Abstract
Disease resistance of plants involves two distinct forms of chemical communication with the pathogen: recognition and defense. Both are essential components of a highly complex, multifaceted defense response, which begins with non-self recognition through the perception of pathogen-derived signal molecules and results in the production, inter alia, of antibiotically active compounds (phytoalexins) and cell wall-reinforcing material around the infection site. To elucidate the molecular details and the genomic basis of the underlying chains of events, we used two different experimental systems: suspension-cultured cells of Petroselinum crispum (parsley) and wild-type as well as mutant plants of Arabidopsis thaliana. Particular emphasis was placed on the structural and functional identification of signal and defense molecules, and on the mechanisms of signal perception, intracellular signal transduction and transcriptional reprogramming, including the structural and functional characterization of the responsible cis-acting gene promoter elements and transacting regulatory proteins. Comparing P. crispum and A. thaliana allows us to distinguish species-specific defense mechanisms from more universal responses, and furthermore provides general insights into the nature of the interactions. Despite the complexity of the pathogen defense response, it is experimentally tractable, and knowledge gained so far has opened up a new realm of gene technology-assisted strategies for resistance breeding of crop plants.
Most plant/pathogen interactions are fierce battles of attack and counterattack. These battles are fought with highly sophisticated means for the survival of the individual and, in the end, of the entire population or species. On the plant side, the most immediate defense response includes the reprogramming of cellular metabolism and highly dynamic, structural rearrangements within and around the attacked cells. In the cases of locally invading, fungal or fungus-like pathogens, the counterstroke of the plant commences in a highly localized fashion with the perception of chemical and physical signals from the intruder and ends with the accumulation of soluble, antibiotically active compounds and wall-bound, barrier-forming substances. The initiating event, attempted penetration of a potential pathogen, immediately activates an elaborate safe-guard system of non-self recognition based on specifically adapted plant receptors. These receptors recognize characteristic pathogen-borne surface molecules and transduce that information to numerous genes through a network of intracellular signaling cascades that orchestrate an extensive, defense-oriented transcriptional reprogramming of the affected cell. Among the major changes in cellular metabolism is the rapid accumulation of various secondary metabolites, some of which are likely to be integral to the complex, multicomponent defense response.
This network of events, from the initial stage of recognition by the plant to the successful confinement or death of the pathogen, is far too fine-meshed to be elucidated by using one single experimental system. For our investigations of a few selected key events, we have used two complementary model systems: suspension-cultured Petroselinum crispum (parsley) cells and Arabidopsis thaliana plants. Cells and protoplasts of P. crispum proved to be ideal tools for analyzing the cell biology, biochemistry, and molecular biology of the defense response. Wild-type and mutant A. thaliana plants were particularly suited for transgenic studies and for investigating defined host plant/pathogen interactions in combination with the plant genetic background. Overlaps at certain focal points enabled us to directly compare these two systems and to infer both species-specific and universal defense mechanisms. In both experimental systems, our analyses of secondary metabolites encompassed aromatic phenylpropanoid as well as indolic compounds, among which antibiotically active phytoalexins and physicochemically active barrier-forming substances were of particular interest.
Here, we combine an overview of earlier findings with data obtained from recent experiments specifically designed to facilitate an interspecies comparison, a summary of the general principles observable so far, and a brief outline of one possible strategy for practical application of the results in crop plant breeding.
From Elicitor Perception to Secondary Metabolite Accumulation
Introductory Overview. The use of cultured P. crispum cells as our preferred experimental system for molecular and cell biological analyses offers several major advantages. First, the exogenously applied, pathogen-derived signal molecule is a chemically defined, small, and highly specific peptide elicitor that enables detailed structural and functional analyses based on specially designed synthetic modifications. Second, protoplasts derived from these cells retain full elicitor responsiveness and hence are a particularly valuable tool for analyzing gene promoter elements and their transcriptional regulators by using a simple and highly reproducible transfection and transient expression assay. Third, the synchronous elicitation of cultured cells, in sharp contrast to the largely asynchronous infections of whole-plant tissue, enables the precise determination of temporal gene expression characteristics. Furthermore, most, if not all, of the readily identifiable, elicitor-induced, soluble and wall-bound aromatic metabolites in P. crispum are phenylpropanoid derivatives; that is, they are members of a single and well characterized class of compounds, in contrast to more heterogeneous chemical responses observed in many other systems, including A. thaliana.
Fig. 1 schematically outlines the various extra- and intracellular events elucidated so far for the P. crispum system. These events extend from physical perception of the pathogen's infection hypha and chemical recognition of one highly active component of a putative mixture of pathogen-derived elicitors to transcriptional reprogramming of the cell's metabolic state and the consequential accumulation of defense-related, aromatic secondary compounds. All of the underlying cell biological experiments were conducted by using either Phytophthora sojae or P. infestans, two closely related pathogenic oomycetes to which P. crispum is nonhost resistant. For most of the molecular analyses, the live pathogen was replaced either with a chemical derivative (elicitor) or with a physical mimic (a sharp needle). As far as applicable to the largely undifferentiated, cultured cells or protoplasts, and as far as individual components have been analyzed, the response to elicitor was essentially the same as that observed with infected, whole-plant tissue, except for the additional occurrence of hypersensitive cell death at true infection sites. It should be noted in this connection that hypersensitive cell death, though not further investigated in P. crispum, is probably among the most efficient defense responses in this and many other systems.
Fig. 1.
Schematic outline of early molecular responses of P. crispum cells to attempted penetration of a Phytophthora sojae hypha. cw, cell wall; pm, plasma membrane; cy, cytoplasm; nu, nucleus.
Signal Perception. Even the earliest steps in the interaction between P. crispum and Phytophthora spp. are highly complex, but a few key events have been identified. After the formation of an appressorium as a tight physical holdfast, the pathogen develops an infection hypha in an attempt to invade the cell below (Fig. 1). This hypha is likely to exert mechanical force during further growth and penetration by virtue of strong physical support from the appressorium, but at the same time exposes its surface to the plant's surveillance mechanism for non-self recognition. The mechanical interaction has been experimentally decoupled from chemical signaling by replacing the hypha with a needle mimic. Even a gentle touch with a tungsten needle induces several, though not all, of the reactions observed after elicitor treatment or true infections, including the activation of some defense-related genes and the migration of nucleus and cytoplasm toward the site of physical contact (1).
In addition to pathogen-borne, exogenous elicitors, attacked plant cells are exposed to numerous breakdown products from their own damaged cell wall, some of which can also have significant roles in signal perception by synergistically acting as so-called endogenous elicitors. We speculate that the overall composition of the respective mixture of elicitor molecules enables the plant to activate the most appropriate defense response against a particular type of attacking pathogen. However, this facet has not been conclusively demonstrated in any pathosystem.
Isolation and purification to homogeneity of the most elicitor-active component from the culture filtrate of Phytophthora sojae yielded a 42-kDa glycoprotein containing an oligopeptide of 13 aa (Pep13) that in pure form is necessary and sufficient to stimulate the same complex defense response as observed either with a crude elicitor preparation or with the live pathogen (2). Elicitor activity of Pep13 is not restricted to P. crispum and leads, for example, to PR gene activation in cultured cells or leaves of potato (Solanum tuberosum) (3). Binding studies using radioiodinated Pep13 as a ligand and P. crispum microsomal membranes or protoplasts as receptor preparations in combination with chemical cross-linking identified a plasma membrane-associated protein complex containing a 91-kDa protein with high binding affinity for Pep13. Studies on structure-activity relationships using various chemically synthesized Pep13 derivatives revealed the particular importance of amino acid residues W2 and P5 for both receptor binding and phytoalexin induction (2, 3). Moreover, Table 1 shows that the nature and the spacing of amino acids N3 and Q4 between these two essential residues are equally important for Pep13-receptor binding. Together, these findings indicate a high degree of structural specificity within this small signal peptide, despite the previous observation that all amino acid residues N- and C-terminal of W2 and P5 can be individually replaced with an alanine residue without significant loss of elicitor activity (2).
Table 1. Competitor activities of Pep13 analogs.
| Peptide sequence | Competitor activity, % |
|---|---|
| VWNQPVRGFKVYE (Pep13) | 100 |
| VWLQPVRGFKVYE | 8 |
| VWLLPVRGFKVYE | 0 |
| VWNEPVRGFKVYE | 1 |
| VWNAQPVRGFKVYE | 2 |
| VWNAAAQPVRGFKVYE | 5 |
| VWQPVRGFKVYE | 0 |
These results were corroborated and extended by recent data showing a high degree of structural conservation, along with an important functional role, of Pep13 as a surface-exposed loop structure within its parent glycoprotein, a Ca2+-dependent transglutaminase (3). This type of transglutaminase occurred in all Phytophthora species analyzed, with the Pep13 motif almost fully conserved in all cases, but absent in all known plant protein sequences. Importantly, the same structural requirements were essential for both elicitor activity of Pep13 (ref. 2; Table 1) and transglutaminase activity of the intact protein (3). Thus, a strong selective pressure for conservation of the Pep13 motif as an essential component of transglutaminase activity, combined with its unique occurrence in pathogens (as opposed to plants) reveals this oligopeptide region as the target against which the highly specific plant receptor for non-self recognition arose during evolution of this defense response.
Some oomycete species, including Phytophthora sojae, possess not only the Pep13-containing transglutaminase, but also a 24-kDa cell-wall protein, necrosis-inducing Phytophthora protein 1 (NPP1), that elicits a defense response in P. crispum cells very similar to that observed with Pep13 (4). Unlike Pep13, however, NPP1 additionally induces the formation of hypersensitive cell death-like lesions in various dicotyledonous plants, including P. crispum and A. thaliana. In P. crispum, the NPP1-mediated defense response does not involve the Pep13 receptor, but employs all of the other signaling components involved in the Pep13-triggered response (Fig. 1), suggesting an early convergence of the two elicitation pathways. It is presently open whether simultaneous recognition of both elicitors leads to synergistic effects.
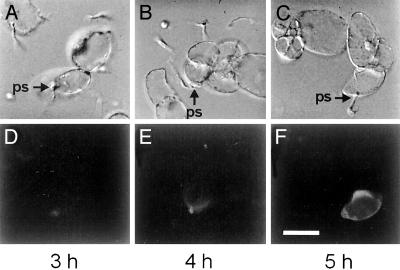
Signal Transduction. Receptor-mediated influx of extracellular Ca2+ is among the first detectable responses of P. crispum cells to treatment with either Pep13 or elicitor-active derivatives thereof (2). This Ca2+ influx is conducted in part by a novel type of Pep13-responsive, plasma membrane-associated ion channel, the activation of which is a prerequisite for the triggering of all subsequent responses (5, 6). Previous results demonstrating the rapid elevation of the cytoplasmic free Ca2+ level in elicitor-stimulated cells by using the bioluminescent Ca2+ indicator, apoaequorin (6), have now been extended by monitoring the spatial progression of intracellular Ca2+ accumulation in the course of the infection process. By using the indicator dye Fluo-4, we could demonstrate a rapid, strong elevation of the Ca2+ level that progressed steadily within a few hours from the initial site of hyphal penetration throughout the entire cell (Fig. 2) and remained high for a prolonged period, as with the measurements using apoaequorin (6). Besides concomitant, functionally unresolved increases in several other ion fluxes (Fig. 1), two additional plasma membrane-associated, Ca2+ influx-dependent, defense-related events occur more or less simultaneously with the onset of Ca2+ accumulation: the generation of reactive oxygen species (ROS) and jasmonate (2, 7).
Fig. 2.
Progressive elevation of the Ca2+ level in Phytophthora sojae-infected P. crispum cells. Hyphal penetration sites (ps) were identified under white light (A-C); Fluo-4 fluorescence was visualized under blue light (D-F). Time points are given in hours postinoculation. (Bar = 100 μm.)
Among these multiple elicitor-induced events within the plasma membrane, the elevated Ca2+ and ROS levels are of particular relevance for the subsequent intracellular signal transduction to the nucleus. However, although both of them are potent mediators of defense-related gene activation, they affect, at least in part, different sets of target genes. One cytoplasmic signaling pathway leads from Ca2+ via the activation of at least three mitogen-activated protein kinases to strong increases in PR gene expression, whereas another ROS-related pathway triggers the activation of phenylpropanoid-biosynthetic genes and thus the induction of the various aromatic compounds to be discussed below (5, 7). These observations indicate the involvement of at least two distinct signaling cascades, one ROS-dependent and the other ROS-independent, in defense-related gene activation in P. crispum. By contrast, the molecular target(s) of a third Pep13-stimulated cascade, the jasmonate pathway (Fig. 1), are still elusive. In cultured P. crispum cells, pharmacological inhibitors of Pep13-induced jasmonate accumulation did not impair phytoalexin production or PR gene activation (7).
Targets of Intracellular Signaling. The cell culture system has revealed at least three major targets of the intracellular signaling pathways: extensive transcriptional reprogramming of the affected cell from ”normal” to defense-oriented metabolism (8, 9); reorganization of the cytoskeleton and translocation of the nucleus, together with a sizable portion of the cytoplasm, to the penetration site (10); and extracellular conversion and extension of the signaling both to locally confined areas around the infection site (10, 11) and systemically throughout the entire affected organ or even the whole organism. In this latter regard, however, only circumstantial evidence exists so far in P. crispum. Among these multiple targets, the two focal points of our studies are the phenomenon of metabolic reprogramming, particularly the mechanisms of defense-related gene activation and inactivation, and the nature and function of the subsequently accumulating aromatic metabolites.
Treatment of cultured P. crispum cells with the Phytophthora sojae-derived peptide elicitor (either Pep13 or Pep25, an equally effective, slightly longer version; ref. 2) leads to rapid transcriptional activation (8) or repression (12) of at least several dozens of genes. Although gene repression is mechanistically as well as metabolically as interesting a phenomenon as is gene activation, our investigations have been focused mainly on the latter, largely because most of the genes analyzed so far in relation to aromatic secondary metabolism are strongly activated by elicitor. Three major outcomes of these studies are particularly noteworthy: the identification of several distinct classes of elicitor-responsive, cis-acting gene promoter elements; the discovery of two new families of regulatory proteins, the trp/arg/lys/tyr (WRKY) (13) and cys/met/pro/gly (CMPG) (14) protein families; and the demonstration of a few cases of exceptionally rapid, immediate-early gene activation, commencing within minutes after elicitor application and thereby possibly revealing immediate target genes of the elicitor-induced, intracellular signaling (13, 14).
In the work summarized up to this point, our studies had been conducted with suspension-cultured P. crispum cells or protoplasts. By contrast, most of the analyses discussed in the following paragraphs were a combination of initiating studies using the cell culture system and subsequent, often more extensive, genome- and mutant-based investigations with A. thaliana plants. Because of this sequential approach, most of the basic new discoveries were made in P. crispum, whereas more general insight was gained by including A. thaliana.
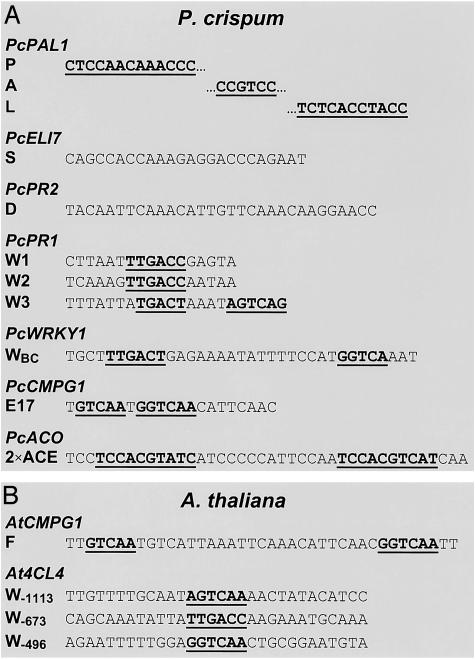
Cis-Acting Promoter Elements. Several elicitor-responsive, cis-acting elements were first identified on P. crispum gene promoters and then served as starting points for comparative, more extensive analyses using the fully sequenced genome of A. thaliana. Equally important was the essential role of these elements in the identification of cognate binding proteins, again initially in P. crispum and then in A. thaliana. The first major finding was the discovery of a set of three almost invariably cooccurring elements specifically on the promoters of phenylpropanoid-biosynthetic genes. This set (boxes P, A, and L) was initially identified on the PcPAL1 gene by ”in vivo footprinting” and has since been shown to occur on every newly discovered phenylalanine ammonia-lyase (PAL) gene (with the occasional exception of the A box), as well as on all other genes constituting the three central steps of ”general phenylpropanoid metabolism” in plants (15). In addition to this P, A, and L box combination, several other elicitor-responsive elements (Fig. 3) were subsequently identified on numerous P. crispum genes (box W and the D, S, and E17 elements; refs. 14 and 16). Of these, certain W box-containing elements, including E17, are particularly interesting because of their apparent involvement in immediate-early gene activation (see below).
Fig. 3.
List of functionally identified, elicitor-responsive cis-acting elements in P. crispum and A. thaliana. See text for references and further explanations.
Notwithstanding that the D and S elements have not been precisely defined, all of the positively or negatively elicitor-responsive elements from P. crispum (Fig. 3) occurred in structurally and functionally related forms in A. thaliana as well. Moreover, whenever elements derived from P. crispum gene promoters were tested in transgenic A. thaliana plants, or whenever A. thaliana-derived elements were tested in transfected P. crispum protoplasts, the results were essentially the same as those obtained within the species of origin.
Regulatory Proteins. Besides the identification of a P-box-binding factor (not sought in A. thaliana) and apart from the recognition of a close structural similarity of boxes P and L both to one another and to the independently defined myeloblastosis recognition element, two more basic discoveries resulting from these studies were the WRKY and CMPG protein families. Three PcWRKY isoforms with particularly interesting, distinct properties were initially identified in P. crispum (13). Their most characteristic features are the presence of the WRKY domain within otherwise poorly conserved proteins, and high affinities as well as high binding specificities for the W box. The most extensively analyzed representative, PcWRKY1, shows exceptionally rapid, immediate-early transcriptional induction by elicitor. Functional, tandemly arranged, elicitor-responsive W-box combinations within the PcWRKY1 gene promoter itself suggest autoregulation of this gene (17).
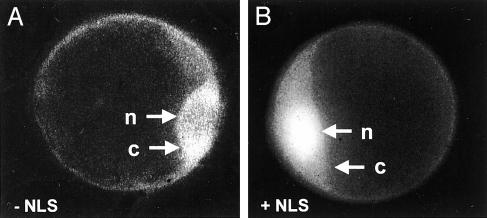
Of the subsequently identified CMPG gene family, only one representative, PcCMPG1, has been analyzed in P. crispum. PcCMPG1 was originally discovered as the fastest responding of all elicitor-responsive genes tested in this species, with an onset of immediate-early mRNA induction even faster (within < 5 min) than that recorded for PcWRKY1 (14). Several lines of circumstantial evidence suggest that CMPG proteins also have regulatory functions, though not as DNA-binding transcriptional regulators, but more likely by exerting regulatory activities involving protein-protein interactions. The occurrence of a putative nuclear localization signal (NLS) within the PcCMPG1 protein and at least partial, NLS-dependent translocation of a fusion product with green fluorescent protein into the nucleus (Fig. 4) suggest that the putative regulatory function is exerted within this cellular compartment.
Fig. 4.
Intracellular localization of a PcCMPG1::GFP fusion protein without (A) and with (B) a putative nuclear localization signal (NLS) within PcCMPG1. n, nucleus; c, cytoplasm.
Simultaneous with the rapid transcriptional activation of defense-related pathways by elicitor or infection, several unrelated metabolic activities, which are probably dispensable under these conditions, are equally rapidly repressed. In P. crispum, a striking example of such putatively compensatory repression is the flavonoid branch of phenylpropanoid metabolism, the products of which serve as UV-protective agents in this and many other species. The responsible genes as well as some of their transcriptional regulators are both strongly activated by UV light and strongly repressed by elicitor (12). Both stimuli act through the same promoter unit, possibly by inducing inversely acting isoform combinations of the differentially regulated common plant regulatory factor (CPRF) family of basic leucine zipper (bZIP) transcriptional regulators (12). Several members of this family had been identified as ACGT-containing element (ACE)-binding proteins with UV-light induction or repression patterns indicative of such a dual regulatory role (18).
These recently discovered or extended protein families were the next obvious targets for comparison between the two species. Similar to the cis-acting elements, all families of regulatory proteins analyzed so far in P. crispum, whether with or without DNA-binding properties, proved to have structurally and, as far as tested, functionally related counterparts in A. thaliana, including the large AtWRKY and AtCMPG protein families, as well as the two independently investigated, similarly large families of AtMYB and AtbZIP proteins. All four of these families can be subdivided into structurally distinct subclasses. In each case, several, but not all, of the family members are positively or negatively responsive to elicitor (or infection), to UV light, or to yet other stimuli that lie outside the scope of this article. A comparative overview of all available data on the intensely investigated WRKY, CMPG and bZIP protein families in A. thaliana and on all presently known family members in P. crispum is given in Fig. 5. Particularly noteworthy in this connection is the striking similarity between PcCMPG1 and AtCMPG1 not only in structural terms, but also with regard to the exceptionally rapid, immediate-early induction by elicitor (14, 19).
Fig. 5.
Schematic representation of the complete WRKY (20), CMPG (19), and common plant regulatory factor/bZIP (21) protein families from A. thaliana and the initially identified members from P. crispum (gray shading). Subdivisions within each family (19-21) are indicated by boxes, and gene codes are given where existing. Filled circles indicate known responsiveness to infection, elicitor, or other functionally related stimuli (19-22).
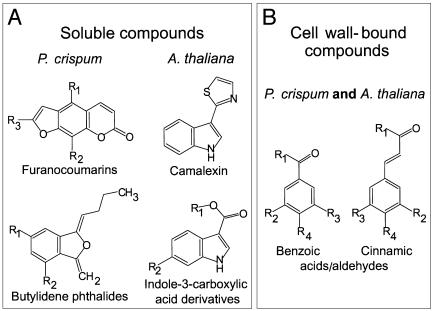
Secondary Products. Among the most abundant aromatic compounds accumulating in the culture fluid of elicitor-treated P. crispum cells or protoplasts, or at infection sites proper, are several structurally related furanocoumarins and various likewise closely related butylidene phthalide aglycones and glycosides (23). Both classes of compounds are biosynthetically derived from phenylalanine by combinations of the three common steps of general phenylpropanoid metabolism with two of the many subsequently diverging branch pathways. Furanocoumarins and butylidene phthalides possess antimicrobial activity and hence are assumed to act as phytoalexins in this species.
However, in contrast to the far-reaching similarities throughout all cis-acting elements and protein families investigated, P. crispum and A. thaliana differ considerably at the secondary metabolite level. In fact, all major aromatic metabolites accumulating in soluble form in infected leaves (24) or roots (P.B., unpublished results) of A. thaliana are indole derivatives, in contrast to their phenylpropanoid or betaketide origin in P. crispum, whereas all major compounds constitutively present in A. thaliana are, notably, phenylpropanoid derivatives. Fig. 6A gives a comparative overview of the elicitor- or infection-induced classes of aromatic compounds identified in the two systems. In parallel with the induction of indolic metabolites, some of the constitutively present phenolics in A. thaliana decline strongly during the infection process, again in leaves as well as in roots. Furthermore, in addition to the various metabolites identified, many less abundant aromatic as well as aliphatic compounds are probably also induced or repressed, but were not monitored in our studies.
Fig. 6.
Chemical structures of major, characteristic classes of compounds accumulating on elicitor treatment or infection in P. crispum and A. thaliana. Soluble (A) and cell wall-bound (B) material was extracted and analyzed by using established protocols (23, 24) with slight modifications.
Remarkably, this striking difference in defense-related, phenylpropanoid and indolic aromatic metabolism between P. crispum and A. thaliana appears to be largely restricted to soluble compounds. In the fraction of wall-bound, alkali-labile substances, only one or two indolic compounds, indole-3-carboxylic acid and possibly the corresponding aldehyde, accumulated strongly in A. thaliana, but not in P. crispum. All other induced wall-bound aromatic compounds are phenylpropanoid (benzoate and cinnamate) derivatives with similar or identical substitution patterns in both A. thaliana and P. crispum (Fig. 6B). Although so far unproven, it seems probable that these wall-bound compounds accumulate, at least in part, locally around the site of attempted penetration of the pathogen, where the papilla (Fig. 1) is formed by apposition of callose and other, largely unidentified materials onto the cell wall.
Causal Connections. Two closely related long-term goals of these studies have been to identify those metabolic junctions that causally connect the numerous individual components of the overall defense response, as well as those final products of the long chains of metabolic events that directly contribute to, or are decisive for, the actual impairment or killing of the pathogen. Three different experimental approaches have recently yielded insights in this direction.
The first approach involves the use of Actinomycin D as a general gene transcription inhibitor. Earlier studies had revealed the total inhibition of PAL induction in UV-irradiated P. crispum cells by low doses of Actinomycin D. Now, we used elicitor (1 μg/ml Pep25) instead of UV light for induction, and observed strong inhibitory effects at three different metabolic levels on addition of Actinomycin D (50 μg/ml) 30 min before the onset of elicitor treatment. Under these conditions, neither CMPG1 or WRKY1 mRNA (as measured 30 min after elicitor addition) nor PAL activity (24-h time point), nor any of the soluble or wall-bound elicitor-inducible compounds listed in Fig. 6 (24-h time point), accumulated significantly above the 0-h control level. Although these results do not demonstrate direct causal connections between any two of the items analyzed, they clearly indicate that in each case induction is the result of transcriptional activation of the responsible gene(s), for the immediate-early induced mRNAs as well as for PAL activity (the first committed step in phenylpropanoid biosynthesis) and for the various metabolic end products. Thus, we can exclude the theoretical possibility that at least some of the monitored effects were caused by transcription-independent mechanisms, an alternative that might, for example, occur in the form of secondary product synthesis by liberation and/or conversion of preformed intermediates. However, we cannot exclude relatively minor but possibly relevant contributions from such sources.
The second approach concerns the extent to which the numerous structural variants of cis-acting elements interact with different members of the large families of cognate binding proteins. This is a difficult question to answer conclusively for the situation in vivo. However, some of the available in vitro assays can give at least valuable hints in this direction. A sensitive tool for determining DNA-protein binding affinities is the analysis of band shifts caused by protein binding of DNA probes on electrophoretic mobility gels. Fig. 7 shows that a few structurally widely different, elicitor-responsive, W box-containing elements, including E17 from PcCMPG1, F from AtCMPG1, and three less precisely defined promoter regions from At4CL4 exhibit distinct binding affinities for one selected WRKY protein, AtWRKY11. Importantly, however, nearly all of these elements are bound more or less efficiently by this particular family member. Considering the large size of the WRKY family of W box-binding proteins and the large number of potential W box-containing sequences and sequence arrangements, this result indicates a vast regulatory potential founded in an almost infinite number of possible combinatorial permutations. Particularly relevant with regard to our goal to identify possible causal connections, it is apparent that any given W box-containing sequence has a high potential to bind with a certain affinity to any given WRKY protein, implicating a similarly wide range of functional relationships in vivo.
Fig. 7.
Gel-shift assay indicating widely differing affinities of a few selected W box-containing elements for WRKY11, a selected member of the WRKY family of cognate DNA-binding proteins. Procedures for heterologous expression and purification of the protein (25) and for gel-shift analysis (13) were followed essentially as described. DNA probes E17-21, 4CL4, and F were those shown in Fig. 3. For probes E17-27 and E17-21m, see ref. 14.
The third case exemplifies one of the most promising and powerful techniques available for the analysis of causal connections: the employment of defined mutants. Among the large and rapidly growing number of A. thaliana knockout mutants, many have been functionally associated with defined steps in defense-related signaling or secondary product formation, or more generally, with the disease resistance phenotype. A presently incomplete analysis employing several such mutants is expected to add substantially to our understanding of the network of defense-related metabolic interconnections.
Pathogen Defense in Plants: Paradigm of Structural and Functional Complexity
At each of the levels investigated, the plant's response to pathogen attack represents a paradigm of biological complexity (9). In addition to the complexity within each individual metabolic level, a hierarchically superimposed complexity pyramid emerges as the contours of the overall picture come into focus (Fig. 8). In accord with the notion of a complexity pyramid ”from the particular to the universal” (26), our results point to at least three, possibly four distinct levels of hierarchical organization. At the top of the pyramid, one universal, multicomponent defense strategy governs several more or less universal, functional modules. Together with the various regulatory motifs and defense-related pathways at the upper and lower central levels of the pyramid, respectively, these modules mediate, coordinate, and execute the strategic response, whereas the bottom harbors the numerous individual, species-specific, defense-related genes and their products.
Fig. 8.
Complexity pyramid illustrating the hierarchical organization and modular structure of the pathogen defense response in higher plants. Note that the examples used to explain the pivotal role of the two central levels of the pyramid in the gradual upward progression from the particular to the universal (26) are an incomplete selection from the modules discussed in the text.
Universal Defense Strategy. In P. crispum, A. thaliana, and all other systems analyzed to date, the overall defense response consists of a series of sequentially activated defense measures. These proceed from the highly localized to the less highly localized and finally to the systemic, with an optional termination of the process at certain stages when pathogen confinement has been achieved. Among the most intensely investigated components of this response are the papilla formed around the site of attempted penetration; the highly localized, hypersensitive death of the immediately affected cell; the local accumulation of antibiotically active substances, including H2O2 and phytoalexins; and the systemic accumulation of hydrolytic enzymes, such as glucanase and chitinase. This strictly ordered, temporally, spatially, and functionally modular defense strategy entails dramatic intracellular rearrangements (10) concomitant with the onset of transcriptional reprogramming at the infection site.
However, the individual functional modules are probably not fully autonomous, but rather interconnected, and in some cases they may even partially overlap. Obvious examples of such metabolic or regulatory overlaps are the various interrelated responses to elicitor stimulation within the plasma membrane; the dual or even manifold roles of Ca2+ in intracellular regulation; the role of H2O2 both as a toxic agent and in cell-wall cross-linking; and the initial, common steps in the biosynthesis of phenylalanine-derived phytoalexins and cell wall-bound compounds. Even though the mechanistic details of such interconnections are presently obscure, the overall response of the plant reveals that they exist.
Regulatory Motifs and Transcription Factor Families. Both the perception of pathogen-derived elicitors and its subsequent intracellular signal transduction employ basic mechanisms that appear to be universal not only in higher plants, but also, at least to a considerable extent, in higher animals (27), suggesting a common evolutionary origin of the general defense strategy throughout all higher eukaryotes.
Numerous, more or less directly defense-related genes are major targets of two of the intracellular signal transduction chains in P. crispum (Fig. 1). Probably all of them contain at least one of a small number of basic types of elicitor-response element, two of which (containing either the W box or the P/A/L set of boxes) occur most frequently on these genes throughout all plants examined. Remarkably, the P/A/L box-containing element has so far been found on all genes encoding functionally identified or putative phenylpropanoid-biosynthetic enzymes. Moreover, these genes share, at least in P. crispum, two additional, remarkable features: they are activated by the  -dependent signaling pathway, and the mRNA accumulation patterns follow identical time courses for all P/A/L-containing genes, in sharp contrast to the uncoordinated behavior of nearly all other elicitor-responsive genes analyzed (8). Most probably, these three common features are causally connected. Whether this extends to the cognate DNA-binding proteins remains to be seen.
-dependent signaling pathway, and the mRNA accumulation patterns follow identical time courses for all P/A/L-containing genes, in sharp contrast to the uncoordinated behavior of nearly all other elicitor-responsive genes analyzed (8). Most probably, these three common features are causally connected. Whether this extends to the cognate DNA-binding proteins remains to be seen.
It presently appears doubtful whether a similar close metabolic relationship exists among the large number of genes bearing W box-containing promoters. The W box is not only by far the most frequently occurring structural element of elicitor-response elements in plants, but is also notorious for its challenging diversity of closely spaced repetitions and/or combinations with other sequences. In such cases, at least one W box is usually essential for conveying the elicitor response, and certain types of repeat structure have been associated with immediate-early gene activation (14, 17, 19). Fig. 7 exemplifies the broad range of binding affinities exerted by just a few structurally distinct representatives of such W-box arrangements toward one selected member of the WRKY family of transcriptional regulators. This sample includes three W box-containing fragments from the At4CL4 gene promoter, the only presently known case in A. thaliana where W boxes cooccur with the characteristic P/A/L-box set on phenylpropanoid-biosynthetic genes. The recently identified At4CL4 gene encodes a rare isoform of 4-coumarate:CoA ligase (4CL) with unusual substrate specificity and may therefore have an exceptional metabolic function and atypical expression mode. Here, the W-box regions from the At4CL4 promoter were used to test binding strength toward WRKY proteins, as predicted from sequence relationships with previously analyzed elements. The results substantiate these predictions and furthermore reveal a large influence of the surrounding sequence on the binding affinity of W box-containing elements.
Although much less is known about the S and D elements, their identification as strong elicitor-responsive elements indicates that the diversity of such elements is by no means confined to those containing P/A/L and W boxes. A particularly interesting recent observation in this context was the repression by elicitor of previously UV light-activated genes through a positively UV light-responsive and negatively elicitor-responsive ACE/ACE element combination, as manifested in the PcACO promoter fragment shown in Fig. 3A. This inverse response of one promoter element to different kinds of stress is yet another example of the complexity and connectivity of regulatory circuits, including the possible involvement of the same family of DNA-binding proteins in both up and down-regulation of genes (12).
Taken together, these results reveal a modularity even at the level of elicitor-responsive gene promoters, with W box- and (metabolically more confined) P/A/L box-containing elements being the most abundant, universally occurring representatives. Recurrent modularity in the fine structure of these basic building blocks at the species and gene levels generates uniqueness and biological specificity. This principle also applies to the cognate DNA-binding proteins and to the peripheral transcriptional regulators. Just as with cis-acting elements, a few distinct classes of transacting factors, each occurring as structurally diversified families, yield discrete but universal modules. Thus, the number of functional combinations for these modules, amplified by homo- and heterodimeric forms, may parallel the number of physiological challenges to which the plant must respond.
Binding of a regulatory protein complex is required for inactivation as well as for activation of a gene. Although the mechanisms of gene inactivation have been studied much less extensively than those of gene activation, it is unlikely that they fundamentally differ. Some of our results require us to presume that it may be the combination of isoforms of a given transcription factor family that changes with up- or down-regulation of a particular gene, whereas the basic type remains unchanged (12).
Metabolic Pathways and Aromatic Secondary Products. A ramified dimension of complexity is reached at the level of secondary plant metabolism. In contrast to the cis-acting elements and transacting factors, many secondary metabolic pathways are unique to certain plant genera, families or even species, and most of them are species-specific at least with regard to the specified composition of the respective product bouquet. Accordingly, all major soluble, elicitor- or pathogen-induced aromatic compounds in P. crispum are species-specific mixtures of differently substituted phenylpropanoid derivatives, whereas in A. thaliana they are exclusively indolic intermediates or end products.
It is therefore all the more surprising that, with the exception of one or two indolic compounds in A. thaliana, all major induced cell-wall constituents are similar or identical phenylpropanoids in these two and several other species. Two alternatives seem equally plausible: either there is greater evolutionary pressure on the conservation of the cell wall-bound compounds or this branch of phenylpropanoid metabolism, whether it has a common evolutionary origin in all plants or has converged from multiple origins, has limited degrees of chemical freedom, in contrast to the various pathways generating phytoalexins that have evolved a greater chemical diversity. Although phytoalexin production and cell-wall reinforcement with phenylpropanoid derivatives can be regarded as two independent functional modules occupying parallel hierarchical positions within the overall defense strategy, their species-specific patterns of diversity could not differ more.
Practical Applications
Our motivation for these investigations has been two-fold: fascination with an exciting combination of molecular, cellular, and ecologically oriented research and the coupling of this excitement with the expectation that scientific discoveries would reveal new approaches to breeding disease resistance in crop plants. To reach this level of practical application, two requirements had to be fulfilled. First, the basic principles of pathogen defense in plants had to be understood. We suppose that this stage has been reached with sufficient clarity, despite many remaining gaps in our knowledge about the underlying mechanisms. Second, a biologically meaningful, heritable, crop plant-adapted strategy must be feasible in every respect, including physiological tolerance by the plant and acceptance by the public. Comparing the largely unexplored efficiency and overwhelming diversity of chemical defense, particularly the difficulty in pinpointing individual, universally applicable (physiologically tolerable as well as consumer-friendly) defense-related compounds, with the proven high efficiency of hypersensitive cell death as a defense mechanism, we decided on probing a new gene technology-based strategy in the latter direction.
Our strategy employs the recently identified, rapidly, strongly, locally, and specifically elicitor-responsive promoter elements (Fig. 3), either alone or in combinations, for example in conjunction with a gene encoding a cell death-conferring principle, such as a broadly acting ribonuclease (28). Transcriptional activation of such a construct on infection of a transgenic plant would cause or intensify hypersensitive suicidal death of the affected cell, and thus confer or augment a particularly efficient defense mechanism. A first successful proof of principle has demonstrated the feasibility of this strategy (28). However, further improvements will be necessary to adapt various details to the special needs of crop plant breeding. For example, strict avoidance of unspecific transgene expression in response to exogenous stimuli other than infections, or to endogenous effectors during plant development, is absolutely essential. This is by no means a trivial obstacle, because most infection-responsive genes also respond to wounding and other stresses, and, in addition, are expressed during certain stages of development, such as flowering, senescence or root-tip growth.
Some of the promoter elements listed in Fig. 3, notably E17 and F, are exceptionally specific in their response to infections (14, 19) and hence may be particularly well suited for the design of artificial defense mechanisms. Recently published (16) as well as unpublished data (A.H.) on the specifics of promoter design and expression modes are aimed at broadening the basis for further improvements of this strategy, including its sophistication in detail. In the long term, for this or any other strategy for breeding disease resistance, multigenic traits will have to be introduced that are not easily overcome by mutations in the pathogens.
Conclusions
The combination of a universal strategy with an almost infinite number of species-specific variations of the pathogen defense response in plants ideally illustrates the interplay of conceptual richness, metabolic options, physiological constraints and ecological demands within which evolution takes place. Hierarchical subdivision of the overall response into multiple functional modules is executed by interconnected, partly universal, partly species-specific signaling pathways that together mediate an extensive, rapid transcriptional reprogramming of exclusively species-specific genes. A few large, highly diversified families of cis-acting elements and transacting factors are the pivotal links between the particular and the universal. The potential for fine tuning through combinatorial permutations accounts for the remarkable interspecies functionality of these elements and their cognate factors in transgenic plants and impels their use in new strategies of crop plant breeding for an environmentally safe disease management.
Acknowledgments
We thank Dr. Bill Martin, Düsseldorf, Germany, and Dr. Dierk Scheel, Halle, Germany, for valuable comments on the manuscript.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, ”Chemical Communication in a Post-Genomic World,” held January 17-19, 2003, at the Arnold and Mabel Beckman Center of the National Academies of Science and Engineering in Irvine, CA.
Abbreviations: ACE, ACGT-containing element; bZIP, basic leucine zipper; 4CL, 4-coumarate:CoA ligase; ROS, reactive oxygen species; CMPG, cys/met/pro/gly; PAL, phenylalanine ammonia-lyase; WRKY, trp/arg/lys/tyr.
References
- 1.Gus-Mayer, S., Naton, B., Hahlbrock, K. & Schmelzer, E. (1998) Proc. Natl. Acad. Sci. USA 95, 8398-8403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nürnberger, T., Nennstiel, D., Jabs, T., Sacks, W. R., Hahlbrock, K. & Scheel, D. (1994) Cell 78, 449-460. [DOI] [PubMed] [Google Scholar]
- 3.Brunner, F., Rosahl, S., Lee, J., Rudd, J. J., Geiler, C., Kauppinen, S., Rasmussen, G., Scheel, D. & Nürnberger, T. (2002) EMBO J. 21, 6681-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fellbrich, G., Romanski, A., Varet, A., Blume, B., Brunner, F., Engelhardt, S., Felix, G., Kemmerling, B., Krzymowska, M. & Nürnberger, T. (2002) Plant J. 32, 1-16. [DOI] [PubMed] [Google Scholar]
- 5.Scheel, D. (1998) Curr. Opin. Plant Biol. 1, 305-310. [DOI] [PubMed] [Google Scholar]
- 6.Blume, B., Nürnberger, T., Nass, N. & Scheel, D. (2000) Plant Cell 12, 1425-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kroj, T., Rudd, J. J., Nürnberger, T., Gäbler, Y., Lee, J. & Scheel, D. (2003) J. Biol. Chem. 278, 2256-2264. [DOI] [PubMed] [Google Scholar]
- 8.Batz, O., Logemann, E., Reinold, S. & Hahlbrock, K. (1998) Biol. Chem. 379, 1127-1135. [DOI] [PubMed] [Google Scholar]
- 9.Somssich, I. E. & Hahlbrock, K. (1998) Trends Plant Sci. 3, 86-90. [Google Scholar]
- 10.Gross, P., Julius, C., Schmelzer, E. & Hahlbrock, K. (1993) EMBO J. 12, 1735-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmelzer, E., Krüger-Lebus, S. & Hahlbrock, K. (1989) Plant Cell 1, 993-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logemann, E. & Hahlbrock, K. (2001) Proc. Natl. Acad. Sci. USA 99, 2428-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rushton, P. J., Tovar Torres, J. T., Parniske, M., Wernert, P., Hahlbrock, K. & Somssich, I. E. (1996) EMBO J. 15, 5690-5700. [PMC free article] [PubMed] [Google Scholar]
- 14.Kirsch, C., Logemann, E., Lippok, B., Schmelzer, E. & Hahlbrock, K. (2001) Plant J. 26, 217-227. [DOI] [PubMed] [Google Scholar]
- 15.Hahlbrock, K., Scheel, D., Logemann, E., Nürnberger, T., Parniske, M., Reinold, S., Sacks, W. R. & Schmelzer, E. (1995) Proc. Natl. Acad. Sci. USA 92, 4150-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rushton, P. J., Rheinstädler, A., Lipka, V., Lippok, B. & Somssich, I. E. (2002) Plant Cell 14, 749-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eulgem, T., Rushton, P. J., Schmelzer, E., Hahlbrock, K. & Somssich, I. E. (1999) EMBO J. 18, 4689-4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weisshaar, B., Armstrong, G. A., Block, A., da Costa e Silva, O. & Hahlbrock, K. (1991) EMBO J. 10, 1777-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heise, A., Lippok, B., Kirsch, C. & Hahlbrock, K. (2002) Proc. Natl. Acad. Sci. USA 99, 9049-9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eulgem, T., Rushton, P. J., Robatzek, S. & Somssich, I. E. (2000) Trends Plant Sci. 5, 199-206. [DOI] [PubMed] [Google Scholar]
- 21.Jakoby, M., Droege-Laser, W., Kroj, T., Tiedemann, J., Vicente-Carbajosa, J., Weisshaar, B. & Parcy, F. (2002) Trends Plant Sci. 7, 106-111. [DOI] [PubMed] [Google Scholar]
- 22.Chen, W., Provart, N. J., Glazebrook, J., Katagiri, F., Chang, H.-S., Eulgem, T., Mauch, F., Luan, S., Zou, G., Whitham, S. A., et al. (2002) Plant Cell 14, 559-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagemeier, J., Batz, O., Schmidt, J., Wray, V., Hahlbrock, K. & Strack, D. (1999) Phytochemistry 51, 629-635. [Google Scholar]
- 24.Hagemeier, J., Schneider, B., Oldham, N. J. & Hahlbrock, K. (2001) Proc. Natl. Acad. Sci. USA 98, 753-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeo, K., Hayashi, S., Kojima-Suzuki, H., Morikami, A. & Nakamura, K. (2001) Biosci. Biotechnol. Biochem. 65, 2428-2436. [DOI] [PubMed] [Google Scholar]
- 26.Oltvai, Z. N. & Barabasi, A.-L. (2002) Science 298, 763-764. [DOI] [PubMed] [Google Scholar]
- 27.Nürnberger, T. & Brunner, F. (2002) Curr. Opin. Plant Biol. 5, 318-324. [DOI] [PubMed] [Google Scholar]
- 28.Strittmatter, G., Janssens, J., Opsomer, C. & Botterman, J. (1995) Bio/Technology 13, 1085-1089. [Google Scholar]