiation of cells contributing to organ formation through epigenetic changes of chromatin organization and differential activation of gene regulatory networks that are coordinated with changes in cell cycle regulation and DNA replication. Stem and progenitor cells gradually restrict their differentiation potential by exiting the cell cycle, regulating their transcriptional program and committing to a specific cell-fate.
Geminin was identified in the Kirschner lab through two parallel lines of investigation: as both a regulator of DNA replication1 and a regulator of differentiation, involved in specifying neural cell fate during Xenopus embryogenesis.2 A number of studies over the past 10 years have shed more light into the function of Geminin at the molecular level and have suggested that Geminin may coordinate proliferation and differentiation decisions through direct interactions with multiple binding partners.3–5
Geminin, a small coiled-coil protein present in metazoa, inhibits licensing of origins for DNA replication through binding and inhibiting Cdtl, a key member of the pre-replicative complex.6–8 Geminin binding to Cdt1 during the S and G2 phases of the cell cycle inhibits re-replication of origins that have already fired, thereby ensuring once per cell cycle replication and genome stability.9–11 Consistent with its role as a central cell cycle regulator, Geminin depletion results in genome over-replication, DNA damage and G2/M cell cycle arrest12–16 while Geminin overexpression causes G1/S arrest or leads to apoptosis. Different cell types however differ in their response to Geminin depletion or overexpression, with cancer cells appearing more sensitive than normal human cells to both Geminin overexpression and knock-down.17,18
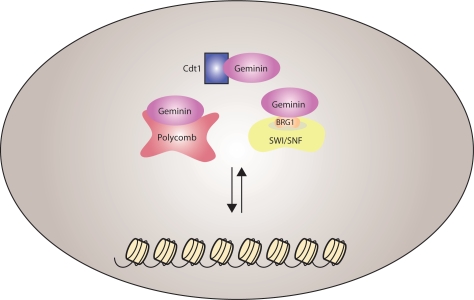
The role of Geminin as a regulator of differentiation and cell fate decisions remains less well understood. During Xenopus early development, Geminin has been shown to bind Brg1 and Brahma (Brm), the catalytic subunits of the chromatin remodelling complex SWI/SNF, and compete with basic helix-loop-helix transcription factors, thereby maintaining neural progenitor cells in an undifferentiated state.19 During neural plate development, Geminin has been shown to regulate Sox2 expression, the earliest definitive marker for the neural plate, through interactions with the chromatin remodelling factor Brm and two novel coiled-coil proteins named ERNI and BERT.20 In Medaka, Geminin has been shown to bind the transcription factor Six3 and antagonize its pro-differentiation function during eye organogenesis.21 Geminin also binds to Hox family members while in addition it regulates expression of the Hox genes through Polycomb-mediated interaction.22 Geminin's role has also been expanded to non-neuronal tissues, as it has been proposed to be involved in maintaining hematopoietic stem cells.23 These lines of work indicate that Geminin interacts with a number of transcription factors and chromatin remodelling activities and regulates transcriptional programs, thereby affecting cell-fate decisions (Fig. 1).
Figure 1.
Balanced interactions of Geminin with Cdt1, SWI/SNF and Polycomb complexes have been implicated in the regulation of proliferation versus differentiation decisions. Geminin binds to the DNA licensing factor Cdt1 and inhibits DNA replication. Direct binding of Geminin to Brg1, the catalytic subunit of the SWI/SNF complex, has been suggested to play a role in the maintenance of neuronal progenitor cells in an undifferentiated state. Interactions of Geminin with Polycomb have been suggested to control homeobox gene expression during embryonic patterning. Geminin might be involved in the coordination of replication and chromatin organization, which are important for progenitor cells proliferation and fate commitment decisions.
Geminin knock-down experiments at the level of the organism are supportive of a central role for Geminin as a regulator of proliferation and differentiation. In the mouse, Geminin-deficient embryos die at the 8 cell stage and fail to form an inner cell mass.24,25 Trophoblast giant cells are present, suggesting a role for Geminin in the establishment of pluripotency.24 Morpholino experiments in Xenopus have shown that reduced Geminin expression leads to cell cycle arrest and embryonic lethality in high doses, while intermediate doses led to downregulation of neural fate markers in a significant percentage of embryos, suggesting that Geminin is required for the acquisition and maintenance of neural fate.12,19 Similar experiments in Medaka revealed optic vesicle enlargement due to increased proliferation.21 While the above experiments have contributed towards our understanding of Geminin's functions, the early lethality associated with a complete Geminin knock-out during embryogenesis hinders an understanding of its role at specific tissues and specific stages during development and homeostasis.
Conditional Inactivation of Geminin in the Mouse Immune System
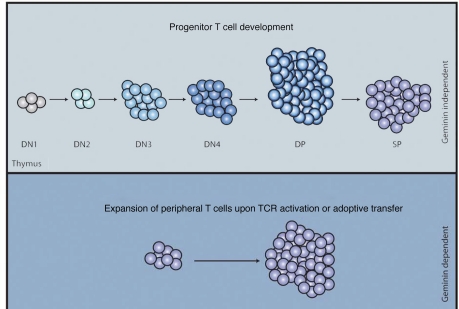
In order to extend our understanding in the in vivo role of Geminin in regulating progenitor cell self-renewal and differentiation decisions in different cellular systems, we have generated mice that allow the conditional inactivation of the mouse Geminin gene, overcoming the lethality observed during the early steps of mouse embryogenesis of constitutive mutants.26 In these mice, exons 3 and 4 of the Geminin gene are flanked by loxP sites, allowing conditional deletion in cells expressing the Cre recombinase. In order to address Geminin's role in the lymphoid system, Geminin loxP mice were crossed to a transgenic mouse line where Cre expression is under the control of the promoter and Locus Control Region of the human CD2 gene. Unexpectedly, Geminin deficiency does not alter development and differentiation of T cells in vivo. Our analysis shows that Geminin-deficient progenitor T-cell populations of the thymus are able to generate all the thymic cell subpopulations and only minor reductions in absolute numbers of double negative 1 (DN1), DN4 and DP T cells were observed (Fig. 2). These data suggest that proliferation and differentiation events associated with T-cell development progress apparently normally in the absence of Geminin. Unpublished observations from our lab indicate that normal development in the absence of Geminin is not a peculiarity of the T-cell compartment: B cell and hepatocyte development also progress apparently unaffected by the absence of Geminin. These findings are challenging our current view of Geminin as indispensible for cell proliferation and differentiation.
Figure 2.
Differential requirements for Geminin in T cells. In the lymphoid system, commitment and differentiation processes of the T-cell lineage in the thymus proceed normally in the absence of Geminin. However, Geminin is required for the proliferation of T cells that take place either in vitro upon TCR receptor activation or in vivo during homeostatic expansion.
In contrast to thymic cell populations, peripheral T-cell populations such as naive, memory and regulatory T cells show a significant reduction in the absence of Geminin. Importantly, mature peripheral T-cells lacking Geminin exhibit significant defects in proliferation both in vitro and in vivo. Following T-cell receptor (TCR) stimulation in vitro, normal peripheral T cells enter the cell cycle and rapidly proliferate, mimicking the activation of peripheral T cells upon infection. Geminin deficient peripheral T cells however show marked cell cycle defects upon TCR stimulation: reduced cell cycle progression and accumulation in S/G2/M with increased protein levels of cyclin B1, cyclin A and tyrosine 15 phosphorylated cdc2. In addition, the number of cells exiting the cell cycle following an attempt to replicate is increased. Similarly, following transfer to a lymphopenic recipient in vivo, Geminin deficient T cells are unable to efficiently proliferate and repopulate the host, showing that Geminin is essential for efficient homeostatic expansion of peripheral T cells (Fig. 2).
These results are supporting the idea that Geminin is an essential cell cycle regulator in certain cells but may be dispensable for cell cycle progression in others. Geminin therefore joins other central cell cycle regulators, such as cdk2, cyclin E and cyclin A, which were unexpectedly shown to be dispensable for cell proliferation, while being required in certain cell types and for certain processes.27–29
Geminin, a Redundant Mechanism for Cdt1 Regulation and Maintenance of Genomic Stability
Our findings for a differential requirement for Geminin in developing thymocytes versus activated peripheral T cells are reminiscent of Geminin depletion experiments in cultured human cells, where DNA re-replication was induced in some cell lines14,15,24,25,30 and not in others.18,31–33 Why do different cells show a differential requirement for Geminin for normal cell cycle progression?
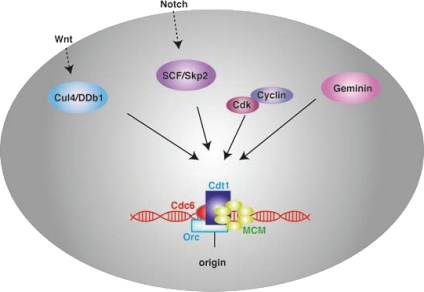
The critical role of Geminin during the cell cycle is believed to be the inhibition of untimely licensing by restricting the activity of Cdt1. The relative expression levels of Cdtl and Geminin might render a particular cell type more sensitive to alterations in the expression of one of the two partners. Consistently, the effects of Cdt1 overexpression also differ between different cell types.15,34–36 In support of this notion, Cdtl protein expression levels in stimulated peripheral T cells that lack Geminin expression is increased compared to the wild-type control, while thymocytes in the absence of Geminin show reduced expression of the Cdtl protein.26 Therefore, the increased sensitivity of peripheral T cells to the absence of Geminin could be attributed to an inability to regulate Cdtl expression levels upon Geminin reduction. In addition to Geminin inhibition, the activity of Cdt1 is controlled through cell cycle phase specific proteolysis mediated by two E3 ubiquitin ligase pathways, SCFSkp2 and CUL4Ddb1Cdt2,37,38 and these two pathways could compensate for Geminin loss in certain cell types or developmental stages. Furthermore, Cyclin A has been shown to act redundantly with Geminin to inhibit genome over-replication in certain cell types (Fig. 3).13,32
Figure 3.
Geminin is one of several factors that regulate Cdt1 activity. the formation on origins of DNA replication of a multisubunit pre-replicative complex consisting of ORC, Cdc6, Cdt1 and MCM proteins licences chromatin for replication. Cdt1 is essential for pre-replicative complex formation and it is regulated by multiple factors. Cdk-dependent phosphorylation and Skp2/SCF-dependent ubiquitination targets Cdt1 for proteolysis. Cdt1 protein levels are also regulated by Cul4/Ddb1/PCNA. Geminin operates as a protein inhibitor through binding and inactivation of Cdt1. these pathways could be modified by extracellular signaling molecules and operate redundantly in order to regulate cell cycle progression and maintenance of genomic integrity.50,51
Timely licensing is controlled at multiple levels and though the Cdt1/Geminin pair appears the critical target for regulation in metazoa, other components of the pre-replicative complex as well as Cyclin Dependent Kinase activity might contribute to the variable phenotypes observed upon Cdtl overexpression or Geminin inhibition in different cell types. The redundant regulation of multiple licensing factors may prevent the deleterious effects from deregulation of any individual factor alone.39,40
Cell Cycle Duration: A Critical Parameter for Geminin Function?
Are there any common traits that could differentiate cell cycles requiring Geminin from those where Geminin function is redundant? In the absence of Geminin in the mouse, inner cell mass (ICM) cells are not formed,24,25 suggesting that Geminin is required for the generation of these cells from uncommitted totipotent embryonic stem cells. Pluripotent stem cells in the preimplantation mouse embryo and embryonic stem cells, which are equivalent to cells comprising the ICM, exhibit very fast cell cycles, lasting approximately 10 hours.41–43 Rapid cell divisions in these cell types are characterized by a very short G1 phase.41,43 As mESCs differentiate, cell cycle duration increases, mainly due to an increase in G1 phase length.43,44 Rapid cell division also characterizes neural stem and progenitor cell populations, while an increase in the length of G1 phase is linked with initiation of fate commitment and differentiation programs.45 Peripheral lymphocytes also have unique properties in division: although mature T cells are quiescent they maintain a high capacity to self-renew to defend the organism from infection by exogenous pathogens. Activated T cells and T cells upon homeostatic proliferation will proliferate very rapidly.46,47 Similarly, cancer cells, which are more sensitive to Geminin depletion than normal cells,18 show increased proliferation rates.
Could the rate of cell proliferation affect a cell's requirement for Geminin? Licensing of replication origins is established during G1 through pre-replicative complex formation, during which MCM proteins associate with chromatin on origins of replication. Rapid cell divisions require licensing to take place in a shorter time, due to the short duration of the G1 phase. Rapidly dividing cells may also need to license a greater number of origins in order to replicate their DNA efficiently. Geminin inhibition of untimely licensing may be more critical under these conditions. During differentiation, the timing of replication of different genomic regions is altered as transcriptional profiles change, while chromatin organization and subnuclear locations are also changing in a coordinate fashion.48,49 Interactions of Geminin with Polycomb group and chromatin remodeling SWI/SNF complexes might be essential in this transition to regulate pluripotency by repressing differentiation.
The differential requirements for cell cycle regulation, the redundant pathways regulating genomic integrity and the relative abundance of Geminin's interacting partners between different cell types may be critical in determining whether Geminin is required for cell proliferation and differentiation.
Conclusions
Conditional inactivation of Geminin in the lymphoid system challenges the prevailing view of Geminin as an essential factor for maintaining genomic stability and controlling progenitor cell proliferation and cell fate acquisition. Geminin has been implicated as a negative regulator of licensing, although there are reports suggesting a positive role as well. Our findings suggest that Geminin is essential for regulating genomic stability and proliferation versus differentiation decisions in a cell type specific context. It is possible that Geminin acts as a redundant mechanism for restricting Cdt1 licensing activity and preserving genomic stability during DNA replication. It is possible that Geminin is required in specific stem and progenitor populations acting as a convergence point for DNA replication, epigenetic changes and transcriptional regulation, while the speed of the cell cycle might be a critical element for determining the role of Geminin. Future experiments are needed to clarify the gene network which operates with Geminin to control self-renewal and commitment in stem and progenitor cells.
Footnotes
Previously published online: www.landesbioscience.com/journals/cc/article/12554
References
- 1.McGarry TJ, Kirschner MW. Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell. 1998;93:1043–1053. doi: 10.1016/s0092-8674(00)81209-x. [DOI] [PubMed] [Google Scholar]
- 2.Kroll KL, Salic AN, Evans LM, Kirschner MW. Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development. 1998;125:3247–3258. doi: 10.1242/dev.125.16.3247. [DOI] [PubMed] [Google Scholar]
- 3.Luo L, Kessel M. Geminin coordinates cell cycle and developmental control. Cell Cycle. 2004;3:711–714. [PubMed] [Google Scholar]
- 4.Kroll KL. Geminin in embryonic development: Coordinating transcription and the cell cycle during differentiation. Front Biosci. 2007;12:1395–1409. doi: 10.2741/2156. [DOI] [PubMed] [Google Scholar]
- 5.Seo S, Kroll KL. Geminin's double life: Chromatin connections that regulate transcription at the transition from proliferation to differentiation. Cell Cycle. 2006;5:374–379. doi: 10.4161/cc.5.4.2438. [DOI] [PubMed] [Google Scholar]
- 6.Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A. Inhibition of eukaryotic DNA replication by geminin binding to Cdt1. Science. 2000;290:2309–2312. doi: 10.1126/science.290.5500.2309. [DOI] [PubMed] [Google Scholar]
- 7.Tada S, Li A, Maiorano D, Mechali M, Blow JJ. Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol. 2001;3:107–113. doi: 10.1038/35055000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishitani H, Taraviras S, Lygerou Z, Nishimoto T. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem. 2001;276:44905–44911. doi: 10.1074/jbc.M105406200. [DOI] [PubMed] [Google Scholar]
- 9.Blow JJ, Gillespie PJ. Replication licensing and cancer—a fatal entanglement? Nat Rev Cancer. 2008;8:799–806. doi: 10.1038/nrc2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishitani H, Lygerou Z. DNA replication licensing. Front Biosci. 2004;9:2115–2132. doi: 10.2741/1315. [DOI] [PubMed] [Google Scholar]
- 11.Petropoulou C, Kotantaki P, Karamitros D, Taraviras S. Cdt1 and Geminin in cancer: markers or triggers of malignant transformation? Front Biosci. 2008;13:4485–4494. doi: 10.2741/3018. [DOI] [PubMed] [Google Scholar]
- 12.McGarry TJ. Geminin deficiency causes a Chk1-dependent G2 arrest in Xenopus. Mol Biol Cell. 2002;13:3662–3671. doi: 10.1091/mbc.E02-04-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mihaylov IS, Kondo T, Jones L, Ryzhikov S, Tanaka J, Zheng J, et al. Control of DNA replication and chromosome ploidy by geminin and cyclin A. Mol Cell Biol. 2002;22:1868–1880. doi: 10.1128/MCB.22.6.1868-1880.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu W, Chen Y, Dutta A. Rereplication by depletion of geminin is seen regardless of p53 status and activates a G2/M checkpoint. Mol Cell Biol. 2004;24:7140–7150. doi: 10.1128/MCB.24.16.7140-7150.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melixetian M, Ballabeni A, Masiero L, Gasparini P, Zamponi R, Bartek J, et al. Loss of Geminin induces rereplication in the presence of functional p53. J Cell Biol. 2004;165:473–482. doi: 10.1083/jcb.200403106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin JJ, Dutta A. ATR pathway is the primary pathway for activating G2/M checkpoint induction after re-replication. J Biol Chem. 2007;282:30357–30362. doi: 10.1074/jbc.M705178200. [DOI] [PubMed] [Google Scholar]
- 17.Shreeram S, Sparks A, Lane DP, Blow JJ. Cell type-specific responses of human cells to inhibition of replication licensing. Oncogene. 2002;21:6624–6632. doi: 10.1038/sj.onc.1205910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W, Depamphilis ML. Selective killing of cancer cells by suppression of geminin activity. Cancer Res. 2009;69:4870–4877. doi: 10.1158/0008-5472.CAN-08-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo S, Herr A, Lim JW, Richardson GA, Richardson H, Kroll KL. Geminin regulates neuronal differentiation by antagonizing Brg1 activity. Genes Dev. 2005;19:1723–1734. doi: 10.1101/gad.1319105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papanayotou C, Mey A, Birot AM, Saka Y, Boast S, Smith JC, et al. A mechanism regulating the onset of Sox2 expression in the embryonic neural plate. PLoS Biol. 2008;6:2. doi: 10.1371/journal.pbio.0060002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Del Bene F, Tessmar-Raible K, Wittbrodt J. Direct interaction of geminin and Six3 in eye development. Nature. 2004;427:745–749. doi: 10.1038/nature02292. [DOI] [PubMed] [Google Scholar]
- 22.Luo L, Yang X, Takihara Y, Knoetgen H, Kessel M. The cell cycle regulator geminin inhibits Hox function through direct and polycomb-mediated interactions. Nature. 2004;427:749–753. doi: 10.1038/nature02305. [DOI] [PubMed] [Google Scholar]
- 23.Ohtsubo M, Yasunaga S, Ohno Y, Tsumura M, Okada S, Ishikawa N, et al. Polycomb-group complex 1 acts as an E3 ubiquitin ligase for Geminin to sustain hematopoietic stem cell activity. Proc Natl Acad Sci USA. 2008;105:10396–10401. doi: 10.1073/pnas.0800672105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez MA, Tachibana KE, Adams DJ, van der Weyden L, Hemberger M, Coleman N, et al. Geminin is essential to prevent endoreduplication and to form pluripotent cells during mammalian development. Genes Dev. 2006;20:1880–1884. doi: 10.1101/gad.379706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara K, Nakayama KI, Nakayama K. Geminin is essential for the development of preimplantation mouse embryos. Genes Cells. 2006;11:1281–1293. doi: 10.1111/j.1365-2443.2006.01019.x. [DOI] [PubMed] [Google Scholar]
- 26.Karamitros D, Kotantaki P, Lygerou Z, Veiga-Fernandes H, Pachnis V, Kioussis D, et al. Differential geminin requirement for proliferation of thymocytes and mature T cells. J Immunol. 184:2432–2441. doi: 10.4049/jimmunol.0901983. [DOI] [PubMed] [Google Scholar]
- 27.Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, et al. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- 28.Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, et al. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet. 2003;35:25–31. doi: 10.1038/ng1232. [DOI] [PubMed] [Google Scholar]
- 29.Kalaszczynska I, Geng Y, Iino T, Mizuno S, Choi Y, Kondratiuk I, et al. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365. doi: 10.1016/j.cell.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu W, Dutta A. An ATR- and BRCA1-mediated Fanconi anemia pathway is required for activating the G2/M checkpoint and DNA damage repair upon rereplication. Mol Cell Biol. 2006;26:4601–4611. doi: 10.1128/MCB.02141-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nishitani H, Lygerou Z, Nishimoto T. Proteolysis of DNA replication licensing factor Cdt1 in S-phase is performed independently of geminin through its N-terminal region. J Biol Chem. 2004;279:30807–30816. doi: 10.1074/jbc.M312644200. [DOI] [PubMed] [Google Scholar]
- 32.Machida YJ, Dutta A. The APC/C inhibitor, Emi1, is essential for prevention of rereplication. Genes Dev. 2007;21:184–194. doi: 10.1101/gad.1495007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulartz M, Knippers R. The replicative regulator protein geminin on chromatin in the HeLa cell cycle. J Biol Chem. 2004;279:41686–41694. doi: 10.1074/jbc.M405798200. [DOI] [PubMed] [Google Scholar]
- 34.Vaziri C, Saxena S, Jeon Y, Lee C, Murata K, Machida Y, et al. A p53-dependent checkpoint pathway prevents rereplication. Mol Cell. 2003;11:997–1008. doi: 10.1016/s1097-2765(03)00099-6. [DOI] [PubMed] [Google Scholar]
- 35.Tatsumi Y, Sugimoto N, Yugawa T, Narisawa-Saito M, Kiyono T, Fujita M. Deregulation of Cdt1 induces chromosomal damage without rereplication and leads to chromosomal instability. J Cell Sci. 2006;119:3128–3140. doi: 10.1242/jcs.03031. [DOI] [PubMed] [Google Scholar]
- 36.Liu E, Lee AY, Chiba T, Olson E, Sun P, Wu X. The ATR-mediated S phase checkpoint prevents rereplication in mammalian cells when licensing control is disrupted. J Cell Biol. 2007;179:643–657. doi: 10.1083/jcb.200704138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishitani H, Sugimoto N, Roukos V, Nakanishi Y, Saijo M, Obuse C, et al. Two E3 ubiquitin ligases, SCF-Skp2 and DDB1-Cul4, target human Cdt1 for proteolysis. EMBO J. 2006;25:1126–1136. doi: 10.1038/sj.emboj.7601002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arias EE, Walter JC. PCNA functions as a molecular platform to trigger Cdt1 destruction and prevent rereplication. Nat Cell Biol. 2006;8:84–90. doi: 10.1038/ncb1346. [DOI] [PubMed] [Google Scholar]
- 39.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 40.Fujita M. Cdt1 revisited: complex and tight regulation during the cell cycle and consequences of deregulation in mammalian cells. Cell Div. 2006;1:22. doi: 10.1186/1747-1028-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burdon T, Smith A, Savatier P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002;12:432–438. doi: 10.1016/s0962-8924(02)02352-8. [DOI] [PubMed] [Google Scholar]
- 42.Savatier P, Huang S, Szekely L, Wiman KG, Samarut J. Contrasting patterns of retinoblastoma protein expression in mouse embryonic stem cells and embryonic fibroblasts. Oncogene. 1994;9:809–818. [PubMed] [Google Scholar]
- 43.Stead E, White J, Faast R, Conn S, Goldstone S, Rathjen J, et al. Pluripotent cell division cycles are driven by ectopic Cdk2, cyclin A/E and E2F activities. Oncogene. 2002;21:8320–8333. doi: 10.1038/sj.onc.1206015. [DOI] [PubMed] [Google Scholar]
- 44.White J, Dalton S. Cell cycle control of embryonic stem cells. Stem Cell Rev. 2005;1:131–138. doi: 10.1385/SCR:1:2:131. [DOI] [PubMed] [Google Scholar]
- 45.Takahashi T, Nowakowski RS, Caviness VS Jr. The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci. 1995;15:6046–6057. doi: 10.1523/JNEUROSCI.15-09-06046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wells AD, Gudmundsdottir H, Turka LA. Following the fate of individual T cells throughout activation and clonal expansion: Signals from T cell receptor and CD28 differentially regulate the induction and duration of a proliferative response. J Clin Invest. 1997;100:3173–3183. doi: 10.1172/JCI119873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Delon J, Germain RN. Information transfer at the immunological synapse. Curr Biol. 2000;10:923–933. doi: 10.1016/s0960-9822(00)00870-8. [DOI] [PubMed] [Google Scholar]
- 48.Hiratani I, Gilbert DM. Replication timing as an epigenetic mark. Epigenetics. 2009;4:93–97. doi: 10.4161/epi.4.2.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aladjem MI. Replication in context: dynamic regulation of DNA replication patterns in metazoans. Nat Rev Genet. 2007;8:588–600. doi: 10.1038/nrg2143. [DOI] [PubMed] [Google Scholar]
- 50.Miranda-Carboni GA, Krum SA, Yee K, Nava M, Deng QE, Pervin S, et al. A functional link between Wnt signaling and SKP2-independent p27 turnover in mammary tumors. Genes Dev. 2008;22:3121–3134. doi: 10.1101/gad.1692808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarmento LM, Huang H, Limon A, Gordon W, Fernandes J, Tavares MJ, et al. Notch1 modulates timing of G1-S progression by inducing SKP2 transcription and p27 Kip1 degradation. J Exp Med. 2005;202:157–168. doi: 10.1084/jem.20050559. [DOI] [PMC free article] [PubMed] [Google Scholar]