Abstract
OBJECTIVE
To determine the relationship between mean sensor glucose concentrations and hemoglobin A1c (HbA1c) values measured in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications laboratory at the University of Minnesota in a cohort of subjects with type 1 diabetes from the Juvenile Diabetes Research Foundation continuous glucose monitoring randomized trial.
RESEARCH DESIGN AND METHODS
Near-continuous glucose sensor data (≥4 days/week) were collected for 3 months before a central laboratory–measured HbA1c was performed for 252 subjects aged 8–74 years, the majority of whom had stable HbA1c values (77% within ±0.4% of the patient mean).
RESULTS
The slope (95% CI) for mean sensor glucose concentration (area under the curve) versus a centrally measured HbA1c was 24.4 mg/dL (22.0–26.7) for each 1% change in HbA1c, with an intercept of −16.2 mg/dL (−32.9 to 0.6). Although the slope did not vary with age or sex, there was substantial individual variability, with mean sensor glucose concentrations ranging from 128 to 187 mg/dL for an HbA1c of 6.9–7.1%. The root mean square of the errors between the actual mean sensor glucose concentration versus the value calculated using the regression equation was 14.3 mg/dL, whereas the median absolute difference was 10.1 mg/dL.
CONCLUSIONS
There is substantial individual variability between the measured versus calculated mean glucose concentrations. Consequently, estimated average glucose concentrations calculated from measured HbA1c values should be used with caution.
Hemoglobin A1c (HbA1c) is a time-honored gold standard measure of overall diabetes control, and HbA1c values serve as the targets for diabetes management (1). The chemistry of glycation predicts a straightforward relationship between mean glucose concentrations and HbA1c values over the average lifespan of a patient’s red cells (2). Because the Diabetes Control and Complications Trial (DCCT) (3) demonstrated that improved glycemic control, measured as HbA1c, decreased the risk of long-term diabetic complications, most HbA1c measurements have been standardized to the DCCT values via the National Glycohemoglobin Standardization Program. Current HbA1c assays can be fast, precise, and accurate (4).
Determining the true relationship between mean glucose concentrations and HbA1c values has been hampered by limitations in accessing mean glucose concentrations in groups of patients over a period of ≥3 months. Discrete glucose measurements obtained infrequently over the day often fail to capture the true magnitude of glycemic excursions commonly found in patients with type 1 diabetes (5) and underestimate the extent and frequency of nocturnal hypoglycemia (6).
In contrast, the recently completed Juvenile Diabetes Research Foundation (JDRF)-sponsored continuous glucose monitoring (CGM) trial provided data to closely examine the relationship between mean glucose concentrations, measured in a near-continuous fashion for 3 months, and the subsequent HbA1c values measured centrally in the DCCT/Epidemiology of Diabetes Interventions and Complications (EDIC) laboratory in patients with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The JDRF CGM randomized trial protocol has been described in detail previously (7–9). Major eligibility criteria included age >8 years, type 1 diabetes for at least 1 year, use of either an insulin pump or at least three daily insulin injections, and an HbA1c value <10.0%. Subjects were randomly assigned to either a CGM group or a control group that used standard home blood glucose monitoring for the first 6 months. After 6 months, both groups used CGM.
Subjects received one of the following CGM devices: the DexCom SEVEN (DexCom, San Diego, CA), the MiniMed Paradigm REAL-Time insulin pump and continuous glucose monitoring system (Medtronic MiniMed, Northridge, CA), or the FreeStyle Navigator (Abbott Diabetes Care, Alameda, CA). Each subject was instructed to wear the sensor on a continuous basis.
HbA1c values were measured at the University of Minnesota using the Tosoh HbA1c 2.2 Plus Glycohemoglobin Analyzer (10). The cohort did not contain enough non–white or Hispanic subjects to evaluate race/ethnicity.
Statistical analysis
We limited our analysis to subjects who averaged ≥4 days per week of CGM use in the 3 months before an HbA1c measurement. To minimize the impact of changing glycemic control after the introduction of CGM, we only analyzed the 3 months of CGM data collected before the 12-month (end-of-study) HbA1c measurement. An HbA1c value was obtained for 436 subjects who completed the 12-month visit. Of these, 252 subjects had worn their CGM device for an average of ≥4 days per week during the prior 3 months and were included in these analyses.
Mean glucose concentrations were calculated over the 91-day period before the HbA1c measurement, giving equal weight to each of the 24 h of the day. Similar calculations were done for the mean glucose values during the 1-month (30-day) and 2-month (61-day) intervals before the HbA1c measurement. Least–squares regression analysis was performed using mean glucose concentration as the dependent variable and HbA1c as the independent variable (linear term). Fitting higher order polynomial terms showed no deviation from linearity. Residual values were examined to verify that they followed an approximate normal distribution. No outliers or overly influential data points were identified. A plot of residuals against predicted values showed no meaningful deviation from the assumption of homoscedasticity.
RESULTS
At the 12-month visit, the 252 subjects in analysis ranged in age from 9 to 74 years (mean ± SD: 32 ± 17), with 21% of subjects <15 years, 24% between 15 and 24 years, and 55% ≥25 years. Median duration of diabetes was 7 years (25th to 75th percentile, 4–9) for children, 8 years (5–10) for adolescents, and 24 years (17–32) for adults; 54% were female and 94% were white. HbA1c values ranged from 5.1 to 9.6% (7.1 ± 0.8%). Approximately half of subjects had a stable HbA1c value, with 55% being within ±0.2% of the HbA1c value measured 3 months prior, 21% improving ≥0.3%, and 24% worsening ≥0.3% over the last 3 months. In total, 346,434 h of CGM glucose values (median 1,433 h per subject) were analyzed.
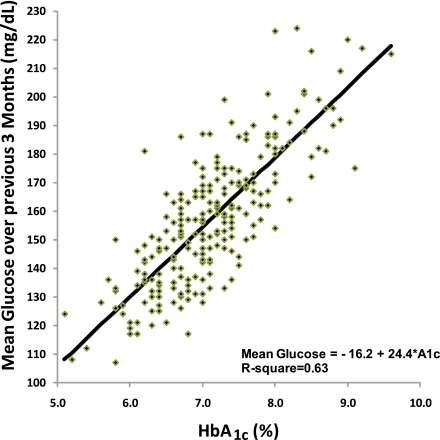
The slope (95% CI) for mean sensor glucose concentration (area under the curve) versus a centrally measured HbA1c was 24.4 mg/dL (22.0–26.7) for each 1% change in HbA1c with an intercept of −16.2 mg/dL (−32.9 to 0.6) (Fig. 1 and Table 1). Using only 1 or 2 months of glucose data before the HbA1c measurement did not alter the slope (Table 1).
Figure 1.
Mean glucose versus HbA1c: mean glucose measured by the CGM device over 3 months (91 days) before the HbA1c measurement (n = 252). Regression line was calculated using least squares. (A high-quality color representation of this figure is available in the online issue.)
Table 1.
Mean glucose versus HbA1c in subgroups
| Slope for mean glucose versus HbA1c (mg/dL per 1%) |
||||
|---|---|---|---|---|
| Subjects (n)* | 3 months† | 2 months† | 1 month† | |
| Overall | 252 | 24.4 ± 2.3 | 25.4 ± 2.4 | 25.7 ± 2.9 |
| Age (years) | ||||
| 8–14 | 54 | 25.0 ± 3.7 | 26.3 ± 4.1 | 26.6 ± 4.9 |
| 15–24 | 60 | 24.6 ± 4.8 | 25.1 ± 5.3 | 26.3 ± 6.9 |
| ≥25 | 138 | 20.7 ± 3.5 | 21.8 ± 3.6 | 20.9 ± 4.1 |
| Treatment group | ||||
| Control | 122 | 22.5 ± 3.7 | 24.2 ± 4.0 | 22.8 ± 4.8 |
| RT-CGM | 130 | 25.7 ± 2.9 | 26.1 ± 3.1 | 27.9 ± 3.6 |
| Sex | ||||
| Female | 137 | 24.8 ± 3.0 | 25.8 ± 3.2 | 25.9 ± 3.9 |
| Male | 115 | 23.5 ± 3.6 | 24.5 ± 3.8 | 25.0 ± 4.5 |
| Insulin delivery | ||||
| Multiple daily injections | 42 | 25.8 ± 6.0 | 26.8 ± 6.1 | 29.3 ± 8.0 |
| Pump | 210 | 23.5 ± 2.5 | 24.3 ± 2.7 | 24.0 ± 3.2 |
| CGM device | ||||
| DexCom | 53 | 25.8 ± 4.9 | 27.5 ± 5.3 | 29.5 ± 7.2 |
| Navigator | 52 | 20.7 ± 4.3 | 22.0 ± 4.8 | 21.1 ± 5.8 |
| Paradigm | 147 | 24.8 ± 3.3 | 25.4 ± 3.4 | 25.4 ± 3.8 |
| Change in HbA1c over the prior 3 months | ||||
| Improved ≥0.5% | 26 | 26.4 ± 5.7 | 25.3 ± 5.7 | 25.2 ± 6.4 |
| Within ±0.4% | 195 | 24.2 ± 3.0 | 24.6 ± 3.2 | 25.5 ± 3.8 |
| Worsened ≥0.5% | 31 | 25.7 ± 5.8 | 27.1 ± 6.1 | 23.6 ± 7.5 |
Data are slopes (± margin of error for 95% CI) unless otherwise indicated.
*One subject was not included in the 1-month analysis because of insufficient data.
†Mean glucose calculated from CGM data taken over 3 months (91 days), 2 months (61 days), and 1 month (30 days) before the HbA1c measurement. To convert slopes to mmol/L per 1%, divide by 18. RT-CGM, real-time continuous glucose monitoring.
The slope of mean glucose concentration versus HbA1c value did not vary meaningfully by age, sex, or type of CGM device (Table 1). Although only 42 of the 252 subjects were not insulin pump users, the mode of insulin delivery did not materially alter the slope. Reanalyzing the data using only the 195 subjects whose HbA1c remained within ±0.4% of the value obtained 3 months earlier or the 138 subjects whose HbA1c remained within ±0.2% did not materially alter the slopes.
Substantial individual variability existed in the relationship between HbA1c and mean glucose concentration. For HbA1c values between 6.9 and 7.1% (n = 46), the average sensor mean glucose concentrations ranged from 128 to 187 mg/dL. For HbA1c values between 7.9 and 8.1% (n = 16), the average sensor mean glucose concentrations ranged from 154 to 223 mg/dL (Fig. 1). The root mean square of the errors between the actual mean sensor glucose concentration versus the value calculated using the regression equation was 14.3 mg/dL, whereas the median absolute difference was 10.1 mg/dL. A total of 91% of subjects had mean glucose concentrations within ±15% of the calculated average glucose concentrations (calculated from HbA1c).
CONCLUSIONS
The estimated slope of the relationship between mean glucose concentration and HbA1c has varied from study to study (Table 2). In studies that used infrequent discrete blood glucose testing, Hempe et al. (11) found a slope of 18.5 mg/dL for each unit (%) change in HbA1c, whereas Rohlfing et al. (12) and Makris et al. (13) found slopes of ∼35 mg/dL for each unit (%) change in HbA1c, a number that was used to describe the relationship for a decade. These investigators also found wide variability between measured mean glucose concentrations and estimated average glucose values calculated using their regression equations.
Table 2.
Summary of published data
| Source | Type 1, type 2, nondiabetes | Subjects (n) | Approximate HbA1c range (National Glycohemoglobin Standardization Program) | Children, adults | Length | Method: discrete or CGM | Curve fit (R2) | Predict HbA1c slope (95% CI) | Intercept (mg/dL) (95% CI) | Range of actual mean glucose at 6.9–7.1%† |
|---|---|---|---|---|---|---|---|---|---|---|
| Discrete | ||||||||||
| Hempe et al. (11) | Type 1 diabetes | 128 | 6.5–18.7% | Children, adolescents, adults | Up to 2.3 years | Discrete | Linear (0.50) | 18.5* | ∼−4.8* | ∼90–235 |
| Rohlfing et al. (12) | Type 1 diabetes | 1,439 | 5.3–13.3%† | Adolescents, adults | 3–9 years | Discrete 7 point quarterly | Linear (0.67) | 35.6 | −77.3 | ∼100–250 |
| Makris et al. (13) | Type 2 diabetes and/or metabolic syndrome | 140 | 5.1–10.9% | Adults 41–81 years | 1 month | Discrete 6 point 12 times in a month | Linear (0.86) | 34.7 (32.5–37.0) | −79.2 | ∼127–207 |
| CGM | ||||||||||
| Nathan et al. (22) | Type 1 and type 2 diabetes and nondiabetes | 15, 7, and 3 | 4.6–10.2%† | Adults | 3 months | CGM | Linear (0.79) | 31.5 | −68.6 | Too few |
| Wilson et al. (16) | Type 1 diabetes | 48 | 5.8–8.8%† | Children, adolescents | 6 months | CGM | Linear | 18 (14–22) | +40 | ∼138–189 |
| Nathan et al. (15) | Type 1 and type 2 diabetes and nondiabetes | 268, 159, and 80 | 3.8–14.3%† | Adults | 3 months | Intermittent CGM >7 days over 3 months discrete | Linear (0.84) | 28.7 | −46.7 | ∼125–205 |
| Mazze (14) | Type 1 and type 2 diabetes and nondiabetes | 124 | 4.9–10.4%† | Adults | 8–75 days | CGM | Linear (0.71) | 26.3* | −32.7* | ∼130–150 |
| Current study | Type 1 diabetes | 252 | 5.1–9.7% | Children, adolescents, adults | 3-month continuous | CGM | Linear (0.63) | 24.4 (22.0−26.7) | −16.2 | 128–187 |
*Study originally reported slope from a model with HbA1c as the independent variable (i.e., HbA1c = slope × mean glucose + intercept). Values were converted to equivalent slope and intercept with mean glucose as the dependent variable using the reported R2 value.
†Estimated from graphs.
In our study, the slope of the regression line was 24–25 mg/dL glucose for every 1% change in HbA1c. This value is lower than values reported earlier using six to seven intermittent sample blood glucose profiles (11,12) but similar to the results of other studies that used CGM (14–16). For example, using CGM, Mazze (14) found a slope of 26.3 with mean glucose concentration as the dependent variable.
Nathan et al. (15) and Borg et al. (17) used a combination of both intermittent discrete and intermittent CGM data from adults with and without diabetes. They found a slope of 28.7 mg/dL glucose for every 1% change in HbA1c using CGM data (15), which was also similar to the value in the current study, and a correlation of 0.89 between HbA1c and mean glucose using CGM and self-monitoring blood glucose data combined (17).
It is not surprising that the relationship between measured glucose concentrations and HbA1c differs with the use of CGM compared with episodic blood glucose monitoring. One might expect that the addition of a more complete 24-h measure of glucose concentrations would provide a tighter and more accurate assessment of the relationship between glucose concentrations and HbA1c values. There are limitations to the determination of the relationship between glucose and HbA1c with the current study. However, we did not find any major differences in the relationships between glucose concentrations and HbA1c values when considering patients whose HbA1c was stable and patients whose HbA1c changed over the time interval of observation. Individual biological variation in erythrocyte survival or glycation rates might contribute to the discrepancy between estimated and measured mean glucose concentrations in individual subjects. Future analysis will examine the consistency of the relationship between glucose and HbA1c in the same patient over time.
Subgroup analyses in our study showed that the slope of mean glucose concentration versus HbA1c value was not clinically or statistically different by age-group, sex, or sensor type. In our study, we did not have a sufficient number of non-white subjects to evaluate the relationships of mean glucose concentration versus HbA1c in other ethnic and racial groups.
It is important to note that all studies have reported substantial variability between the measured mean glucose concentrations and the estimated values calculated from regression equations (Table 2). As an example, Nathan et al. (15) reported that only slightly <90% of subjects had measured glucose concentrations within ±15% mean glucose concentrations predicted by HbA1c. We found a similar value in the current study (91%).
CGM typically has a relative error ranging from 14 to 20% (18–21). Quality control samples conducted during this study for HbA1c, in contrast, showed that 99% of repeat measurements were within ±0.1% of the original value. This result suggests that the measurement error for HbA1c is negligible compared with that for CGM used to calculate the mean glucose in this analysis.
Although there are challenges in measuring mean glucose concentrations with CGMS as well, the errors with these devices are generally unbiased, with mean errors typically centered around zero. Moreover, CGMS can provide an unprecedented view across time. In the current study, we had nearly complete glycemic data, day and night, for the entire 3 months of glucose concentrations before an HbA1c measurement. Consequently, our findings of considerable discrepancies between actual and estimated mean glucose concentrations lead us to disagree with the conclusions of Nathan et al. (15) that a calculated mean glucose is clinically equivalent to a measured mean glucose. HbA1c measures are extremely precise, and there are substantial individually persistent variations in the ratio between HbA1c and mean glucose. Thus, estimated mean glucose values calculated from measured HbA1c values should be used with caution.
Supplementary Material
Acknowledgments
This study was supported by JDRF (Grants 22-2006-1107, 22-2006-1117, 22-2006-1112, 22-2006-1123, and 01-2006-8031).
J.B. has received consulting fees from Abbott Medtronic MiniMed. B.B. has received consulting fees, honoraria, travel reimbursement, and research funds from Medtronic MiniMed and grant support from DexCom. I.H. has received consulting fees and travel reimbursement from Abbott Diabetes Care. C.K. has received consulting fees from Medtronic MiniMed. L.L. has received consulting fees and a speaker honorarium from Abbott Diabetes Care and consulting fees and research funding from Medtronic MiniMed. W.V.T. has received consulting fees from Medtronic MiniMed. No other potential conflicts of interest relevant to this article were reported.
D.M.W. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. D.X. contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. R.W.B. contributed to discussion and reviewed and edited the manuscript. J.B. contributed to discussion. B.B. researched data, contributed to discussion, and reviewed and edited the manuscript. L.A.F. researched data, contributed to discussion, and reviewed and edited the manuscript. I.H. researched data, contributed to discussion, and reviewed and edited the manuscript. C.K. contributed to discussion and reviewed and edited the manuscript. L.L. researched data, contributed to discussion, and reviewed and edited the manuscript. K.J.R. researched data, contributed to discussion, and reviewed and edited the manuscript. M.S. researched data and reviewed and edited manuscript. W.V.T. researched data, contributed to discussion, and reviewed and edited the manuscript.
The study was designed and conducted by the investigators. The writing group collectively wrote the manuscript and vouch for the data. The investigators had complete autonomy to analyze and report the trial results. There were no agreements concerning confidentiality of the data between the JDRF, the authors, or their institutions. The Jaeb Center for Health Research had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Continuous glucose monitors and sensors were purchased at a bulk discount price from DexCom (San Diego, CA), Medtronic MiniMed (Northridge, CA), and Abbott Diabetes Care (Alameda, CA). Home glucose meters and test strips were provided to the study by LifeScan and Abbott Diabetes Care. The companies had no involvement in the design, conduct, or analysis of the trial or the manuscript preparation.
The JDRF CGM study group would like to recognize the efforts of the subjects and their families and thank them for their participation.
APPENDIX
Co-authors: Darrell M. Wilson, MD1; Dongyuan Xing, MPH2; Roy W. Beck, MD, PHD2; Jennifer Block, RN, CDE1; Bruce Bode, MD3; Larry A. Fox, MD4; Irl Hirsch, MD5; Craig Kollman, PHD2; Lori Laffel, MD, MPH6; Katrina J. Ruedy, MSPH2; Michael Steffes, MD, PHD7; William V. Tamborlane, MD8, of the JDRF Continuous Glucose Monitoring Study Group.
1Stanford University, Stanford, California; the 2Jaeb Center for Health Research, Tampa, Florida; 3Atlanta Diabetes Associates, Atlanta, Georgia; the 4Nemours Children’s Clinic, Jacksonville, Florida; the 5University of Washington, Seattle, Washington; the 6Joslin Diabetes Center, Boston, Massachusetts; the 7University of Minnesota, Minneapolis, Minnesota; 8Yale University, New Haven, Connecticut.
Footnotes
Clinical trial reg. no. NCT00406133, clinicaltrials.gov.
The article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1054/-/DC1.
Members of the writing committee are listed in APPENDIX. A full listing of the members of the study group is included in the Supplementary Data online.
References
- 1.American Diabetes Association Standards of medical care in diabetes—2009. Diabetes Care 2009;32:S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen RM, Franco RS, Joiner CH. Is poor glycemic control associated with reduced red blood cell lifespan? Diabetes Care 2004;27:1013–1014 [DOI] [PubMed] [Google Scholar]
- 3.Diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Tamborlane WV, Kollman C, Steffes MW, et al. Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Research in Children Network (DirecNet) Study. Pediatr Diabetes 2005;6:13–16 [DOI] [PubMed] [Google Scholar]
- 5.Fiallo-Scharer R, Diabetes Research in Children Network Study Group Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab 2005;90:3387–3391 [DOI] [PubMed] [Google Scholar]
- 6.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858–1862 [DOI] [PubMed] [Google Scholar]
- 7.JDRF CGM Study Group JDRF randomized clinical trial to assess the efficacy of real-time continuous glucose monitoring in the management of type 1 diabetes: research design and methods. Diabetes Technol Ther 2008;10:310–321 [DOI] [PubMed] [Google Scholar]
- 8.Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 9.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group The effect of continuous glucose monitoring in well-controlled type 1 diabetes. Diabetes Care 2009;32:1378–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibb I, Parnham A, Fonfrède M, Lecock F. Multicenter evaluation of Tosoh glycohemoglobin analyzer. Clin Chem 1999;45:1833–1841 [PubMed] [Google Scholar]
- 11.Hempe JM, Gomez R, McCarter RJ, Jr, Chalew SA. High and low hemoglobin glycation phenotypes in type 1 diabetes: a challenge for interpretation of glycemic control. J Diabetes Complications 2002;16:313–320 [DOI] [PubMed] [Google Scholar]
- 12.Rohlfing CL, Wiedmeyer HM, Little RR, England JD, Tennill A, Goldstein DE. Defining the relationship between plasma glucose and HbA(1c): analysis of glucose profiles and HbA(1c) in the Diabetes Control and Complications Trial. Diabetes Care 2002;25:275–278 [DOI] [PubMed] [Google Scholar]
- 13.Makris K, Spanou L, Rambaouni-Antoneli A, et al. Relationship between mean blood glucose and glycated haemoglobin in type 2 diabetic patients. Diabet Med 2008;25:174–178 [DOI] [PubMed] [Google Scholar]
- 14.Mazze RS. The future of self-monitored blood glucose: mean blood glucose versus glycosylated hemoglobin. Diabetes Technol Ther 2008;10:s93–s101 [Google Scholar]
- 15.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group Translating the A1C assay into estimated average glucose values. Diabetes Care 2008;31:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DM, Kollman, Diabetes Research in Children Network (DirecNet) Study Group Relationship of A1C to glucose concentrations in children with type 1 diabetes: assessments by high-frequency glucose determinations by sensors. Diabetes Care 2008;31:381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borg R, Kuenen JC, Carstensen B, et al. Associations between features of glucose exposure and A1C: the A1C-Derived Average Glucose (ADAG) study. Diabetes 2010;59:1585–1590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bode B, Gross K, Rikalo N, et al. Alarms based on real-time sensor glucose values alert patients to hypo- and hyperglycemia: the Guardian Continuous Monitoring System. Diabetes Technol Ther 2004;6:105–113 [DOI] [PubMed] [Google Scholar]
- 19.DexCom Inc. DexCom™ STS™ Continuous Glucose Monitoring System User's Guide. 2006, p. 86–102 [Google Scholar]
- 20.Diabetes Research in Children Network (DirecNet) Study Group. The accuracy of the Guardian RT continuous glucose monitor in children with type 1 diabetes. Diabetes Technol Ther 2008;10:266–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diabetes Research in Children Network Study Group. The accuracy of the FreeStyle Navigator™ Continuous Glucose Monitoring System in children with type 1 diabetes. Diabetes Care 2007;30:59–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nathan DM, Turgeon H, Regan S. Relationship between glycated haemoglobin levels and mean glucose levels over time. Diabetologia 2007;50:2239–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.