Abstract
The standard treatment for advanced, androgen-responsive prostate cancer is androgen deprivation therapy with or without a nonsteroidal antiandrogen, such as bicalutamide. Although maximal androgen blockade exhibits favorable responses in the majority of patients, prostate cancer eventually progresses to an androgen-refractory stage. The mechanism underlying bicalutamide resistance in the course of prostate cancer progression is incompletely understood. However, interleukin-6 (IL-6) plays a critical role in the development and progression of CRPC. Herein, we explored an association between IL-6 and bicalutamide resistance. To study this, series of lower and higher passages of LNCaP cell sublines generated by long-term exposure to IL-6 were used. The cells from higher passages of LNCaP treated with IL-6 developed resistance to bicalutamide treatment compared with parental LNCaP cells. The levels of transcriptional intermediary factor 2 (TIF2) in IL-6-treated LNCaP cells were found to be significantly higher than parental LNCaP cells. Down-regulation of TIF2 expression via short hairpin RNA in IL-6-treated LNCaP cells sensitized these cells to bicalutamide treatment, whereas overexpression of TIF2 in the parental LNCaP cells increased resistance to bicalutamide. Furthermore, overexpression of IL-6 attenuated bicalutamide-mediated blockage of androgen-induced androgen receptor nuclear translocation and recruitment. These results show that overexpression of IL-6 increases the resistance of prostate cancer cells to bicalutamide via TIF2. Overexpression of IL-6 not only plays an important role in prostate cancer progression but also contributes to bicalutamide resistance. Our studies suggest that bicalutamide-IL-6-targeted adjunctive therapy may lead to a more effective intervention than bicalutamide alone.
Introduction
Androgen signaling through the androgen receptor (AR) plays an important role not only in maintaining the function of the prostate but also in promoting the development of castration-resistant prostate cancer (CRPC). A common treatment modality for prostate cancer is androgen deprivation, which can be achieved by surgical or medical castration (1). Chemical castration using luteinizing hormone-releasing hormone analogues (e.g., leuprolide acetate or goserelin acetate) may be combined with nonsteroidal antiandrogens (e.g., flutamide, nilutamide, or bicalutamide; ref. 1). The goal of androgen deprivation treatment is to reduce androgen levels, whereas antiandrogens block androgen binding to AR. The nonsteroidal antiandrogen bicalutamide is often used with androgen deprivation therapy (2–4). Although bicalutamide treatment initially exhibits favorable responses, prostate cancers eventually become refractory and develop resistant to bicalutamide (5, 6). Great effort has focused on understanding CRPC and targeting therapies against it. Unfortunately, despite these research efforts, little therapeutic progress for advanced prostate cancer has been made in the past 50 years.
Interleukin-6 (IL-6) is a glycoprotein and has been implicated in the modulation of growth and differentiation in many cancers including prostate (7–9). The expression of IL-6 and its receptor has been consistently shown in human prostate cancer cell lines and clinical specimens of prostate cancer and benign prostate hyperplasia (10–12). Multiple studies have shown that IL-6 is elevated in the sera of patients with metastatic prostate cancer and that the levels of IL-6 correlate with tumor burden, serum prostate-specific antigen, clinically evident metastases, and CRPC (13, 14). In addition to the clinical data that IL-6 is associated with CRPC, experimental studies show that IL-6 plays a critical role in prostate cancer cell growth and differentiation. IL-6 functions as a paracrine growth factor for the human LNCaP androgen-sensitive prostate cancer cells and an autocrine growth factor for the human DU145 and PC3 androgen-insensitive prostate cancer cells (15). IL-6 activates AR-mediated gene expression by activation of the AR through a Stat3 pathway in LNCaP cells (16–18). Further studies showed that overexpression of IL-6 enhanced prostate-specific antigen mRNA expression in LNCaP cells and can partially rescue LNCaP cells from growth arrest induced by androgen deprivation therapy (19, 20). In addition, overexpression of IL-6 protects LNCaP cells from undergoing apoptosis induced by androgen deprivation therapy (20). Collectively, these findings suggest that IL-6 can regulate the expression of androgen-responsive genes in an androgen-independent manner and induces castration resistant growth of androgen-dependent human prostate cancer cells.
Previous studies suggest a dynamic nature of prostate cancer cells such as LNCaP in response to IL-6 (21, 22). Prolonged passage of LNCaP cells in the presence of IL-6 selects a population that is now adapted to IL-6 and grows in a castration resistant manner (22). We showed previously that whereas short-term treatment with IL-6 inhibits LNCaP cell growth by a paracrine mechanism associated with neuroendocrine differentiation, long-term treatment promotes LNCaP cell growth by an autocrine mechanism accompanied by an activation of AR signaling (22). In the lower passages (<28 passages) of LNCaP cells treated with IL-6, cell growth was severely retarded with neuroendocrine-like morphology and increased expression of neuroendocrine markers, such as neuron-specific enolase and chromogranin A, and loss of AR expression. However, in the higher passages (>42 passages) of LNCaP cells treated with IL-6, cells started to express endogenous IL-6. At the same time, neuroendocrine characteristics disappeared, AR signaling was activated, and cell growth was accelerated with development of castration resistance. These cells thus offer a unique opportunity to study the “evolution” of CRPC and the molecular and cellular changes responsible for the conversion.
In this study, we investigated whether overexpression of IL-6 in androgen-responsive human prostate cancer cells confers resistance to bicalutamide treatment and the potential mechanisms involved in IL-6-mediated bicalutamide resistance.
Materials and Methods
Cell Culture
Parental LNCaP cells were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS) and 100 units/mL penicillin and 0.1 mg/mL streptomycin. LNCaP-IL-6+ cells were cultured in medium containing 10% FBS, penicillin/streptomycin and 5 ng/mL IL-6 (R&D Systems) as described previously (22). The cells were maintained at 37°C in a humidified atmosphere with 5% carbon dioxide.
Growth Assays
LNCaP cells passaged at the same time in the absence of IL-6 were used as controls. Control LNCaP and LNCaP-IL-6+ (passage of ~58; ref. 22) cells were seeded into 12-well plates at a density of 1 × 105 per well in RPMI 1640 containing 10% FBS or 10% charcoal-stripped FBS (CS-FBS). The cells were treated with increasing doses of bicalutamide (1–5 µmol/L). The cells were counted after 48 h treatment.
Plasmids and Cell Transfection
A human transcriptional intermediary factor 2 (TIF2) plasmid was described previously (23). The human TIF2 short hairpin RNA was purchased from Open Biosystems. For transfection, 1 × 105 cells were seeded into 12-well plates with RPMI 1640 containing 10% FBS. Cells were transfected with either TIF2 plasmid (0, 0.1, and 0.2 µg) or TIF2 shRNA (0, 0.1, and 0.2 µg) using Tfx 20 (Promega) according to the manufacturer’s instructions. The total amount of DNA was kept constant with vector control at 0.2 µg/well.
Northern Blot Analysis
Total RNA was extracted from cells with TRIzol reagent (Life Technologies). Each sample (20 µg) was electrophoresed on 1.2% denaturing agarose gels and transferred to a nylon membrane (MSI). TIF2 cDNA was labeled with [α-32P]dCTP (3,000 Ci/mmol; ICN) using the Ready-to-Go DNA Labeling Beads (Amersham Pharmacia Biotech). Hybridization was carried out for 3 h at 65°C in Rapid-hyb buffer (Amersham). Membranes were washed for 15 min at 65°C in 2× SSC, 0.1% SDS (twice), 0.5× SSC, 0.1% SDS, and 0.1× SSC, 0.1% SDS. Radioactivity in the membranes was analyzed with a Storm Phosphoimager System.
Total and Nuclear Lysate Preparation
Total protein lysates were obtained using high-salt buffer with freeze-thaw procedure (19). Briefly, cells were lysed in a high-salt buffer containing 10 mmol/L HEPES (pH 7.9), 0.25 mol/L NaCl, 1% NP-40, and 1 mmol/L EDTA, and total protein in the lysates was determined with the Coomassie Blue Protein Assay Reagent (Pierce). For nuclear lysate preparation, cells were harvested, washed with PBS once, and resuspended in a hypotonic buffer [10 mmol/L HEPES-KOH (pH 7.9), 10 mmol/L KCl, 0.1 mmol/L EDTA, 0.4% NP-40, 0.5 mmol/L phenylmethylsulfonyl fluoride, 0.5 mmol/L DTT, 1 mmol/L NaV, 20 mmol/L NaF, and 1 µg/mL aprotinin] and incubated on ice for 20 min. Nuclei were precipitated by centrifugation at 6,000 rpm at 4°C for 10 min. After washing once with hypotonic buffer, the nuclei were lysed in a high-salt buffer [10 mmol/L HEPES-KOH (pH 7.9), 400 mmol/L NaCl, 1 mmol/L EDTA, 1 mmol/L DTT, 0.5 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L NaV, 20 mmol/L NaF, 20% glycerol, and 1 µg/mL aprotinin] and incubated at 4°C for 30 min with vigorous shaking. The nuclear lysate was cleared by 12,000 rpm centrifugation at 4°C for 15 min. Protein concentration was determined using the Coomassie Plus protein assay kit (Pierce).
Western Blot Analysis
Whole-cell protein extracts or nuclear extracts were resolved on 8% or 10% SDS-PAGE. Proteins were then transferred to nitrocellulose membranes. After blocking for 1 h at room temperature in 5% milk in PBS/0.1% Tween 20, membranes were incubated overnight at 4°C with antibodies against TIF2 (Santa Cruz Biotechnology) or AR (Santa Cruz Biotechnology) in 1% milk in PBS with Tween 20. Following secondary antibody incubation, immunoreactive proteins were visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
Chromatin Immunoprecipitation Assay
LNCaP and LNCaP-IL-6+ cells were cultured in phenol red-free RPMI 1640 supplemented with 10% CS-FBS for 3 days. Cells were treated overnight with or without 5 µmol/L bicalutamide in the presence of 1 nmol/L dihydrotestosterone (DHT). The AR-DNA complexes were cross-linked inside the cells by the addition of formaldehyde (1% final concentration) to the cells in culture. Whole-cell extracts were prepared using sonication and an aliquot of the cross-linked receptor protein complexes was immunoprecipitated by incubation with the AR-specific antibody (AR441; Santa Cruz Biotechnology) overnight at 4°C with rotation. Chromatin-antibody complexes were isolated from solution by incubation with protein G-Sepharose beads for 1 h at 4°C with rotation. The Sepharose-bound immune complexes were washed and eluted from beads with elution buffer (1% SDS and 0.1 mol/L NaHCO3) and DNA was extracted. DNA samples from chromatin immunoprecipitation preparations were analyzed by PCR using primers spanning prostate-specific antigen gene in the region of promoter (forward 5′-CCTAGATGAAGTCTCCATGAGCTACA and reverse 5′-GGGAGGGAGAGCTAGCACTTG).
Statistical Analysis
Data are shown as mean ± SE. Multiple group comparison was done by one-way ANOVA followed by the Scheffe procedure for comparison of means. P < 0.05 was considered statistically significant.
Results
Effect of IL-6 on the Growth of LNCaP Cells in Androgen-Deprived Condition In vitro
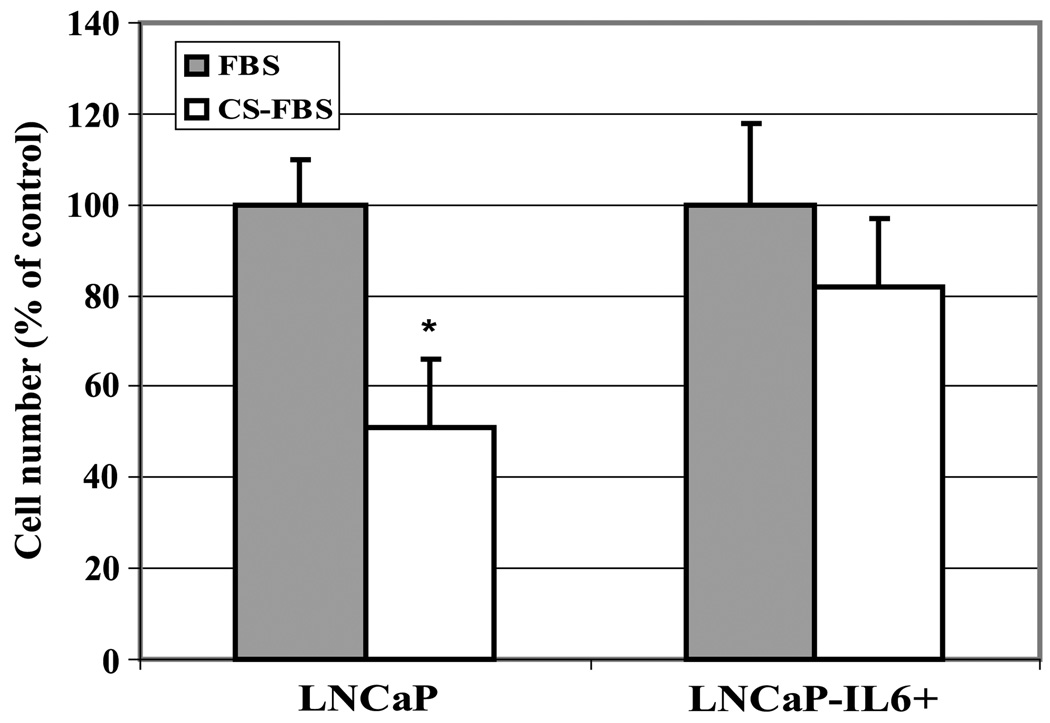
Previous studies showed that long-term treatment of IL-6 promotes LNCaP cell growth by an autocrine mechanism accompanied by the activation of AR signaling (22). Growth of high passages (>58 passages) of LNCaP cells treated with IL-6 (LNCaP-IL-6+58) was accelerated accompanied by overexpression of IL-6 in these cells (22). To determine whether LNCaP-IL-6+58 cells developed castration-resistant growth in vitro, parental LNCaP and LNCaP-IL-6+58 cells were cultured in the presence and absence of androgen, and cell growth was determined. As shown in Fig. 1, the growth of androgen-responsive LNCaP cells in culture was reduced by ~50% after 72 h in androgen-deprived charcoal-stripped serum condition (Hyclone; testosterone concentration is <10−11 mol/L, in which prostate epithelial cells do not respond to testosterone stimulation; ref. 24) compared with that in the normal serum condition (testosterone concentration is ~10−9 mol/L). Addition of dihydrotestosterone (10−9 mol/L) in the charcoal-stripped serum restored growth of LNCaP cells to levels similar to that of the complete normal serum (data not shown). In LNCaP-IL-6+58 cells, however, there was only a 15% to 20% decrease in growth under androgen-deprived conditions compared with growth in normal serum, suggesting that long-term treatment of LNCaP cells with IL-6 can enhance their growth in androgen-deprived conditions in vitro.
Figure 1.
Comparison of cell growth between LNCaP and LNCaP-IL-6+ cells in androgen-deprived condition in vitro. Cells were cultured in RPMI 1640 supplemented with 10% FBS. After 24 h, cells were switched to either 10% FBS or 10% CS-FBS. Cell number was counted after incubating another 72 h. Cell numbers in the complete FBS were expressed as 100%, and cell numbers in the CS-FBS were expressed as percentage relative to those in complete FBS. Mean ± SD of triplicate samples. Three independent experiments were done. *, P < 0.05.
High Passages of LNCaP-IL-6+ Cells Are Resistant to Bicalutamide Compared with Parental LNCaP Cells
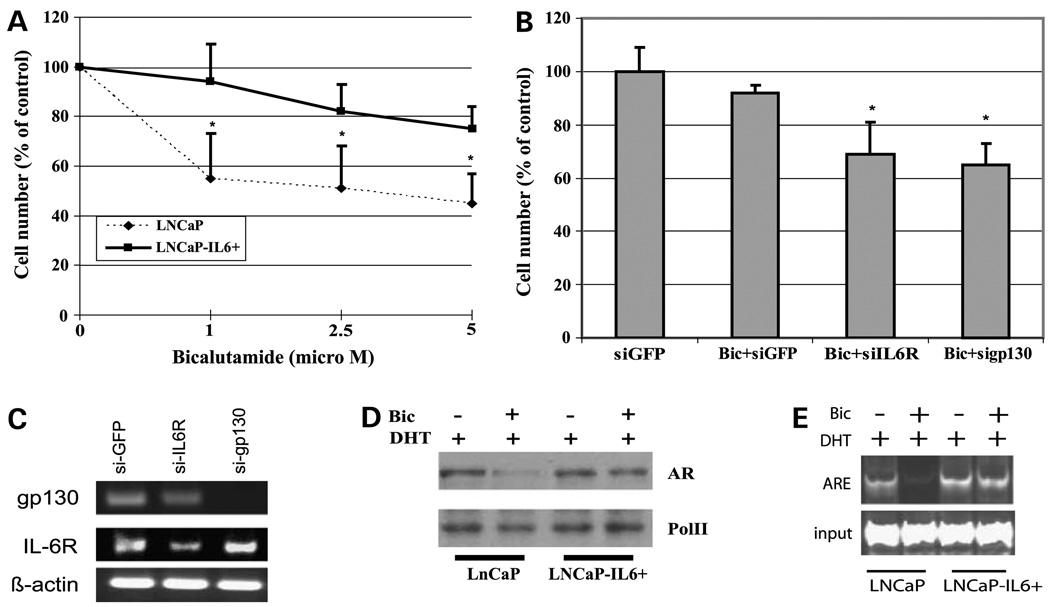
Clinical studies showed that development of CRPC is accompanied by elevated levels of IL-6. To determine whether expression of IL-6 affects the response of prostate cancer cells to antiandrogen treatment, LNCaP-IL-6+ cells were treated with increasing doses of bicalutamide, a clinically used nonsteroidal antiandrogen. Parental LNCaP cells were relatively sensitive to bicalutamide. The growth of LNCaP cells was reduced by ~40% at 1 µmol/L bicalutamide, which reached 55% inhibition at 5 µmol/L bicalutamide (Fig. 2A). In contrast, LNCaP-IL-6+ cells were considerably more resistant to bicalutamide. The growth of LNCaP-IL-6+ cells was reduced by ~10% at 1 µmol/L bicalutamide, which was reduced further by 20% at 5 µmol/L bicalutamide (Fig. 2A).
Figure 2.
Effect of bicalutamide on cell growth and AR nuclear translocation. A, LNCaP-IL-6+ cells are more resistant to bicalutamide treatment compared with parental LNCaP cells. Cells were seeded in 12-well plates at 1 × 105 per well. After 24 h, increasing doses of bicalutamide (0–5 µmol/L) were administered to the cells. Cell number was counted after 48 h. Cell numbers in the control (without bicalutamide) were expressed as 100%, and cell numbers in the treated groups were expressed as percentage relative to the control without bicalutamide. Mean ± SD of triplicate samples. Three independent experiments were done. *, P < 0.05, compared with LNCaP cells at each point. B, knockdown of IL-6 signaling resensitized LNCaP-IL-6+ cells to bicalutamide treatment. LNCaP-IL-6+ cells were transfected with shRNA specific to IL-6 receptor and gp130 and treated with 5 µmol/L bicalutamide for 48 h. Cell number was determined, and gp130 and IL-6 receptor mRNA expression was determined by reverse transcription-PCR analysis (C). D, effect of IL-6 on AR nuclear translocation. LNCaP and LNCaP-IL-6+ cells were cultured in CS-FBS condition in the presence of 1 nmol/L DHT and treated with or without 5 µmol/L bicalutamide. Nuclear proteins were isolated after overnight treatment and subjected to Western blot analysis using AR antibody. Pol II was used as protein loading control. E, Chromatin immunoprecipitation assay of AR recruitment to the ARE site in the presence and absence of bicalutamide (Bic) treatment. LNCaP and LNCaP-IL-6+ cells were cultured in CS-FBS for 3 days, and 1 nmol/L DHT was then added to the cells overnight in the presence and absence of 5 µmol/L bicalutamide. Cell extracts were subjected to chromatin immunoprecipitation assay as described in Materials and Methods.
IL-6 signaling is through its receptor composed of an IL-6-specific receptor subunit (α chain) and a signal transducer, gp130 (β chain). Both subunits belong to the cytokine receptor superfamily (8). The binding of IL-6 to an α chain results in the formation of a hexametric complex containing two molecules of each component: IL-6 α chain and gp130 (8). To examine whether the IL-6-mediated resistance to bicalutamide in LNCaP-IL-6+ cells requires IL-6 signaling, we down-regulated the expression levels of IL-6 receptor and its signal transducer gp130, respectively, using their specific small interfering RNAs. Knockdown of IL-6 receptor or gp130 expression sensitized LNCaP-IL-6+ cells to bicalutamide treatment (Fig. 2B and C). These results suggest that acquisition of IL-6 expression by prolonged IL-6 treatment in LNCaP cells confers a significant level of bicalutamide resistance.
Interestingly, bicalutamide treatment blocked AR nuclear translocation induced by DHT in LNCaP cells (Fig. 2D) but failed to block AR nuclear translocation induced by DHT in LNCaP-IL-6+ cells (Fig. 2D). Consistent with AR nuclear translocation, bicalutamide treatment reduced DHT-mediated AR binding to the ARE site (ARE I/II of prostate-specific antigen promoter gene) in LNCaP cells but failed to block DHT-mediated AR binding to the ARE site in LNCaP-IL-6+ cells (Fig. 2E). Collectively, these results suggest that persistent activation of AR signaling by IL-6 may contribute to bicalutamide resistance.
TIF2 Expression Is Associated with Bicalutamide Resistance by LNCaP-IL-6+ Cells
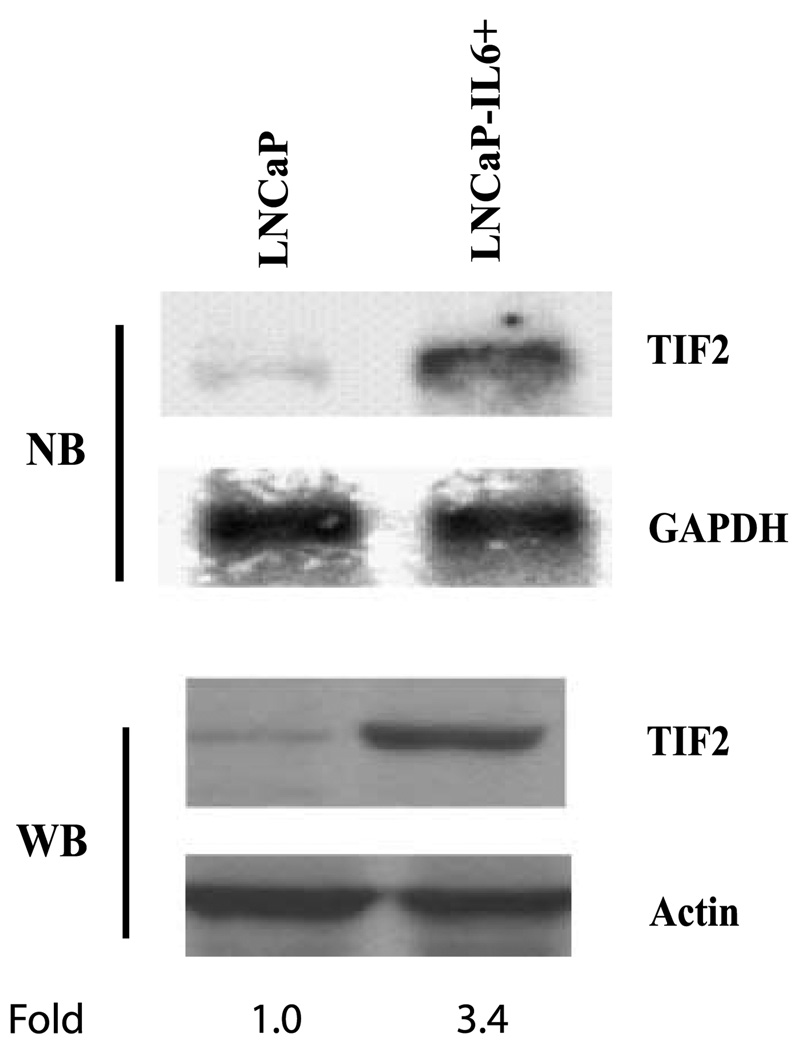
TIF2 interacts with AR to enhance ligand-dependent transactivation of AR. The expression of TIF2 is increased in recurrent prostate cancer after medical or surgical castration (25), suggesting that TIF2 may be involved in the recurrence of prostate cancer. To determine whether TIF2 expression is elevated in LNCaP-IL-6+ cells compared with LNCaP cells, Northern blot analysis was done. As shown in Fig. 3, the level of TIF2 mRNA was increased in LNCaP-IL-6+ cells compared with LNCaP cells. Consistent with mRNA levels, the levels of TIF2 protein expression were considerably elevated compared with the parental LNCaP cells (Fig. 3).
Figure 3.
TIF2 expression in LNCaP and LNCaP-IL-6+ cells. Total RNA and protein were extracted from LNCaP and LNCaP-IL-6+ cells. TIF2 mRNA expression was analyzed by Northern blot (NB), and TIF2 protein expression was examined by Western blot (WB) using antibody against TIF2. GAPDH and actin were used as loading controls for RNA and protein, respectively. Levels of TIF2 protein were normalized to those of actin and expressed as fold change relative to LNCaP cells.
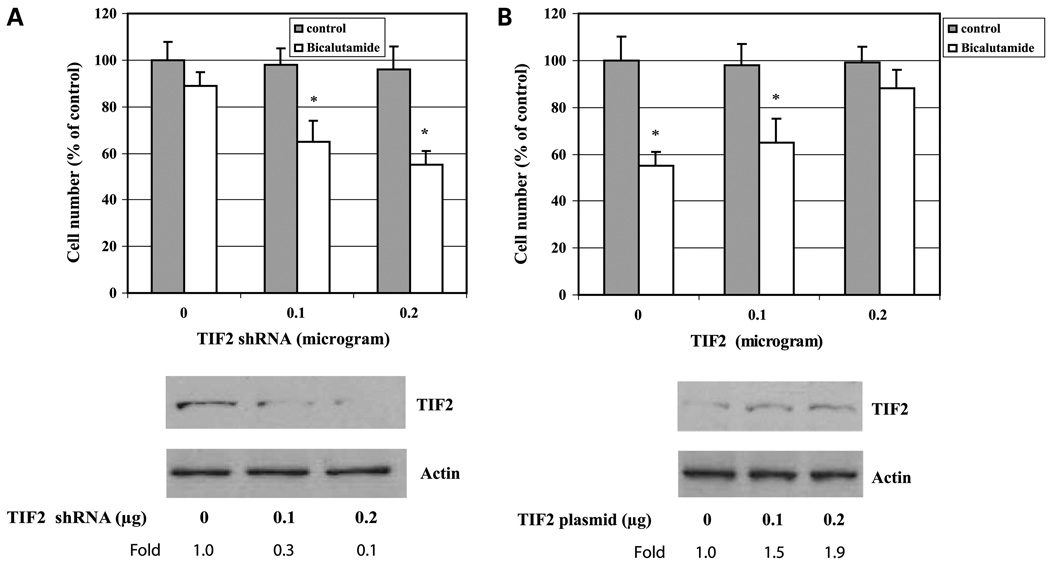
Having shown that the level of TIF2 protein is elevated in LNCaP-IL-6+ cells compared with the parental LNCaP cells, we next determined whether TIF2 expression is associated with the acquisition of resistance to bicalutamide by LNCaP-IL-6+ cells. LNCaP-IL-6+ cells were transfected with shRNA specific to TIF2 and the cells were then treated with bicalutamide and the cell number was determined. Knockdown of TIF2 expression by TIF2-specific shRNA in LNCaP-IL-6+ cells sensitized their response to bicalutamide (Fig. 4A), whereas overexpression of TIF2 in LNCaP cells by transfection of a plasmid encoding TIF2 cDNA increased their resistance to bicalutamide treatment (Fig. 4B). Collectively, these results indicate that TIF2 expression plays a critical role in IL-6-mediated bicalutamide resistance in LNCaP prostate cancer cells.
Figure 4.
Effect of TIF2 expression on the response of cells to bicalutamide. A, LNCaP-IL-6+ cells were transfected with increasing doses of TIF2 specific shRNA (0–0.2 µg). Cells were then treated with 5 µmol/L bicalutamide for 48 h. Cells without bicalutamide treatment were used as control. Cell number was counted. Bottom, TIF2 protein expression by Western blot analysis. B, LNCaP cells were transfected with increasing doses of TIF2 expression plasmid (0–0.2 µg). Cells were then treated with 5 µmol/L bicalutamide for 48 h. The controls are the untreated cells. Cell number was counted. Bottom, TIF2 protein expression by Western blot analysis. Levels of TIF2 protein were normalized to those of actin and expressed as fold induction relative to the controls. Mean ± SD of triplicate samples. *, P < 0.05.
Discussion
CRPC is the eventually inevitable outcome, despite initial high response rates and palliative clinical benefits of androgen deprivation therapy combined with bicalutamide. Although bicalutamide lacks cross-reactivity with other steroid receptors and has improved oral bioavailability (2–4) and less diarrhea than flutamide, bicalutamide resistance still exhibits a challenge to prostate cancer treatment (5, 6). In the present study, we showed that long-term treatment of androgen-responsive LNCaP cells with IL-6 confers bicalutamide resistance mediated by TIF2.
Bicalutamide is effective at blocking AR activity and tumor growth in androgen-responsive prostate cancer. However, prostate cancer cells eventually develop resistance to bicalutamide therapy as shown by the limited response to secondary addition or withdrawal of bicalutamide (5, 6). Both bicalutamide withdrawal syndrome and bicalutamide resistance represent clinical dilemmas in advanced prostate cancer. Bicalutamide withdrawal syndrome occurs in a small fraction of patients who are treated with bicalutamide as part of their initial androgen deprivation therapy. This phenomenon was recapitulated in a cell culture model in which long-term growth of LNCaP cells in medium with bicalutamide selected for cells that are bicalutamide stimulated (26). One mechanism responsible for agonist activity of bicalutamide is the AR mutation in codon W741L (27, 28). Mutation of codon 741 in LNCaP cells confers agonist activity to bicalutamide (27, 28), suggesting that the mutation of W741L in AR is likely involved to be in bicalutamide withdrawal syndrome (bicalutamide agonist). Bicalutamide resistance occurs in the majority of cases of CRPC (6). One of the mechanisms associated with bicalutamide resistance in CRPC may be the decreased bicalutamide binding to AR and hypersensitivity to low levels of endogenous androgens by enhancing the recruitment of coactivator proteins (6, 29). Consistent with this mechanism, in LNCaP-IL-6+ cells, acquisition of IL-6 expression by LNCaP cells resulted in increased resistance to bicalutamide treatment, which was accompanied by increasing levels of TIF2 protein expression and enhanced recruitment of TIF2 to the androgen-responsive elements in androgen-responsive genes. Additionally, IL-6 expression attenuated bicalutamide-mediated blockage of androgen-induced AR nuclear translocation, thus allowing AR to translocate to nucleus and activate androgen-responsive genes even in the presence of bicalutamide. These results provide evidence that IL-6 may directly participate in bicalutamide resistance.
TIF2/glucocorticoid receptor-interacting protein (TIF2/GRIP1/SRC2) is a member of the steroid receptor coactivator (SRC/p160) family of coactivators, which also include SRC-1 and SRC-3 and function as enhances of AR transactivation (30). Prostate cancer patients with increased expression of TIF2 have more aggressive tumors than those with lower expression, and significantly higher TIF2 mRNA levels were coincident with biochemical recurrence (25, 31–33). In the present study, the levels of TIF2 mRNA and protein were elevated in LNCaP-IL-6+ cells compared with parental LNCaP cells. Knockdown of TIF2 expression by TIF2 shRNA in LNCaP-IL-6+ cells sensitized the cells to bicalutamide treatment, whereas overexpression of TIF2 in LNCaP cells increased cellular resistance to bicalutamide. One of the mechanisms contributing to AR activation in androgen recurrent prostate cancer might involve TIF2 in maintaining AR activity and inducing lower-affinity steroids such as adrenal androgens to form AR-TIF2 complexes that increases the ligand-bound AR in the absence of testicular androgens (25, 31). It is reported that bicalutamide may stimulate AR binding to DNA but in a transcriptionally inactive conformation by failing to mediate the AR NH2/COOH-terminal interaction or recruitment of TIF2 (29). Our results suggest that overexpression of IL-6 may overcome bicalutamide-mediated inhibition of TIF2 recruitment through increasing TIF2 expression and enhancing the recruitment of TIF2 to the active sites.
In summary, our studies show that overexpression of IL-6 in androgen-responsive LNCaP prostate cancer cells increases resistance to bicalutamide treatment. IL-6 induces TIF2 expression and recruitment to the androgen-responsive genes and attenuates bicalutamide-mediated blockage of androgen-induced AR nuclear translocation, which may contribute to bicalutamide resistance.
Acknowledgments
Grant support: NIH grants CA90271 and CA109441.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–396. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolvenbag GJ, Furr BJ. Relative potency of bicalutamide (Casodex) and flutamide (Eulexin) Urology. 1999;54:194–197. [PubMed] [Google Scholar]
- 3.Kolvenbag GJ, Blackledge GR. Worldwide activity and safety of bicalutamide: a summary review. Urology. 1996;47:70–79. doi: 10.1016/s0090-4295(96)80012-4. discussion 80-4. [DOI] [PubMed] [Google Scholar]
- 4.Blackledge G, Kolvenbag G, Nash A. Bicalutamide: a new antiandrogen for use in combination with castration for patients with advanced prostate cancer. Anti Cancer Drugs. 1996;7:27–34. [PubMed] [Google Scholar]
- 5.Scher HI, Steineck G, Kelly WK. Hormone-refractory (D3) prostate cancer: refining the concept. Urology. 1995;46:142–148. doi: 10.1016/s0090-4295(99)80182-4. [DOI] [PubMed] [Google Scholar]
- 6.Hodgson MC, Astapova I, Hollenberg AN, Balk SP. Activity of androgen receptor antagonist bicalutamide in prostate cancer cells is independent of NCoR and SMRT corepressors. Cancer Res. 2007;67:8388–8395. doi: 10.1158/0008-5472.CAN-07-0617. [DOI] [PubMed] [Google Scholar]
- 7.Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- 8.Murakami M, Hibi M, Nakagawa N, et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Science. 1993;260:1808–1810. doi: 10.1126/science.8511589. [DOI] [PubMed] [Google Scholar]
- 9.Simpson RJ, Hammacher A, Smith DK, Matthews JM, Ward LD. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siegall CB, Schwab G, Nordan RP, FitzGerald DJ, Pastan I. Expression of the interleukin 6 receptor and interleukin 6 in prostate carcinoma cells. Cancer Res. 1990;50:7786–7788. [PubMed] [Google Scholar]
- 11.Siegsmund MJ, Yamazaki H, Pastan I. Interleukin 6 receptor mRNA in prostate carcinomas and benign prostate hyperplasia. J Urol. 1994;151:1396–1399. doi: 10.1016/s0022-5347(17)35267-9. [DOI] [PubMed] [Google Scholar]
- 12.Hobisch A, Rogatsch H, Hittmair A, et al. Immunohistochemical localization of interleukin-6 and its receptor in benign, premalignant and malignant prostate tissue. J Pathol. 2000;191:239–244. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH633>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Drachenberg DE, Elgamal AA, Rowbotham R, Peterson M, Murphy GP. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–133. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 14.Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC. Elevated levels of circulating interleukin-6 and transforming growth factor-β1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 15.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 16.Hobisch A, Eder IE, Putz T, et al. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58:4640–4645. [PubMed] [Google Scholar]
- 17.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells [In Process Citation] Cancer Res. 2000;60:2132–2135. [PubMed] [Google Scholar]
- 18.Lou W, Ni Z, Dyer K, Tweardy DJ, Gao AC. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 19.Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Interleukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9:370–376. [PubMed] [Google Scholar]
- 20.Lee SO, Lou W, Johnson CS, Trump DL, Gao AC. Interleukin-6 protects LNCaP cells from apoptosis induced by androgen deprivation through the Stat3 pathway. Prostate. 2004;60:178–186. doi: 10.1002/pros.20045. [DOI] [PubMed] [Google Scholar]
- 21.Hobisch A, Ramoner R, Fuchs D, et al. Prostate cancer cells (LNCaP) generated after long-term interleukin 6(IL-6) treatment express IL-6 and acquire an IL-6 partially resistant phenotype. Clin Cancer Res. 2001;7:2941–2948. [PubMed] [Google Scholar]
- 22.Lee SO, Chun JY, Nadiminty N, Lou W, Gao AC. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67:764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- 23.De Miguel F, Lee SO, Onate SA, Gao AC. Stat3 enhances transactivation of steroid hormone receptors. Nucl Recept. 2003;1:3. doi: 10.1186/1478-1336-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Foster BA, Cunha GR. Efficacy of various natural and synthetic androgens to induce ductal branching morphogenesis in the developing anterior rat prostate. Endocrinology. 1999;140:318–328. doi: 10.1210/endo.140.1.6435. [DOI] [PubMed] [Google Scholar]
- 25.Gregory CW, He B, Johnson RT, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–4319. [PubMed] [Google Scholar]
- 26.Culig Z, Hoffmann J, Erdel M, et al. Switch from antagonist to agonist of the androgen receptor bicalutamide is associated with prostate tumour progression in a new model system. Br J Cancer. 1999;81:242–251. doi: 10.1038/sj.bjc.6690684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hara T, Miyazaki J, Araki H, et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 2003;63:149–153. [PubMed] [Google Scholar]
- 28.Bohl CE, Gao W, Miller DD, Bell CE, Dalton JT. Structural basis for antagonism and resistance of bicalutamide in prostate cancer. Proc Natl Acad Sci U S A. 2005;102:6201–6206. doi: 10.1073/pnas.0500381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masiello D, Cheng S, Bubley GJ, Lu ML, Balk SP. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J Biol Chem. 2002;277:26321–26326. doi: 10.1074/jbc.M203310200. [DOI] [PubMed] [Google Scholar]
- 30.Ye X, Han SJ, Tsai SY, et al. Roles of steroid receptor coactivator (SRC)-1 and transcriptional intermediary factor (TIF) 2 in androgen receptor activity in mice. Proc Natl Acad Sci U S A. 2005;102:9487–9492. doi: 10.1073/pnas.0503577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agoulnik IU, Vaid A, Nakka M, et al. Androgens modulate expression of transcription intermediary factor 2, an androgen receptor coactivator whose expression level correlates with early biochemical recurrence in prostate cancer. Cancer Res. 2006;66:10594–10602. doi: 10.1158/0008-5472.CAN-06-1023. [DOI] [PubMed] [Google Scholar]
- 32.Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Expression and function of androgen receptor coactivators in prostate cancer. J Steroid Biochem Mol Biol. 2004;92:265–271. doi: 10.1016/j.jsbmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Fujimoto N, Miyamoto H, Mizokami A, et al. Prostate cancer cells increase androgen sensitivity by increase in nuclear androgen receptor and androgen receptor coactivators; a possible mechanism of hormoneresistance of prostate cancer cells. Cancer Invest. 2007;25:32–37. doi: 10.1080/07357900601130698. [DOI] [PubMed] [Google Scholar]