Abstract
OBJECTIVE
The surgical option could represent a valid alternative to medical therapy in some diabetic patients. However, no data are available on long-term effects of metabolic surgery on diabetic complications. We aimed to determine whether patients with newly diagnosed type 2 diabetes who underwent bilio-pancreatic diversion (BPD) had less micro- and macrovascular complications than those who received conventional therapy.
RESEARCH DESIGN AND METHODS
This was an unblinded, case-controlled trial with 10-years’ follow-up, conducted from July 1998 through October 2009 at the Day Hospital of Metabolic Diseases, Catholic University, Rome, Italy. A consecutive sample of 110 obese patients (BMI >35 kg/m2) with newly diagnosed type 2 diabetes was enrolled. The study was completed by 50 subjects. The main outcome measure was long-term effects (10 years) of BPD versus those associated with conventional therapy on microvascular outcome, micro- and macroalbuminuria, and glomerular filtration rate (GFR). Secondary measures included macrovascular outcomes, type 2 diabetes remission, glycated hemoglobin, and hyperlipidemia.
RESULTS
Ten-year GFR variation was −45.7 ± 18.8% in the medical arm and 13.6 ± 24.5% in the surgical arm (P < 0.001). Ten-year hypercreatininemia prevalence was 39.3% in control subjects and 9% in BPD subjects (P = 0.001). After 10 years, all BPD subjects recovered from microalbuminuria, whereas microalbuminuria appeared or progressed to macroalbuminuria in control subjects. Three myocardial infarctions, determined by electrocardiogram, and one stroke occurred in control subjects. After the 10-year follow-up, coronary heart disease (CHD) probability was 0.22 ± 0.10 and 0.05 ± 0.04 in the medical and surgical groups, respectively (P < 0.001). Remission from type 2 diabetes was observed in all patients within 1 year of surgery. Surgical and medical subjects had lost 34.60 ± 10.25 and 0.38 ± 6.10% of initial weight at the 10-year follow-up (P < 0.001).
CONCLUSIONS
Renal and cardiovascular complications were dramatically reduced in the surgical arm, indicating long-term benefits of BPD on diabetic complications, at least in the case of morbid obesity with decompensated type 2 diabetes.
Type 2 diabetes is associated with serious complications, including cardiovascular disease, premature death, blindness, renal failure, amputations, and cognitive decline (1). Recently, the efficacy of the stringent glycemic control by medical therapy was found to be ineffective in reducing major macrovascular complications, suggesting the presence of other causative factors. In fact, intensive treatment, targeted at attaining normal glycated hemoglobin levels, i.e., <6.0%, not only was found to be ineffective in reducing cardiovascular events but also was found to be associated with significantly higher mortality, leading to the decision to terminate the intensive regimen after 3.5 years of follow-up in the Action to Control Cardiovacular Risk in Diabetes (ACCORD) trial (2). Also, the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROactive) (3), where pioglitazone was added to ongoing glucose-lowering therapy in type 2 diabetic patients at high cardiovascular risk, did not show significant reduction of cardiovascular events, in spite of a significant improvement of HbA1c. As shown in the Veterans Affairs Diabetes Trial (VADT) (4), although a comparison of intensive versus standard therapy did not reveal significant effects on either death rate or microvascular complications, strict glycemic control seems to be relevant in decreasing the conversion from normo- to micro- or macroalbuminuria. Similar results, i.e., a decreased incidence of albuminuria, were also obtained in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial (5).
Importantly, when intensive glucose control strategy was started at the time of diagnosis, a significantly decreased risk of myocardial infarction and death from any cause, in addition to a large reduction in the risk of microvascular disease, was observed, suggesting that it is crucial to start adequate medical treatment and lifestyle modification as soon as possible (6). Unfortunately, obese diabetic patients have a limited compliance to the diet, which is essential to obtain a reasonable metabolic control. There is evidence in the literature that type 2 diabetes is controlled in fewer than 50% of patients in the U.S. (7). Even with the most recent therapy, liraglutide in monotherapy, the American Diabetes Association’s HbA1c goal of <7% was reached only in the 51% of patients with the highest daily dose of 1.8 mg (8). Therefore, bariatric surgery can represent a valid option to the medical treatment failure, at least in morbidly obese subjects.
A recent systematic review and meta-analysis of the literature (9) showed that the clinical and laboratory manifestations of type 2 diabetes are resolved in 78.1% and are improved in 86.6% of patients after bariatric surgery. However, a gradation of effectiveness exists among the different bariatric procedures, with bilio-pancreatic diversion (BPD) and duodenal switch providing the greatest effect (9).
The Swedish Obese Subjects (SOS) study (10), in which gastric bypass, vertical banded gastroplasty, and banding bariatric surgery were performed in obese subjects, was associated with significant reduction in overall mortality after 10 years of follow-up compared with conventional treatment. These data were so impressive that diabetologists have proposed the surgical option in type 2 diabetes as soon as possible (11).
In previous studies, we have shown that, similarly to what has been observed after Roux-en-Y gastric bypass (12), after BPD, type 2 diabetes also rapidly reverses to normal glucose tolerance independently of weight loss (13,14). To our knowledge, no study is available in the literature about the long-term effect of bariatric surgery on diabetic complications. In the current study, we report 10-year follow-up effects of BPD on micro- and macrovascular outcomes in newly diagnosed type 2 diabetic patients with severe obesity.
RESEARCH DESIGN AND METHODS
Between July 1998 and September 1999, 110 obese subjects with newly diagnosed type 2 diabetes, aged 25–60 years, were enrolled in a longitudinal study at the Day Hospital of Metabolic Diseases of the Catholic University in Rome, Italy, with 10 years of follow-up ending in October 2009. The study was approved by the Catholic University ethics committee in accordance with the Helsinki Declaration. All participants provided written informed consent to participate in the study. Additional specific written informed consent was obtained prior to surgical procedure.
Design of the study
The study was designed as an open case-control study targeting patients with newly diagnosed type 2 diabetes. The aim was to assess the effect of bariatric surgery versus that of conventional medical therapy on diabetic complications. Although a randomization of diabetic participants to the two different treatment modalities (BPD vs. conservative therapy) would be the most desirable study design, this was not approved by the ethics committee of our institution, in particular because a long follow-up period was planned. However, the subjects enrolled in the study were matched, according to the method of sequential treatment assignment (15), for sex, age, BMI, serum cholesterol and triglyceride levels, and smoking habits. The investigators had no influence on the computerized matching process.
Inclusion criteria
Subjects were aged 25–60 years, had a BMI >35 kg/m2, and were able to understand and comply with the study process. Fasting plasma glucose >7.0 mmol/L on two mornings or glycemia >11 mmol/L at 2 h after a 75-g oral glucose tolerance-test (OGTT) were diagnostic criteria for diabetes. Subjects with cardiovascular events during the previous 6 months, advanced congestive heart failure, severe angina, serum creatinine >1.6 mg/dL, internal malignancy, or portal hypertension were excluded.
Assessment and run-in period
An energy-restricted diet rich in complex carbohydrates and fibers and low in saturated fats was prescribed. After 3 months on the diet, eligible patients matching the above criteria for diabetes were asked to participate in the study by choosing to be part of the surgical or the medical group (Supplementary Fig. 1), the latter following a treatment with sulphonylurea or insulin and/or metformin, according to diabetes severity. Baseline height, weight, body fat distribution, blood pressure, and biochemical data (fasting plasma glucose and insulin, HbA1c, lipid profile, blood urea nitrogen, plasma creatinine, and microalbuminuria) were measured immediately prior to the study.
Criteria for defining health/disease conditions
Nephropathy was excluded (Supplementary Materials) if patients did not have micro- or macroalbuminuria or a persistently elevated plasma creatinine. Nephropathy was classified as microalbuminuria 50–299 mg/L on two consecutive visits, plasma creatinine <175 μmol/L, macroalbuminuria ≥300 mg/L at two consecutive visits, plasma creatinine <175 μmol/L, or elevated plasma creatinine (≥175 μmol/L) on two consecutive visits.
The glomerular filtration rate (GFR) was estimated according to the reexpressed four-variable Modification of Diet in Renal Disease (MDRD) study equation (GFR = 175 × standardized serum creatinine [Scr]−1.154 × age−0.203 × 0.742 [if female]) (Supplementary Materials). GFR <60 mL/min per 1.73 m2 indicated chronic nephropathy.
Hypertension was assessed as systolic blood pressure ≥130 mmHg and diastolic ≥85 mmHg, hypercholesterolemia as total cholesterol ≥5.21 mmol/L, hypertriglyceridemia as 1.70 mmol/L, and low level of HDL cholesterol as ≤1.01 mmol/L. The Framingham model was used to compute the 10-year predicted probability for CHD (Supplementary Materials). Homeostasis model assessment of insulin resistance index (fasting plasma insulin [mUI/L] × fasting plasma glucose [mg/dL])/405) (Supplementary Materials) and Quicky index {1/log(fasting plasma insulin [mUI/L] + fasting plasma glucose [mg/dL])} (Supplementary Materials) monitored insulin resistance evolution.
Conventional therapy and surgical programs
Treatment guidelines, based on recommendations of the American Diabetes Association, for blood pressure and lipid control, as well as for dietary, exercise, and diabetes education, were provided. Patients had open access to a diabetologist every 3 months. Medical therapies, including pharmaceutical agents, were assigned on an individual basis. In addition to all aspects of the conventional therapy program, the surgical group underwent laparotomic BPD (16) within 2 month of recruitment.
Primary and secondary end points
The primary end point of the study was the relative percentage variation of GFR (ratio of difference between final and baseline values on baseline value [ΔGFR%]). Secondary end points were the incidence of other signs of nephropathy, hypertension, hyperlipidemia, and cardiovascular events as well as the fraction recovering from diabetes in the two studied groups during the 10 years of follow-up. Changes over time of HbA1c levels, plasma glucose concentration, weight, blood pressure, and CHD risk as well as changes of levels of fasting lipids, including total cholesterol, triglycerides, and HDL cholesterol, were also evaluated. Changes over time of insulin sensitivity were considered only in the BPD group because in the other group insulin therapy made the evaluation of insulin sensitivity unreliable.
Sample size
According to a previous published study (Supplementary Materials), GFR per-year variation in diabetic patients was 5.2 ± 4.1%. Supposing an exponential decay of GFR with time, the relative percentage variation of the studied variable at the end of 10-year follow-up is expected to be ∼40%. Assuming a variance model with constant coefficient of variation, the SD is ∼32%. According to the hypotheses above, 60 subjects (30 in the conventional therapy and 30 in the BPD group) would have provided 90% power to detect a difference of ΔGFR% of 25% (which means to assume a percentage variation of GFR in the treated group of −15%), with P = 0.05 with a one-sided test. Having supposed that 40% of subjects could not meet inclusion criteria after the first 3 months of diet regimen and that 30% could drop out, 110 subjects were enrolled in the study.
Data analysis
Statistical analysis was performed with R (version 2.10; The R Project; R-Foundation). Subjects were analyzed on a per-protocol criterion. Continuous variables baseline characteristics were compared by t tests or Mann-Whitney U tests according to their compliance with normal distribution assumptions, whereas χ2 tests or Fisher exact tests were used to compare categorical variables. Continuous variables were means ± SD while categorical variables were percentages with respect to sample size. ANOVA for repeated measures (RMANOVA) was used to test changes of continuous variables over time, as depending on treatment group, using sex, age, and BMI at time 0 as covariates. Logistic regression was used to study the association of diabetes remission with the studied variables and to compare the rates of recovery between treatment and control groups. P < 0.05 was considered significant.
RESULTS
The baseline characteristics of the two groups are reported in Table 1 (Supplementary Materials); no significant differences were found in the variables analyzed. In the control group, therapy for diabetes was distributed as follows: 8 subjects with metformin, 10 with metformin plus sulfonylurea, and 10 with metformin plus insulin. As per protocol, no subject in the BPD group was assigned to a therapeutic regimen for diabetes treatment.
Table 1.
Baseline characteristics of participants
| Conventional therapy | BPD | |
|---|---|---|
| n | 28 | 22 |
| Age (years) | 43.71 ± 6.782 | 43.77 ± 8.257 |
| Sex | ||
| Men | 12 (42.86) | 10 (45.45) |
| Women | 16 (57.14) | 12 (54.55) |
| Smoker | 16 (57.14) | 13 (59.10) |
| Hypolipidemic treatment | 17 (60.71) | 14 (63.64) |
| Antihypertensive treatment | 13 (46.43) | 14 (63.64) |
| BMI (kg/m2) | 51.53 ± 6.19 | 50.47 ± 8.46 |
| Weight (kg) | 142.91 ± 16.45 | 142.50 ± 29.34 |
| Blood pressure (mmHg) | ||
| Systolic | 155.93 ± 36.38 | 155.23 ± 32.30 |
| Diastolic | 93.93 ± 16.69 | 93.09 ± 16.13 |
| HbA1c (%) | 0.08 ± 0.013 | 0.08 ± 0.014 |
| Plasma glucose (mmol/L) | 8.69 ± 2.80 | 8.69 ± 2.04 |
| Plasma insulin (pmol/L) | 109.66 ± 49.46 | 140.04 ± 52.13 |
| Creatinine (μmol/L) | 89.98 ± 12.97 | 90.41 ± 20.94 |
| Triglycerides (mmol/L) | 2.35 ± 0.85 | 2.35 ± 0.52 |
| Total cholesterol (mmol/L) | 6.14 ± 1.59 | 5.96 ± 1.77 |
| HDL cholesterol (mmol/L) | 1.03 ± 0.20 | 1.03 ± 0.26 |
| Microalbuminuria (g/L) | 0.49 ± 0.066 | 0.49 ± 0.11 |
| HOMA [(mUI/L)(mg/dL)] | 6.00 ± 3.42 | 7.72 ± 2.95 |
| Quicky {1/log[(mUI/L)(mg/dL)]} | 0.30 ± 0.02 | 0.29 ± 0.01 |
| 10-year predicted probability for CHD | 0.16 ± 0.11 | 0.17 ± 0.12 |
| GFR (mL/min per 1.73 m2) | 69.50 ± 14.34 | 71.42 ± 17.54 |
| GFR (mL/min) | 98.22 ± 27.20 | 100.83 ± 31.23 |
Data are n (%) or means ± SD unless otherwise indicated.
HOMA, homeostasis model assessment.
Early complications included one pulmonary (4.5%) and one wound infection (4.5%) (Supplementary Materials). There was no mortality. As to late complications, three patients had incisional hernias (13.6%) and two had a peptic ulcer (9.1%) on the intestinal side of the gastroenteric anastomosis, which required medical treatment.
Clinical outcomes
Nephropathy study: relative percentage variation of GFR.
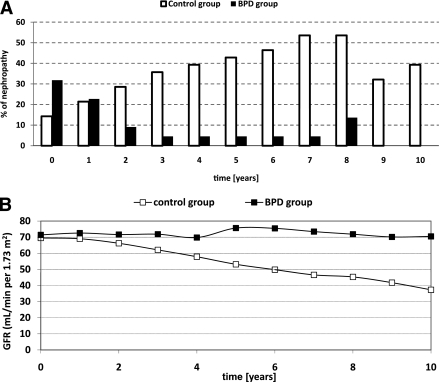
The relative percentage variation of GFR was significantly different between the two groups (P < 0.001): it was negative in the control group (−45.6 ± 18.7%) and positive in the BPD group (4.2 ± 31.3%). The RMANOVA results were also significant (P < 0.001) for time, time × treatment, and treatment. When non-body surface area (BSA)–corrected GFR values were considered, relative variations were −45.7 ± 18.8 vs. +13.6 ± 24.5% (P < 0.001).
Microalbuminuria, macroalbuminuria, and elevated serum creatinine prevalence.
At diagnosis of diabetes, 14.3% of the control patients versus 31.8% of the BPD patients had microalbuminuria (P = 0.178). After 2 years, in the control group the percentage increased to 28.6%, whereas in the BPD group it decreased to 9.1% (P = 0.154). After 10 years, all subjects in the BPD group recovered from microalbuminuria, whereas in the control group microalbuminuria cases worsened and a prevalence of 39.3% of hypercreatininemia (plasma creatinine ≥1.98 mg/dL, equivalent to 175 μmol/L) (P = 0.001) was observed.
Annual nephropathy transition rates from stage to stage.
Fifty percent of subjects in the control group versus nine percent in the BPD group (P = 0.002) progressed from no nephropathy at the time of diabetes diagnosis to nephropathy at the end of the follow-up period. These percentages were 39.3 vs. 9% when only progression to microalbuminuria was considered (P = 0.02). In the control group, starting from time 0, the average incidence of any nephropathy condition was 8.4% per year: 5.9% per year was the average annual progression to microalbuminuria, and 2.5% per year was the average progression to hypercreatininemia. Average transition rate from either health or microalbuminuria to hypercreatininemia was 4.8%. In the BPD group, only 0.95% on average developed a disease condition: two new cases of microalbuminuria were indeed recorded at the eighth year. Figure 1A reports the prevalence of diabetic nephropathy over the 10-year follow-up; the GFR trend is reported in Fig. 1B.
Figure 1.
A: Prevalence of nephropathy over the 10 years of follow-up. □, conventional therapy; ■, surgical therapy. B: GFR trend in the conventional (□) and in the surgical (■) therapy over the 10 years of follow-up.
Cardiovascular events
Four major events in four different patients occurred in the control group: three electrocardiogram-determined myocardial infarctions at years 5, 7, and 9, respectively, and one stroke at year 6.
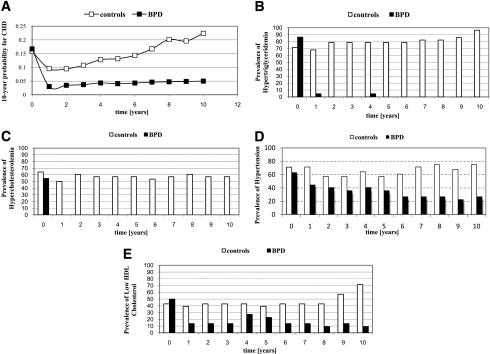
Ten-year CHD–predicted probability was computed at baseline and at each of the following years, estimating the risk for CHD up to 20 years from the study beginning. Figure 2A reports the trend of the probability to develop CHD. RMANOVA was significant for time, time × treatment, and treatment (P < 0.001). Whereas in the BPD group the CHD probability decreased and then remained quite constant over the follow-up, in the control group the probability decreased during the first 2 years and then increased over time to reach 0.22 ± 0.10 vs. 0.05 ± 0.04 in the BPD group (P < 0.001).
Figure 2.
A: Trend of 10-year probability for CHD in the conventional (□) and the surgical (■) therapy over the 10 years of follow-up. B–E: Prevalence of hypertriglyceridemia, hypercholesterolemia, hypertension, and low HDL cholesterol.
Hypertension
At time 0, 34 subjects (20 in the control group vs. 14 in the BPD group; P = 0.8) were hypertensive. A logistic model was used to study the probability of recovery from the disease at 1, 2, and 10 years in the 34 subjects affected. The model used included age and BMI at baseline; sex and use of antihypertensive medications were covariates. The variables were retained in the model only if significant at 0.10. After 1 and 2 years, the recovery rates for the two groups were not significantly different. At the end of follow-up, the recovery rate was higher in the BPD group, with an odds ratio of 23 (P = 0.01). Hypertension incidence in the two groups is reported in Fig. 2. The association of treatment with hypertension was significant only after the sixth year. At 10 years, the prevalence of hypertensive subjects in the control group was 75 vs. 27.3% in the treated-group (P = 0.001).
Hyperlipidemia
The prevalence of hyperlipidemia over the 10-year follow-up period is reported in Fig. 2. As expected, in all likelihood as a consequence of lipid malabsorption, circulating triglycerides and cholesterol levels decreased in the BPD group. Notably, HDL cholesterol increased over time, possibly as a result of the substantial weight loss.
Diabetes recovery
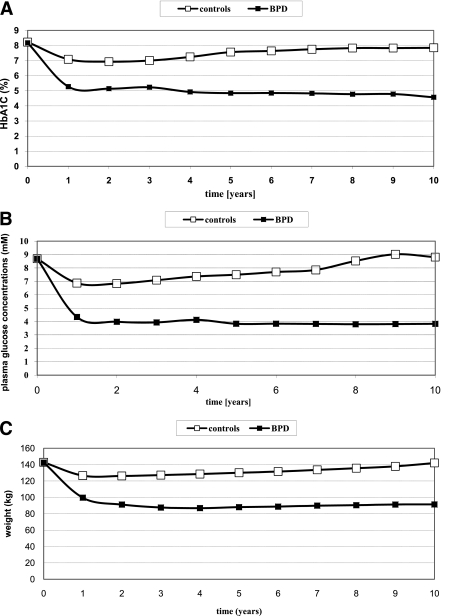
At year 1 of follow-up, all subjects in the BPD group experienced remission from diabetes compared with 45% in the control group (P < 0.001). In the former group, the recovery was definitive because none of the patients experienced diabetes again in the course of the study. The trend of glycated hemoglobin closely resembled that of glucose (Fig. 3A and B), and both treatment and the interaction time × treatment were significant (P < 0.001).
Figure 3.
Trends over the 10-year follow-up of glycated hemoglobin (A), plasma glucose concentrations (B), and weight (C) in the conventional (□) and in the surgical (■) therapy.
Insulin sensitivity
Baseline homeostasis model assessment of insulin resistance was not significantly different between groups (6.0 ± 3.4 vs. 7.7 ± 2.9 mUI/L [mg/dL] in the control and BPD groups, respectively; P = 0.07). Changes over time of insulin resistance were tested only in the BPD group because these subjects did not undergo any pharmacological treatment (insulin in particular): the effect of time was significant (RMANOVA, P < 0.001); insulin resistance decreased significantly throughout the study, reaching a value of 1.8 ± 0.9.
The improvement of insulin sensitivity in the BPD group was also apparent from the analysis of the Quicky index. Starting from a value very similar to that of the control group (0.30 ± 0.02 in the BPD group vs. 0.29 ± 0.01 log−1 mUI/L [mg/dL] in controls), the BPD group showed a notable improvement, reaching a value of 0.36 ± 0.03 at the end of follow-up (P < 0.001).
Weight changes
Weight changes were significantly different between groups (P levels for treatment and interaction time × treatment were both <0.001). Contrasts within the time × treatment factor were all significant: while BPD group subjects lost a large amount of weight in the first 2 years, remaining stable thereafter for a long time and showing finally a modest trend to recover some weight, the control group—after some initial decrease—recovered all lost weight by the end of the follow-up period (Fig. 3C).
CONCLUSIONS
This study, to our knowledge, is the first controlled trial focusing on the effect of metabolic surgery, in particular malabsorptive BPD, on diabetic complications. In addition to prolonged effects of BPD in improving glycemic control, the trial showed that there were large differences in outcomes between metabolic surgery and an intensive medical glucose-control strategy in patients with newly diagnosed type 2 diabetes. The major results concern not only the attainment of an optimal glycemic control, as shown by the sustained reduction of glycated hemoglobin, which reached normal levels already by 1 year after surgery, but also the net advantage of metabolic surgery in comparison with medical therapy regarding diabetic complications, particularly with regard to the progressive loss of GFR, which is indicative of diabetic nephropathy.
Recently, an agreement was reached about the definition of the effect of metabolic surgery on diabetes (17), with “remission” defined as the achievement of glycemia below the diabetes range in the absence of active pharmacological or additional surgical therapy. Furthermore, in relation to its duration, a remission was characterized as partial, complete, or prolonged, with this last applying to periods of 5 years or more. Therefore, a prolonged type 2 diabetes remission was attained in our diabetic patients undergoing BPD.
The incidence of nephropathy progressively increased over time in the medical arm while it decreased in the surgical arm, suggesting that nephropathy is a reversible feature of glomerular dysfunction that is possibly linked to increased intra-abdominal pressure in severe obesity, which has been documented as 10 times higher than that in normal weight subjects (18).
The absence of episodes of myocardial infarction and stroke and the net reduction of the cardiovascular risk in the surgical group suggest that metabolic surgery had a strong impact on abating cardiovascular disease. In fact, the estimated 10-year probability for CHD was about 16% in both groups. However, while in the conventional therapy group four subjects (14%) developed cardiovascular diseases by 9 years of follow-up, no cardiovascular episodes occurred in the surgical group.
In a 10-year longitudinal study of 1,268 patients with type 2 diabetes, Sjöström et al. (19) showed that the 2- and 10-year incidence rates of diabetes, hypertriglyceridemia, and hyperuricemia were more favorable in the surgery group than in the control group; however, differences between the groups in the incidence of hypercholesterolemia and hypertension were not statistically significant. Sjöström et al. found a lower rate of recovery from diabetes in comparison with our study; in particular, they found 72% of recovery at 2 years after surgery and 36% at 10 years. It is likely that these differences arise mainly from the different surgical procedures used. In fact, in the study of Sjöström et al., 156 subjects underwent banding, 451 vertical banded gastroplasty, and 34 Roux-en-Y gastric bypass, whereas in our study the smaller but homogeneous group of diabetic subjects studied underwent malabsorptive bariatric surgery. Our results are in agreement with two meta-analyses (9,20) reported in the literature. We should also highlight that in the study of Sjöström et al. (19) the subjects were not diabetic at the beginning of the study, whereas the subjects in our investigation had newly diagnosed diabetes.
In spite of the good results regarding diabetic complications after BPD, it is necessary to point out that this operation is attended by both surgical and medical complications, which should be taken into consideration when diabetic patients are advised to undergo BPD. In particular, early complications after BPD include 0.4% operative mortality and 1.2% wound dehiscence and infection; late surgical complications are incisional hernia (8.7%) and intestinal obstruction (1.2%) (21). However, medical complications are related to malabsorption and include hypoproteinemia, anemia, and hypovitaminosis (22–24).
The major weaknesses of the current study are a large dropout, lack of randomization, and a large average BMI (~50 kg/m2). As a result of these factors, the population studied does not represent the general population of patients with diabetes.
In conclusion, this study indicates that metabolic surgery represents a favorable option in the treatment of type 2 diabetes, at least in subjects with severe obesity. In particular, it shows that renal and cardiovascular complications are dramatically reduced in the surgical group, indicating long-term benefits of metabolic surgery on diabetic complications. This observation is particularly relevant because it has been shown that the attainment of an optimal glycemic control is not the major factor in reducing the risk of coronary events in type 2 diabetic subjects (2).
Supplementary Material
Acknowledgments
Funds were provided by the Catholic University of Rome.
No potential conflicts of interest relevant to this article were reported.
A.I. participated in the study coordination, participant enrollment, medical oversight of participants, and data collection. S.P. participated in the conception and design of the study and performed statistical analysis. A.D.G. participated in the conception and design of the study and performed statistical analysis. M.M. participated in the study coordination, participant enrollment, medical oversight of participants, and data collection. C.G. participated in the study coordination, participant enrollment, medical oversight of participants, and data collection. L.L. participated in the study coordination, participant enrollment, medical oversight of participants, and data collection. D.G. participated in the study coordination, participant enrollment, medical oversight of participants, and data collection. G.N. performed surgical operations. M.C. performed surgical operations and provided critical revisions. G.G. provided critical revisions. G.M. participated in the conception and design of the study, participated in data interpretation, and wrote the article.
The authors thank Mrs. Anna Caprodossi, Catholic University, Department of Medicine in Rome, for her precious technical assistance.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1761/-/DC1.
References
- 1.Goff DC, Jr, Gerstein HC, Ginsberg HN, et al. ACCORD Study Group Prevention of cardiovascular disease in persons with type 2 diabetes mellitus: current knowledge and rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Am J Cardiol 2007;99:4i–20i [DOI] [PubMed] [Google Scholar]
- 2.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dormandy JA, Charbonnel B, Eckland DJ, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet 2005;366:1279–1289 [DOI] [PubMed] [Google Scholar]
- 4.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 5.ADVANCE Collaborative Group Patel A, MacMahon S, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 6.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008;359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 7.Resnick HE, Foster GL, Bardsley J, Ratner RE. Achievement of American Diabetes Association clinical practice recommendations among U.S. adults with diabetes, 1999-2002: the National Health and Nutrition Examination Survey. Diabetes Care 2006;29:531–537 [DOI] [PubMed] [Google Scholar]
- 8.Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD-3 MONO): randomized, 52-week, phase III, double blind, parallel-treatment trial. Lancet 2009;373:473–481 [DOI] [PubMed] [Google Scholar]
- 9.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med 2009;122:248–256 [DOI] [PubMed] [Google Scholar]
- 10.Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–752 [DOI] [PubMed] [Google Scholar]
- 11.Laville M, Disse E. Bariatric surgery for diabetes treatment: why should we go rapidly to surgery. Diabetes Metab 2009;35:562–563 [DOI] [PubMed] [Google Scholar]
- 12.Pories WJ, Caro JF, Flickinger EG, Meelheim HD, Swanson MS. The control of diabetes mellitus (NIDDM) in the morbidly obese with the Greenville Gastric Bypass. Ann Surg 1987;206:316–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guidone C, Manco M, Valera-Mora E, et al. Mechanisms of recovery from type 2 diabetes after malabsorptive bariatric surgery. Diabetes 2006;55:2025–2031 [DOI] [PubMed] [Google Scholar]
- 14.Salinari S, Bertuzzi A, Asnaghi S, Guidone C, Manco M, Mingrone G. First-phase insulin secretion restoration and differential response to glucose load depending on the route of administration in type 2 diabetic subjects after bariatric surgery. Diabetes Care 2009;32:375–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics 1975;31:103–115 [PubMed] [Google Scholar]
- 16.Scopinaro N, Gianetta E, Civalleri D, Bonalumi U, Bachi V. Bilio-pancreatic bypass for obesity. II. Initial experience in man. Br J Surg 1979;66:618–620 [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Caprio S, Cefalu WT, et al. How do we define cure of diabetes? Diabetes Care 2009;32:2133–2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert DM, Marceau S, Forse RA. Intra-abdominal pressure in the morbidly obese. Obes Surg 2005;15:1225–1232 [DOI] [PubMed] [Google Scholar]
- 19.Sjöström L, Lindroos AK, Peltonen M, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–2693 [DOI] [PubMed] [Google Scholar]
- 20.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA 2004;292:1724–1737 [DOI] [PubMed] [Google Scholar]
- 21.Scopinaro N, Gianetta E, Adami GF, et al. Biliopancreatic diversion for obesity at eighteen years. Surgery 1996;119:261–268 [DOI] [PubMed] [Google Scholar]
- 22.de Luis DA, Pacheco D, Izaola O, Terroba MC, Cuellar L, Martin T. Clinical results and nutritional consequences of biliopancreatic diversion: three years of follow-up. Ann Nutr Metab 2008;53:234–239 [DOI] [PubMed] [Google Scholar]
- 23.Balsa JA, Botella-Carretero JI, Peromingo R, et al. Role of calcium malabsorption in the development of secondary hyperparathyroidism after biliopancreatic diversion. J Endocrinol Invest 2008;31:845–850 [DOI] [PubMed] [Google Scholar]
- 24.Mingrone G. Role of the incretin system in the remission of type 2 diabetes following bariatric surgery. Nutr Metab Cardiovasc Dis 2008;18:574–579 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.