Abstract
OBJECTIVE
Stimulated serum C-peptide (sCP) during a mixed-meal tolerance test (MMTT) is the gold standard measure of endogenous insulin secretion, but practical issues limit its use. We assessed urine C-peptide creatinine ratio (UCPCR) as an alternative.
RESEARCH DESIGN AND METHODS
Seventy-two type 1 diabetic patients (age of diagnosis median 14 years [interquartile range 10–22]; diabetes duration 6.5 [2.3–32.7]) had an MMTT. sCP was collected at 90 min. Urine for UCPCR was collected at 120 min and following a home evening meal.
RESULTS
MMTT 120-min UCPCR was highly correlated to 90-min sCP (r = 0.97; P < 0.0001). UCPCR ≥0.53 nmol/mmol had 94% sensitivity/100% specificity for significant endogenous insulin secretion (90-min sCP ≥0.2 nmol/L). The 120-min postprandial evening meal UCPCR was highly correlated to 90-min sCP (r = 0.91; P < 0.0001). UCPCR ≥0.37 nmol/mmol had 84% sensitivity/97% specificity for sCP ≥0.2 nmol/L.
CONCLUSIONS
UCPCR testing is a sensitive and specific method for detecting insulin secretion. UCPCR may be a practical alternative to serum C-peptide testing, avoiding the need for inpatient investigation.
The mixed-meal tolerance test (MMTT) is the gold standard measure of endogenous insulin secretion in type 1 diabetes, but practical issues restrict testing to the hospital setting (1,2). Ninety-minute stimulated serum C-peptide (sCP) ≥0.2 nmol/L (≥0.6 ng/L) is related to improved clinical outcomes (3) and is used to indicate significant endogenous insulin secretion (4–6). We have recently shown urine C-peptide creatinine ratio (UCPCR) to be both reproducible and stable for 3 days at room temperature using boric acid as a preservative (7). Here, we assessed whether UCPCR is a noninvasive alternative to the 90-min sCP response during the MMTT in type 1 diabetes.
RESEARCH DESIGN AND METHODS
Additional information about study design, ethical considerations, and laboratory methods can be found in Supplementary Materials. We studied 72 children (n = 21) and adults with type 1 diabetes without known renal impairment (estimated glomerular filtration rate <60 mL/min/1.73 m2) (Supplementary Tables 1 and 2).
MMTT
Patients underwent a standard MMTT (1). sCP was collected at 0 and 90 min. Additional samples were taken at 30, 60, and 120 min in pediatric patients (n = 18), allowing area under the curve (AUC) to be calculated. Urine was collected as a fasting second morning void immediately before the start of the MMTT (0 min) and after 120 min.
Significant endogenous insulin secretion was defined as 90-min sCP ≥0.2 nmol/L, in accordance with the Diabetes Control and Complications Trial (8).
Home urine collections
Urine was collected in boric acid 120 min after the evening meal following a premeal void. Adult patients collected further home urine samples 120 min after a standard 60-g carbohydrate breakfast and following the patients’ own lunch. Urine samples were brought to the research center within 24 h, measured in aliquots, and frozen at −80°C.
Statistical analysis
We assessed the association between 90-min sCP (1) and both the MMTT 120-min UCPCR and after the home evening meal (Spearman rank correlation coefficient). In the pediatric cohort, correlations were also determined between AUC sCP and 120-min UCPCR. UCPCR cutoffs equivalent to 90-min sCP ≥0.2 nmol/L were derived using linear regression equations. UCPCR (120 min) following a home evening meal was compared with that after a MMTT (Wilcoxon test for paired samples).
RESULTS
UCPCR correlations with serum C-peptide
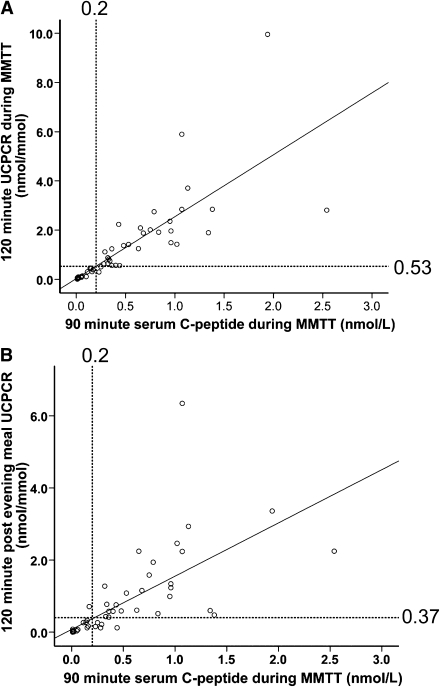
MMTT 120-min UCPCR was highly correlated with the 90-min sCP (r = 0.97; P < 0.0001). The equivalent 120-min MMTT UCPCR cutoff for significant endogenous insulin secretion (90-min sCP ≥0.2 nmol/L) was ≥0.53 nmol/mmol, with 94% sensitivity and 100% specificity (Fig. 1A). A strong correlation was also seen between AUC for sCP and 120-min UCPCR during the MMTT (r = 0.96; P < 0.0001).
Figure 1.
Scatter diagram showing the relationship between 90-min sCP and 120-min UCPCR in the MMTT (A) and following the patients’ own evening meal at home (B). A: 120-min UCPCR is well correlated with 90-min sCP in the MMTT (r = 0.97; P < 0.0001). UCPCR ≥0.53 nmol/mmol is equivalent to 90-min sCP ≥0.2 nmol/L (linear regression), with 94% sensitivity and 100% specificity. B: 120-min postprandial UCPCR is well correlated with 90-min sCP in the MMTT (r = 0.91). UCPCR ≥0.37 nmol/mmol is equivalent to 90-min sCP ≥0.2 nmol/L (linear regression), with 84% sensitivity and 97% specificity.
Home postprandial evening meal UCPCR (120 min) was well correlated with 90-min sCP (r = 0.91; P < 0.0001) (Fig. 1B). The equivalent UCPCR cutoff was ≥0.37 nmol/mmol, with 84% sensitivity and 97% specificity (Fig. 1B).
Using the UCPCR cutoff ≥0.53 nmol/mmol in the home evening meal samples yielded lower levels of sensitivity (71%) and specificity (97%) for significant endogenous insulin secretion. This is probably explained by a lower stimulus, as shown by the lower 120-min UCPCR in the home postprandial samples than in those in the MMTT (0.16 nmol/mmol [interquartile range 0.01–0.76] vs. 0.35 nmol/mmol [0.04–1.41]; P < 0.0001).
The correlations were similar in adults and children when analyzed separately (Supplementary Tables 4 and 5). Result tables for combined (Supplementary Table 3) and separate analysis of adults (Supplementary Table 4) and children (Supplementary Table 5) are given in the Supplementary Materials.
CONCLUSIONS
UCPCR measured during an MMTT or after a home meal is highly correlated with MMTT sCP. UCPCR offers a sensitive and specific method of detecting insulin secretion.
UCPCR as a practical alternative to serum C-peptide measurement
Our results showed strong correlations between stimulated UCPCR and serum C-peptide (r = 0.91–0.97) during an MMTT. UCPCR, while not superior, has some clear practical advantages over sCP. sCP requires separating the serum by spinning rapidly and subsequent freezing (2). This effectively limits testing to the hospital setting. Because UCPCR is stable at room temperature for 3 days in boric acid preservative (7), home samples could be collected following a liquid mixed meal or the patients’ own home meal and a spot urine sample collected and posted for analysis directly. This would allow assessment to be done at home and to be noninvasive—a particular advantage for children.
As would be predicted, UCPCR values were lower after a meal compared with the standard MMTT, and so a lower concentration of UCPCR was required to suggest clinically significant insulin deficiency. The slight loss of precision compared with the standard MMTT needs to be balanced by the practicality of this approach because it would remove the need for inpatient testing and allow widespread screening.
Other measures of urinary C-peptide
The strong correlation of UCPCR with serum C-peptide in the MMTT is supported by previous studies that have shown that timed measures of urinary C-peptide are a useful marker of endogenous insulin secretion (7,9–13). We used UCPCR to correct for dilution by measuring creatinine. This allowed spot samples to be taken rather than sampling over 24 h, in which case complete collection is difficult. This is similar to the practical reason why spot albumin creatinine ratio is used as opposed to 24 h urine collections in the assessment of renal protein excretion.
Study limitations
A strong correlation between AUC C-peptide and 120-min UCPCR was demonstrated (r = 0.96); however, numbers were small (n = 18) and further work is needed to explore this. Inclusion was limited to patients who could void on demand. The test can be difficult in young children, especially those who are still in nappies. Our results also only apply to patients without renal impairment. Further studies are needed to confirm our findings in this subgroup.
Implications
The ease of use means that, if used in conjunction with formal MMTT, UCPCR may be useful for screening patients for initial inclusion and also follow-up during intervention trials. In conclusion, our study demonstrates that in children and adults with type 1 diabetes, UCPCR may be a practical noninvasive alternative to the MMTT for use in routine clinical practice.
Supplementary Material
Acknowledgments
We acknowledge the support of Diabetes UK for this project through funding (through a clinical training fellowship) to R.E.J.B. Other funding was from the Peninsula National Institute for Health Research Clinical Research Facility and from the European Community FP7 program Collaborative European Effort to Develop Diabetes Diagnostics (CEED3) (HEALTH-F2-2008-223211). The study was also supported by Barndiabetesfonden (The Swedish Child Diabetes Foundation) and the Swedish Research Council.
No potential conflicts of interest relevant to this article were reported.
R.E.J.B. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. J.L. researched data, contributed to discussion, and reviewed and edited the manuscript. A.G.J. contributed to discussion and reviewed and edited the manuscript. T.J.M., B.M.S., B.A.K., and A.T.H. researched data, contributed to discussion, and reviewed and edited the manuscript.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-2114/-/DC1.
References
- 1.Greenbaum CJ, Mandrup-Poulsen T, McGee PF, et al. Type 1 Diabetes Trial Net Research Group. European C-Peptide Trial Study Group Mixed-meal tolerance test versus glucagon stimulation test for the assessment of beta-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008;31:1966–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark PM. Assays for insulin, proinsulin(s) and C-peptide. Ann Clin Biochem 1999;36:541–564 [DOI] [PubMed] [Google Scholar]
- 3.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 4.Palmer JP, Fleming GA, Greenbaum CJ, et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21-22 October 2001. Diabetes 2004;53:250–264 [DOI] [PubMed] [Google Scholar]
- 5.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes 2005;54:2060–2069 [DOI] [PubMed] [Google Scholar]
- 6.Hattersley A, Bruining J, Shield J, Njolstad P, Donaghue K, International Society for Pediatric and Adolescent Diabetes ISPAD Clinical Practice Consensus Guidelines 2006-2007. The diagnosis and management of monogenic diabetes in children. Pediatr Diabetes 2006;7:352–360 [DOI] [PubMed] [Google Scholar]
- 7.McDonald TJ, Knight BA, Shields BM, Bowman P, Salzmann MB, Hattersley AT. Stability and reproducibility of a single-sample urinary C-peptide/creatinine ratio and its correlation with 24-h urinary C-peptide. Clin Chem 2009;55:2035–2039 [DOI] [PubMed] [Google Scholar]
- 8.The Diabetes Control and Complications Trial Research Group Effect of intensive therapy on residual beta-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. Ann Intern Med 1998;128:517–523 [DOI] [PubMed] [Google Scholar]
- 9.Aurbach-Klipper J, Sharph-Dor R, Heding LG, Karp M, Laron Z. Residual B cell function in diabetic children as determined by urinary C-peptide. Diabetologia 1983;24:88–90 [DOI] [PubMed] [Google Scholar]
- 10.Gjessing HJ, Matzen LE, Frøland A, Faber OK. Correlations between fasting plasma C-peptide, glucagon-stimulated plasma C-peptide, and urinary C-peptide in insulin-treated diabetics. Diabetes Care 1987;10:487–490 [DOI] [PubMed] [Google Scholar]
- 11.Koskinen P, Viikari J, Irjala K, Kaihola HL, Seppälä P. Plasma and urinary C-peptide in the classification of adult diabetics. Scand J Clin Lab Invest 1986;46:655–663 [DOI] [PubMed] [Google Scholar]
- 12.Huttunen NP, Knip M, Käär ML, Puukka R, Akerblom HK. Clinical significance of urinary C-peptide excretion in children with insulin-dependent diabetes mellitus. Acta Paediatr Scand 1989;78:271–277 [DOI] [PubMed] [Google Scholar]
- 13.Sasaki N, Miyamoto S, Niimi H, Nakajima H. C-peptide/creatinine ratio in early morning urine as an indicator of residual B-cell function in insulin-dependent diabetes. Acta Paediatr Jpn 1991;33:375–380 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.