Abstract
OBJECTIVE
Hypoglycemia causes recurrent morbidity in patients with type 2 diabetes. This study evaluated if exenatide twice daily (BID) was noninferior to premixed insulin aspart 70/30 BID (PIA) for glycemic control and associated with less hypoglycemia.
RESEARCH DESIGN AND METHODS
In this open-label study, metformin-treated adults with type 2 diabetes were randomized to 26-week treatment with exenatide BID (4 weeks 5 μg, then 10 μg) or PIA.
RESULTS
Exenatide BID (n = 181) was noninferior to PIA (n = 173) for A1C control (least squares [LS] mean change −1.0 vs. −1.14%; difference [95% CI] 0.14 [−0.003 to 0.291]) and associated with a lower risk for hypoglycemia (8.0 vs. 20.5%, P < 0.05). LS mean weight decreased by 4.1 kg and increased by 1.0 kg with PIA (P < 0.001). A total of 39.2 vs. 20.8% of patients reached the composite end point of A1C <7.0%, no weight gain, and no hypoglycemia (P < 0.001; post hoc analysis).
CONCLUSIONS
In metformin-treated patients, exenatide BID was noninferior to PIA for glycemic control but superior for hypoglycemia and weight control.
The well-known limitations of insulin therapy are weight gain and increased risk of hypoglycemia (1,2). Results of cardiovascular outcome trials (3,4) have triggered a discussion about the association between hypoglycemia and increased mortality (5). The American Diabetes Association defines the prevention of hypoglycemia as a critical component of diabetes management (6). This study was specifically designed to compare hypoglycemia with exenatide twice daily (BID) versus premixed insulin aspart 70/30 BID (PIA) (70% protamin crystallized, 30% soluble) at a noninferior level of glycemic control in type 2 diabetic patients on metformin treatment. An additional smaller patient cohort previously treated with metformin plus sulfonylurea or meglitinides was enrolled into an exploratory arm; data have been published separately (7).
RESEARCH DESIGN AND METHODS
This 26-week randomized open-label study was conducted at 68 sites in Germany. Metformin-treated adults with type 2 diabetes (A1C 6.5–10.0%) received exenatide BID (4 weeks, 5 μg, then 10 μg) or PIA. PIA was titrated to glucose targets of 5.0–7.2 mmol/L (fasting) and <10 mmol/L (2 h postprandial) after each main meal (8), without a structured insulin dosing algorithm. Metformin was continued unchanged. Randomization was stratified by baseline A1C (≤8.0 or >8.0%).
A1C was measured by a central laboratory (Tosoh HPLC analyzer; Interlab, Munich, Germany), and hypoglycemic episodes were collected from patient diaries. Patients were encouraged to measure blood glucose levels twice daily and immediately in case of hypoglycemic symptoms.
The primary objective was to test if exenatide BID was noninferior to PIA for A1C control, and superior to PIA regarding hypoglycemia (blood glucose ≤3.9 mmol/L or severe episode). Severe episodes were defined as episodes requiring assistance of another person, with symptoms recovering after treatment (8). A hierarchical testing procedure was used. For noninferiority of exenatide BID, the upper limit of the 95% CI of the group difference in A1C change was required to be <0.4% (exenatide BID minus PIA; mixed model repeated measurement [MMRM] adjusting for baseline A1C). Only if noninferiority was shown, the second test on the risk for the first hypoglycemic episode (blood glucose ≤3.9 mmol/L or severe; Kaplan-Meier analysis) was done. The analysis included all randomized patients who received the study drug (full analysis population). Body weight was compared using an MMRM model similar to the primary analysis. Post hoc, the proportion of patients reaching A1C <7.0% without weight gain and hypoglycemic episodes (composite end point) was compared by χ2 test. Frequencies of adverse events were compared using χ2 tests.
RESULTS
Of 363 randomized patients (exenatide/PIA 182/181), 354 (181/173) received study drug (full analysis population); 272 (135/137) completed the study. Patient characteristics were similar in both groups (exenatide/PIA mean ± SD): age 57 ± 10/57 ± 9.9 years, BMI 33.4 ± 4.2/32.9 ± 4.4 kg/m2, diabetes duration 5 ± 4/5 ± 5 years, baseline A1C 7.9 ± 0.8%/7.9 ± 0.9%.
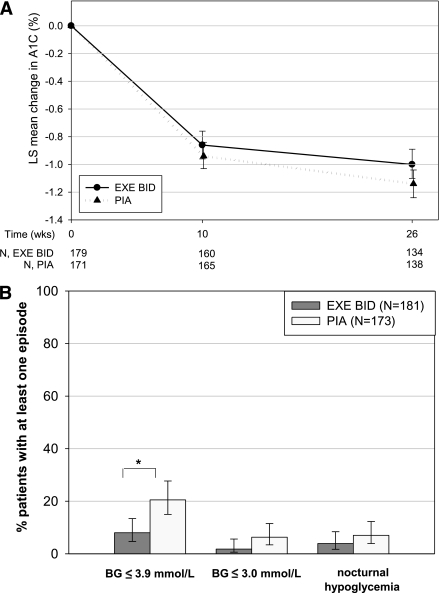
After 26 weeks, LS mean A1C had decreased by −1.00% with exenatide BID and −1.14% with PIA (Fig. 1A); exenatide BID was noninferior (group difference 0.14; 95% CI −0.003 to 0.291). A1C targets of <7.0% (<6.5%) were achieved by 49.2% (27.6%) of exenatide BID patients and 56.6% (24.9%) of PIA patients. The risk for the first hypoglycemic episode (blood glucose ≤3.9 mmol/L or severe) was significantly lower with exenatide BID than with PIA (P < 0.05); 8.0% (95% CI 4.7–13.4%) vs. 20.5% (15.0–27.7%) of patients experienced at least one episode (Fig. 1B). There was no severe hypoglycemia. Nocturnal episodes were reported for 3.9% of exenatide BID and 7.0% of PIA patients. Hypoglycemic episodes with blood glucose ≤3.0 mmol/L were also less frequent with exenatide BID (1.8 vs. 6.3%, Fig. 1B).
Figure 1.
LS mean change in A1C (A) and risk of hypoglycemic episodes (B) up to week 26 (full analysis set, all patients treated). A: Data were derived from an MMRM analysis, adjusting for baseline A1C. B: *P < 0.05 for second step of hierarchical testing procedure. The risk for the first hypoglycemic episode (blood glucose [BG] ≤3.9 mmol/L or severe, but no severe observed) to occur up to week 26 was tested for superiority of exenatide (EXE) BID over PIA by nonoverlapping 95% CIs from Kaplan-Meier analysis (two-sided, α = 0.05). Vertical bars indicate 95% CIs.
After 26 weeks, exenatide BID patients had lost 4.1 ± 0.22 kg of weight, while PIA patients had gained 1.0 ± 0.22 kg (LS mean ± SEM; P < 0.001 for group difference).
The proportion of patients reaching the post hoc composite end point of A1C <7.0%, no weight gain, and no hypoglycemia was significantly higher with exenatide BID than with PIA (39.2 vs. 20.8%; P < 0.001).
The mean final total insulin dose (PIA) was 28.4 IU/day (0.29 IU/kg/day). Metformin doses remained unchanged in both groups (median 2,000 mg/day).
Most common adverse events (≥5% of patients) with the exenatide BID group were nausea (18.8%), nasopharyngitis (14.9%), diarrhea (10.5%), vomiting (9.9%), headache (8.3%), and dyspepsia (6.1%). With PIA, nasopharyngitis (19.1%), headache (13.3%), diarrhea (8.1%), and back pain (5.2%) were reported most frequently. More patients on exenatide BID discontinued because of adverse events (7.2 vs. 0.6%; P = 0.0014). The main reasons for discontinuation of exenatide BID were nausea (3.9%) and diarrhea (1.1%).
CONCLUSIONS
In metformin-treated type 2 diabetic patients, exenatide BID was noninferior to PIA in terms of glycemic control and superior in terms of hypoglycemia. In addition, exenatide patients achieved significant mean weight reduction. With exenatide BID, twice as many patients reached the clinically relevant composite end point of A1C <7.0%, no weight gain, and no hypoglycemia (post hoc analysis). Two previous studies had also compared exenatide BID versus PIA. The 52-week trial by Nauck et al. (9) showed that in a similar patient population, exenatide BID was noninferior to PIA for glycemic control, but without any difference in hypoglycemia. The mean final daily insulin dose was lower than in our trial (24.4 vs. 28.4 IU/day). Patients received concomitant sulfonylurea, and this may explain the lower insulin dose and lack of differences in hypoglycemia.
In contrast, the 24-week trial by Bergenstal et al. (10) showed superior glycemic control with PIA versus exenatide BID in poorly controlled patients with advanced disease (baseline A1C 10.2%, diabetes duration 10 years). In late-stage diabetes, insulin may improve glycemic control better; GLP-1 therapy may have limited effects because of the impaired β-cell function (10). Hypoglycemia was less frequent with exenatide BID (20.2 vs. 52.1%) in spite of concomitant sulfonylurea, possibly because of aggressive insulin titration (mean final dose 96.1 IU/day).
In our study, sulfonylurea treatment was excluded, decreasing the risk of hypoglycemia in both arms and enabling us to assess the full benefit of exenatide on the risk of hypoglycemia. The hierarchical testing required noninferior glycemic control before testing for hypoglycemia. Insulin titration might be criticized as not being aggressive enough (11). The mean final insulin dose (28.4 IU/day) was higher than in the study by Nauck et al. (9), but lower than in two studies using other premixed insulins (12,13). However, A1C reductions were similar to previous trials, and a mean end point A1C of 6.8% was among the best results reported (12,13).
Acknowledgments
K.H., N.P., and O.B. are employed by Lilly Deutschland, Germany. H.P. is employed by Eli Lilly, Austria. No other potential conflicts of interest relevant to this article were reported.
B.G., M.B., T.S., A.M., K.M., B.B., and K.H. reviewed and edited the manuscript. B.G., K.H., H.P., N.P., and O.B contributed to the interpretation of the results. K.H. and O.B. drafted the introduction and the discussion sections of the manuscript, respectively. N.P. conducted the study. H.P. conducted the statistical analysis.
The authors thank Birgit Eschweiler, Medical Writing Services, Lage, Germany, for preparing the draft of the methods and the results sections of the manuscript. The authors also thank Claudia Nicolay, Lilly Deutschland GmbH, Bad Homburg, Germany, for contributions to the study design and Axel Haupt, Lilly Deutschland GmbH, Bad Homburg, Germany, for contributions to the discussion section. Finally, the authors thank all the investigators and subinvestigators who participated in this clinical trial.
Footnotes
Clinical trial reg. no. NCT00434954, clinicaltrials.gov.
References
- 1.Matthaei S, Bierwirth R, Fritsche A, et al. German Diabetes Association Medical antihyperglycaemic treatment of type 2 diabetes mellitus: update of the evidence-based guideline of the German Diabetes Association. Exp Clin Endocrinol Diabetes 2009;117:522–557 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes—2008 (Position Statement). Diabetes Care 2008;31(Suppl. 1):S12–S54 [DOI] [PubMed]
- 3.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Diabetes Care 2009;32:187–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonds DE, Miller ME, Bergenstal RM, et al. The association between symptomatic, severe hypoglycemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 2010;340:b4909 [DOI] [PMC free article] [PubMed]
- 6.American Diabetes Association Standards of medical care in diabetes—2010 (Position Statement). Diabetes Care 2010;33(Suppl. 1):S11–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallwitz B, Böhmer M, Segiet T, et al. Exenatide vs. insulin aspart in patients with type 2 diabetes: results of a randomised, open-label study (Abstract). 45th Annual Meeting of the European Association for the Studies of Diabetes (EASD), 20–24 September 2010, Stockholm, Sweden. Available from http://www.easd.org
- 8.Workgroup on Hypoglycemia, American Diabetes Association Defining and reporting hypoglycemia in diabetes: a report from the American Diabetes Association Workgroup on Hypoglycemia. Diabetes Care 2005;28:1245–1249 [DOI] [PubMed] [Google Scholar]
- 9.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 2007;50:259–267 [DOI] [PubMed] [Google Scholar]
- 10.Bergenstal R, Lewin A, Bailey T, Chang D, Gylvin T, Roberts V, NovoLog Mix-vs.-Exenatide Study Group Efficacy and safety of biphasic insulin aspart 70/30 versus exenatide in subjects with type 2 diabetes failing to achieve glycemic control with metformin and a sulfonylurea. Curr Med Res Opin 2009;25:65–75 [DOI] [PubMed] [Google Scholar]
- 11.Home PD. Comment on: Nauck MA, Duran S, Kim D, et al. (2007) A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia 50:259–267. Diabetologia 2007;50:1561–1562; author reply 1563–1564 [DOI] [PubMed] [Google Scholar]
- 12.Esposito K, Ciotola M, Maiorino MI, et al. Addition of neutral protamine lispro insulin or insulin glargine to oral type 2 diabetes regimens for patients with suboptimal glycemic control: a randomized trial. Ann Intern Med 2008;149:531–539 [DOI] [PubMed] [Google Scholar]
- 13.Fogelfeld L, Dharmalingam M, Robling K, Jones C, Swanson D, Jacober S. A randomized, treat-to-target trial comparing insulin lispro protamine suspension and insulin detemir in insulin-naive patients with type 2 diabetes. Diabet Med 2010;27:181–188 [DOI] [PubMed] [Google Scholar]