Abstract
OBJECTIVE
There is no general agreement regarding the use of the first or second drop of blood for glucose monitoring. This study investigated whether capillary glucose concentrations, as measured in the first and second drops of blood, differed ≥10% compared with a control glucose concentration in different situations.
RESEARCH DESIGN AND METHODS
Capillary glucose concentrations were measured in two consecutive drops of blood in the following circumstances in 123 patients with diabetes: without washing hands, after exposing the hands to fruit, after washing the fruit-exposed hands, and during application of different amounts of external pressure around the finger. The results were compared with control measurements.
RESULTS
Not washing hands led to a difference in glucose concentration of ≥10% in the first and in the second drops of blood in 11% and 4% of the participants, respectively. In fruit-exposed fingers, these differences were found in 88% and 11% of the participants, respectively. Different external pressures led to ≥10% differences in glucose concentrations in 5–13% of the participants.
CONCLUSIONS
We recommend washing the hands with soap and water, drying them, and using the first drop of blood for self-monitoring of blood glucose. If washing hands is not possible, and they are not visibly soiled or exposed to a sugar-containing product, it is acceptable to use the second drop of blood after wiping away the first drop. External pressure may lead to unreliable readings.
Self-monitoring of blood glucose (SMBG) is an important part of diabetes care. The purpose of SMBG is to provide a timely and reliable assessment of blood glucose concentrations in an individual in order to be able to make adequate decisions in relation to diet, exercise, and medication (1,2).
There are several aspects concerning SMBG that need attention. For example, there is no general agreement regarding the use of the first or the second drop of blood for glucose monitoring. In the Netherlands, there are three different recommendations. Firstly, using the first drop of blood after washing the hands with soap and water or after disinfecting the finger and waiting until the finger is dry (3). Secondly, using the first drop of blood after washing the hands with soap and water and using the second drop of blood when the patient has not washed the hands (4). Thirdly, always using the second drop of blood after washing the hands with soap and water (5). Furthermore, in one of these recommendations, patients are advised not to squeeze the finger to obtain a drop of blood as this could potentially influence the blood glucose concentration (3).
To address the questions raised by these different recommendations, we conducted a study with a cross-sectional design to investigate whether capillary glucose concentrations, as measured in the first and second drops of blood, differed 10% or more compared with a control capillary glucose concentration, in the following situations:
without washing hands
after handling fruit
after washing the fruit-exposed fingers
during the application of different amounts of external pressure around the finger (squeezing)
RESEARCH DESIGN AND METHODS
Patients were recruited from the outpatient clinic of the Department of Internal Medicine of the Isala Clinics in Zwolle, the Netherlands. Eligibility criteria were a diagnosis of type 1 or type 2 diabetes, treated with insulin, SMBG, and age >18 years.
Eligible patients received a letter with information about the study and an invitation to participate at their next outpatient clinic visit. Recruitment took place between September 2009 and February 2010. Approval for the study was obtained from the local medical ethics committee. All patients gave written informed consent.
Data on glycemic control and BMI were collected from hospital records, and the hematocrit value was assessed.
Intervention
Capillary glucose concentrations for two consecutive drops of blood were measured in four different circumstances: 1) without washing hands, 2) after handling with fruit, 3) after washing the fruit-exposed hands, and 4) during the application of different amounts of pressure around the finger. All capillary blood glucose concentrations were determined without squeezing (“milking”) except in the intervention where this aspect was investigated. All glucose measurements were nonfasting and were performed at times depending on scheduled outpatient clinic visit.
Description of the interventions
Intervention 1: not washing hands.
The participant arrived at the research setting and did not wash the hands prior to the first finger puncture. The capillary blood glucose concentrations were determined from the first and second drops of blood, and the puncture site was wiped by a tissue in between obtaining the first and second drops.
Intervention 2: finger exposed to fruit.
The participant washed the hands with soap and water and dried them. The participant then handled either an apple or a banana. This fruit is generally used in the Dutch population. The participant either cut part of an apple (jonagold) into three pieces with a knife and broke the pieces into two smaller pieces with the hands, or peeled a piece of a ripe banana and broke the piece into two smaller pieces with the hands. After handling the fruit, the capillary blood glucose concentrations were determined in the first and second drops of blood, and the finger was wiped off with a tissue in between obtaining the two drops.
Intervention 3: washing the fruit-exposed finger.
As in intervention 2, the participant’s other forefinger was exposed to a piece of fruit. The participant then washed the hands with soap and water and dried them. The tests were repeated.
Intervention 4: different external pressures.
The participant washed the hands with soap and water and dried them. The cuff of the hand blood pressure meter was put around the middle phalanx of the middle finger. The pressure was increased to 240 mmHg. Immediately, a finger puncture was performed, and the capillary glucose concentration was determined in the first and in the second drops of blood, again wiping the finger with a tissue in between the two drops. Thereafter, the finger cuff was put around the middle phalanx of the ring finger of the same hand. The cuff was inflated to 40 mmHg. A finger puncture was performed after 1 min to achieve venous stasis, and the tests were repeated.
Control measurement
The patient washed the hands with soap and water and dried them. A finger puncture was performed. A mean capillary blood glucose concentration was obtained by averaging the result obtained from the first and second drops of blood (the finger was wiped off after the first drop was obtained). This result was used as the control after instrument combined with strip and performance bias were excluded. A separate control was calculated for each of interventions 1, 2, and 3 with the control for intervention 1 being performed after the intervention, and the control for intervention 3 also being used for intervention 4.
Time interval during measurements
Capillary glucose measurements were performed directly following the finger puncture with a maximum delay of 90 s between measurements.
Measuring equipment
All capillary glucose values were determined with the Accu-Chek Compact plus meter with plasma-calibrated test strips (Roche, Almere, the Netherlands). A Speidel and Keller hand blood pressure meter was used to achieve different external pressures. The regular cuff was replaced by a neonatal cuff. One of two available sizes was used depending on the thickness of the finger (Philips, M1866A neonatal disposable cuff #1 and M1868A neonatal disposable cuff #2). The meter was calibrated prior to the start of the study as well as halfway through the study. No significant changes were observed.
Statistical analyses
Descriptive statistics include mean (SD) and median (interquartile range). All data were reviewed for normality using Q-Q plots, and parametric and nonparametric tests were used as appropriate. The Wilcoxon signed rank test was used to test for differences in glucose concentrations. Bland-Altman plots were produced and intraclass correlation coefficients were calculated for assessing agreement between measurements and for the reliability of the control measurement (6).
A difference of ≥10% between control and intervention values or a difference of 0.82 mmol/L in the case of a glucose concentration <4.2 mmol/L was considered to be clinically relevant. An intervention was considered to lead to reliable readings when 95% of the readings were within 10% differences. The 10% is based on the external quality assessment scheme, the quality mark for self-test glucose meters, assessing analytical quality and technical quality (7). The total allowable error in the quality mark is 9.4%, based on the inter- and intraperson variation concept of Fraser and Peterson. (8).
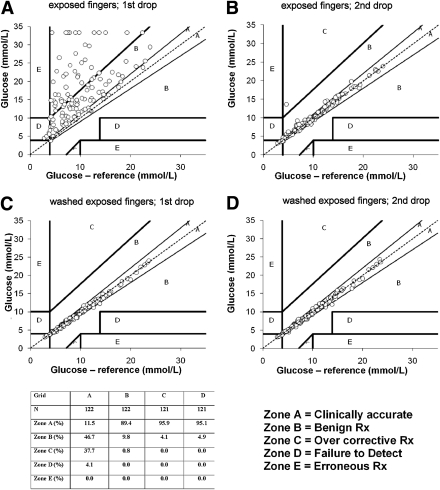
Clarke error grids were used to investigate how often the outcome would lead to a different interpretation and/or action (9). They were originally developed to evaluate the accuracy of capillary blood glucose testing systems using a relevant difference of 20% between reference and measured values. For the study, the error grids were adapted to the 10% differences. The grid is subdivided into five zones: A, B, C, D, and E. Zone A represents values that differ from the reference value by <10%. Zone B represents values that differ >10% from the reference value. Results in zones A and B will lead to the same treatment decision. Zone C represents values that would result in overcorrecting acceptable glucose values. Zone D represents values that are erroneously uncorrected, and zone E represents values that would result in the inverse treatment.
To detect a 10% difference between the glucose concentrations with a power of 90%, α 0.025 (one-sided equivalence test), a total sample size of 100 participants is required. SPSS software (version 15.0) was used for all the analyses.
RESULTS
The study population consisted of 123 patients; 63 (51%) were men, and 66 (54%) were patients with type 1 diabetes. Mean age was 54.4 years (SD 14.2), mean HbA1c was 59 mmol/mol (SD 14) (or 7.5% SD 1.3), and mean BMI was 29 kg/m2 (SD 6.2). Mean hematocrit values were 0.45 L/L (SD 0.05). All values were within the hematocrit ranges of the Accu-Chek Compact plus meter (0.25–0.65 L/L).
Control measurements
Intraclass correlations of the first and second drops of blood of the three control measurements were 0.996, 0.995, and 0.996, respectively. In 2–4% of these three control measurements, the second drop of blood differed 10% or more compared with the first drop of blood.
Table 1 shows median and interquartile ranges of the glucose concentration in various testing sequences in different circumstances.
Table 1.
Glucose concentrations in different sequential drops of blood
| First drop | Second drop | Control | |
|---|---|---|---|
| Not washing hands (n = 123) | 8.9 (6.4–12.6) | 8.9 (6.5–12.2) | 8.6 (6.1–12.2) |
| Washing hands (n = 123) | 8.5 (6.3–12.2) | 8.7 (5.9–12.2) | 8.6 (6.1–12.2) |
| Finger exposed to fruit, no washing (n = 122) | 15.0 (10.5–21.7) | 8.9 (6.5–12.5) | 8.9 (6.4–12.2) |
| After washing the fruit-exposed finger (n = 121) | 8.4 (6.3–11.9) | 8.3 (6.4–12.0) | 8.5 (6.2–12.0) |
| Pressure 40 mmHg (n = 102) | 8.4 (6.1–11.9) | 8.2 (5.5–11.4)* | 8.5 (6.2–12.0) |
| Pressure 240 mmHg (n = 102) | 8.3 (6.1–11.6) | 8.4 (5.9–11.1)** | 8.5 (6.2–12.0) |
Data are median (interquartile range). Glucose is in mmol/L.
*n = 96;
**n = 100.
Intervention 1: not washing hands.
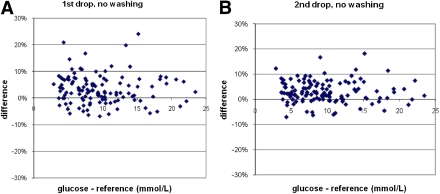
Not washing hands led to a ≥10% difference in glucose concentrations compared with the control measurement in the first and the second drops of blood in 11% (P < 0.001) and 4% (P < 0.001) of the participants, respectively. Two glucose concentrations in the first drop of blood were even more than 20% higher than the control measurement (Fig. 1A). Wiping away the first drop led to 96% of the values within the 10% differences (Fig. 1B).
Figure 1.
The deviation in glucose concentrations of the first (A) and second (B) drops of blood when the patient had not washed the hands vs. control measurement. (A high-quality color representation of this figure is available in the online issue.)
Intervention 2: fruit-exposed finger.
Exposing the finger to fruit led to 10% or higher glucose concentrations in the first drop of blood in 88% of the patients (P < 0.001) compared with the control measurements. Wiping the first drop away with a tissue considerably improved readings. In 11% of cases, however, the glucose readings from the second drop of blood were still ≥10% higher than the control measurements (P < 0.001).
Intervention 3: washing the fruit-exposed finger.
After washing their hands with soap and water, 4% (P < 0.001) and 5% (P = 0.189) of the participants showed a difference of ≥10% in the glucose concentrations compared with the controls, respectively.
Figure 2 shows modified Clarke error grids of fruit-exposed fingers and after washing fruit-exposed fingers. In the intervention with fruit-exposed fingers, 11 glucose concentrations were higher than 33.3 mmol/L. Thirty-eight percent of the capillary glucose points of the fruit-exposed fingers were in zone C, which would result in an overcorrection of acceptable glucose concentrations. Wiping away the first drop of blood from the fruit-exposed finger led to one point falling in zone C and 11% in zone B. Washing the fruit-exposed fingers with soap and water led to 95% of glucose concentrations falling within zone A.
Figure 2.
Modified Clarke error grids of fruit-exposed fingers and after washing fruit-exposed fingers.
Intervention 4: different external pressures.
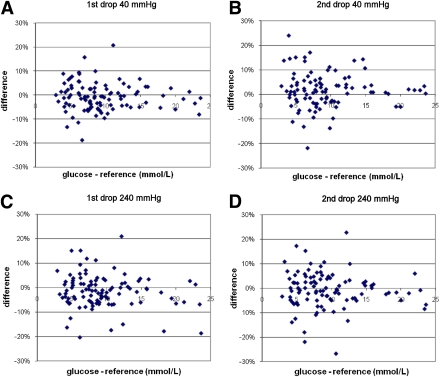
Figure 3 shows the deviation between the glucose concentrations for different pressures. The deviation between the glucose concentrations and the control measurements increased when the pressure was increased. Pressure with 40 mmHg led to ≥10% differences in glucose concentrations compared with the controls in the first drop and the second drop of blood in 5% (P = 0.055) and 10% (P = 0.009) of the participants, respectively. Pressure of 240 mmHg led in 12% (P = 0.018) and 13% (P = 0.217) of the participants to ≥10% differences in glucose concentrations in the first and second drops of blood compared with the controls, respectively.
Figure 3.
The deviation in glucose concentrations of the first and second drops of blood vs. control measurement for different pressures. (A high-quality color representation of this figure is available in the online issue.)
Blood glucose concentrations in fingers of different hands
When the measurements were performed at the same time in fingers of different washed and dried hands, only one glucose concentration (1%) differed ≥10% (data not shown).
CONCLUSIONS
The first drop of blood can be used for self-monitored glucose testing, but only after washing hands. If washing hands is not possible and they are not visibly soiled or exposed to a sugar-containing product, it is acceptable to use the second drop of blood after wiping away the first drop. It does not matter which finger is used for glucose measurements. External pressure may lead to unreliable readings.
Many insulin-treated patients have to perform SMBG for a lifetime—some of them every day. Discarding the first drop of blood and refraining from squeezing the finger makes measurements more complex and necessitates deeper and more painful punctures. International guidelines and studies about SMBG (e.g., the American Diabetes Association [ADA] and the Diabetes UK guidelines) recommend using the first drop of blood after washing the hands (10–12). Some also allow squeezing or milking the finger. The manufacturer’s instructions of the meter used in the study include washing hands with warm water and soap and drying the hands. The first drop of blood can be used after gently squeezing the finger. In daily practice, patients cannot or do not always wash their hands before performing SMBG (1). In international guidelines, these situations are not discussed.
Only two studies investigated the differences between glucose concentrations in the first and the second drops of blood. Both of these studies, however, involved volunteers without diabetes. In one study of 53 volunteers, no differences were found in the readings when the hands were clean (13). Glucose readings for 25 volunteers in the other study were shown to be greatly affected when the fingers were exposed to glucose (i.e., fruit). Even the third drop of blood cannot be used in these cases (14). Our study also shows that the first drop of blood should not be used when the patient has not washed the hands. Use of the second drop of blood leads to reliable values when the finger is wiped by a tissue in between obtaining the two drops. However, this does not apply to fingers exposed to glucose products as the glucose concentrations in the second drop still differed ≥10% from the control measurements in 11% of the patients. Therefore, patients should always wash their hands when they have touched a sugar-containing product.
Fruhstorfer and Quarder (13) also investigated the influence of milking the finger in 10 volunteers without diabetes and concluded that milking the finger gives correct glucose values. In our study, we used two pressures to explore whether there would be any influence on the capillary glucose concentration. Venous stasis is achieved with a pressure of 40 mmHg. A pressure of 240 mmHg is above the systolic pressure of the participants. Our study shows more deviation between the glucose concentrations with the higher pressure.
The differences used in this study are more strict than the 20% difference in the International Organization for Standardization (ISO) standard (15), or the 15% difference or a difference of 1 mmol/L in cases when the glucose concentration is <6 mmol/L in the Dutch guideline (16). Patients expect meters to provide high analytical quality of blood glucose measurements (17). Furthermore, these differences may cause errors in insulin dose when using strict insulin algorithm (18). Based on the article by Jansen and Slingerland (7), a difference of 10% cannot be neglected.
A standardized method of squeezing of the finger in daily practice is difficult because the necessity for squeezing varies strongly between individuals, depending on the structure of the skin. A limitation of our study is that the method of squeezing does not fully mimic daily practice, so the results should be interpreted with some caution. The strength of the study is that a standardized method of squeezing was used. The use of one meter by one experienced person limited variability. On the other hand, it limits generalization of the findings to other equipment. There are several aspects that could affect readings, such as the time of the last insulin dosage. Therefore, a separate control measurement was performed for each intervention. The time interval between measurements was maximal 90 s, but in most of the interventions the time interval was 30–60 s. Using this design, it is not likely that these aspects have relevantly influenced the results. However, we cannot completely exclude an effect of this time delay. Multivariate analyses show that in none of the interventions, sex or HbA1c had a statistically significant influence on the results. Finally, because of the selection of the patients, the results cannot be generalized to the hospital setting.
Our study investigated important and underexposed aspects concerning SMBG in people with diabetes to acquire a reliable glucose concentration. Based on this study, the first choice is to wash the hands with soap and water, dry them, and use the first drop of blood for SMBG. If washing hands is not possible, and they are not visibly soiled or exposed to a sugar-containing product, it is acceptable to use the second drop of blood after wiping away the first drop. Firm squeezing of the finger should be avoided.
Acknowledgments
The authors acknowledge sanofi-aventis and Roche Diagnostics Nederland BV for their support.
The sponsors had no role in the study design, data collection, analysis, interpretation, or writing of this article. No other potential conflicts of interest relevant to this article were reported.
J.H. researched data, contributed to discussion, and wrote the manuscript. R.J.S. contributed to discussion and reviewed and edited the manuscript. N.K. contributed to discussion and reviewed and edited the manuscript. S.J.J.L. reviewed and edited the manuscript. K.H.G. researched data and reviewed and edited the manuscript. S.T.H. contributed to discussion and reviewed and edited the manuscript. H.J.G.B. contributed to discussion and reviewed and edited the manuscript.
The authors would like to thank Marion Fokkert, point-of-care coordinator, and Wim Muller, technician, both working in the Department of Clinical Chemistry, Isala Clinics, Zwolle, the Netherlands, for their support in the study.
References
- 1.Bergenstal R, Pearson J, Cembrowski GS, Bina D, Davidson J, List S. Identifying variables associated with inaccurate self-monitoring of blood glucose: proposed guidelines to improve accuracy. Diabetes Educ 2000;26:981–989 [DOI] [PubMed] [Google Scholar]
- 2.Dutch Diabetes Federation. Recommendations for Self-Monitoring of Blood Glucose Amersfoort, the Netherlands, 2003
- 3.Dutch Association of Diabetic Care Professionals. Guidelines: Performing Self-Monitoring of Blood Glucose Utrecht, the Netherlands, 2004
- 4.Dutch Diabetes Association. Self-monitoring of blood glucose [Internet]. Amersfoort, the Netherlands. Available from http://www.dvn.nl Accessed 1 May 2010
- 5.The Netherlands Health Care Inspectorate. Recommendations for self-monitoring of blood glucose [Internet], 2008. Available from http://www.igz.nl/zoeken/download.aspx?download=Circulaire_bloedsuikermetingen.pdf Accessed 1 May 2010
- 6.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310 [PubMed] [Google Scholar]
- 7.Jansen RTB, Slingerland RJ. SKML-Quality Mark for point-of-care test (POCT) glucose meters and glucose meters for home-use. Clin Chem Lab Med 2010;48:1021–1027 [DOI] [PubMed] [Google Scholar]
- 8.Fraser CG, Petersen PH. Desirable standards for laboratory tests if they are to fulfill medical needs. Clin Chem 1993;39:1447–1453 [PubMed]
- 9.Clarke WL, Cox D, Gonder-Frederick LA, Carter W, Pohl SL. Evaluating clinical accuracy of systems for self-monitoring of blood glucose. Diabetes Care 1987;10:622–628 [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Living with diabetes: checking your blood glucose [Internet]. Available from http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/checking-your-blood-glucose.html Accessed 1 May 2010
- 11.Diabetes UK. Self-blood glucose testing [Internet]. Available from http://www.diabetes.org.uk/Guide-to-diabetes/Monitoring/Blood_glucose/Self_blood_glucose_testing/. Accessed 1 May 2010
- 12.Baum JM, Monhaut NM, Parker DR, Price CP. Improving the quality of self-monitoring blood glucose measurement: a study in reducing calibration errors. Diabetes Technol Ther 2006;8:347–357 [DOI] [PubMed] [Google Scholar]
- 13.Fruhstorfer F, Quarder O. Blood glucose monitoring: milking the finger and using the first drop of blood give correct glucose values (Letter). Diabetes Res Clin Pract 2009;85:e14–e15 [DOI] [PubMed] [Google Scholar]
- 14.Hortensius J, Kleefstra N, Slingerland RJ, et al. The influence of a soiled finger in capillary blood glucose monitoring. Neth J Med 2010;68:330–331 [PubMed] [Google Scholar]
- 15.International Organization for Standardization. ISO 15197:2003: In vitro diagnostics test systems–requirements for blood-glucose monitoring systems for self-testing in managing diabetes mellitus. [Internet], 2003. Available from www.iso.org
- 16.Post H, van Keulen GJ, Muller WJ, Miedema K. TNO Quality Guideline PG/TG/2001.045: portable in-vitro blood monitoring systems for (self)-monitoring–blood glucose monitors–particular requirements and test methods. Netherlands Organisation for Applied Scientific Research, Leiden, the Netherlands, November 2001 (No. 011.51275/01.02)
- 17.Skeie S, Thue G, Sandberg S. Patient-derived quality specifications for instruments used in self-monitoring of blood glucose. Clin Chem 2001;47:67–73 [PubMed] [Google Scholar]
- 18.Boyd JC, Bruns DE. Quality specifications for glucose meters: assessment by simulation modeling of errors in insulin dose. Clin Chem 2001;47:209–214 [PubMed] [Google Scholar]