Abstract
OBJECTIVE
To compare the pharmacokinetics, pharmacodynamics, and safety of insulin lispro or regular human insulin (RHI) with or without recombinant human hyaluronidase (rHuPH20) administered before a standardized meal.
RESEARCH DESIGN AND METHODS
In this four-way, crossover study, 22 patients with type 1 diabetes received injections of individually optimized doses of lispro or RHI with and without rHuPH20 before a liquid meal.
RESULTS
With rHuPH20 coadministration, early insulin exposure (0–60 min) increased by 54% (P = 0.0011) for lispro and 206% (P < 0.0001) for RHI compared with the respective insulin alone. Peak blood glucose decreased 26 mg/dL for lispro (P = 0.002) and 24 mg/dL for RHI (P = 0.017), reducing hyperglycemic excursions (area under the curve for blood glucose >140 mg/dL) by 79% (P = 0.09) and 85% (P = 0.049), respectively. Rates of hypoglycemia were comparable for lispro with or without rHuPH20, whereas coadministration of RHI and rHuPH20 reduced hypoglycemia.
CONCLUSIONS
Lispro or RHI with rHuPH20 produced earlier and greater peak insulin concentrations and improved postprandial glycemic control.
Effective control of postprandial glycemic excursions is increasingly recognized as a crucial element in achieving optimum diabetes control (1,2). Nonetheless, many patients with diabetes fail to meet target blood glucose levels (3,4), suggesting that the pharmacokinetic and efficacy profiles of available prandial insulin products should be improved. Results from a recently conducted study demonstrated that recombinant human hyaluronidase (rHuPH20) accelerates the pharmacokinetics of the insulin analog lispro and regular human insulin (RHI) in healthy volunteers (5). The aim of this study was to confirm these findings in patients with type 1 diabetes using patient-specific, optimized doses of insulin and to explore the impact of these pharmacokinetic effects on glycemic response to a standardized meal.
RESEARCH DESIGN AND METHODS
In this phase II, single-blind (patient), institutional review board–approved study, patients first participated in up to three dose-finding visits to identify an optimum lispro plus rHuPH20 dose after consuming a standardized liquid test meal. The same dose of lispro alone was administered after another test meal. Separately optimized doses of RHI plus rHuPH20 with two additional test meals were then administered, followed by a final test-meal visit using the identical dose of RHI. For each test-meal visit, patients withheld prandial insulin injections for >8 h and basal insulin for >12 h. Blood glucose levels were stabilized to 100–120 mg/dL using intravenous glucose or insulin. The intervention-free, 30-min period before treatment administration was used to establish a baseline blood glucose concentration. The optimum insulin dose was defined as one that did not allow postprandial blood glucose levels to increase >160 mg/dL for >30 min during a 4-h postinjection period and did not cause blood glucose to fall <60 mg/dL. Intravenous glucose (20%) was administered at the investigator’s discretion when blood glucose levels fell <60 mg/dL or if symptoms of hypoglycemia were observed.
Study solutions with rHuPH20 were combined at the study site to final concentrations of 91 units/mL of lispro (100 units/mL from Humalog; Eli Lilly, Indianapolis, IN) with 18.2 μg/mL of rHuPH20 and 100 units/mL RHI (500 units/mL from Humulin R; Eli Lilly) with 20.0 μg/mL of rHuPH20. Study drugs were injected subcutaneously by the investigative staff in the abdominal wall region.
Twenty-two patients (15 were male; 4 Hispanic; 19 white, 2 black, and 1 Asian) with type 1 diabetes (ages 18–65 years, mean age 40.7 years; BMI 18–29 kg/m2, mean BMI 24.2 kg/m2) who provided written informed consent were enrolled and comprised the safety population. Efficacy outcomes were determined from patients who had paired data for lispro with or without rHuPH20 (pharmacokinetic data: n = 21; glycemic data: n = 22) or RHI with or without rHuPH20 (pharmacokinetic data: n = 16; glycemic data: n = 18). Pharmacokinetic parameters were assessed using baseline-subtracted data with noncompartmental analyses. Comparisons between lispro with or without rHuPH20 and RHI with or without rHuPH20 were conducted using a repeated-measures ANOVA to control for the crossover design. No adjustments were made for multiple statistical tests. Fractional exposure and temporal results are presented as arithmetic means and peak and total exposure as geometric means.
RESULTS
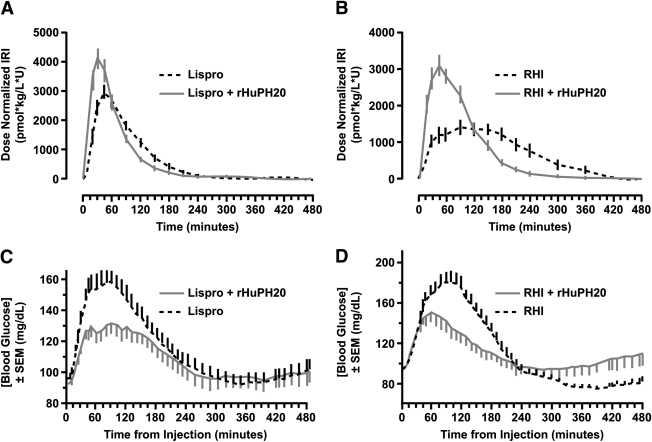
Coadministration of rHuPH20 accelerated the absorption of lispro and RHI (Fig. 1A and B), producing an earlier and greater peak in insulin exposure. With rHuPH20, peak insulin exposure increased by 35% (P = 0.006) and 66% (P < 0.0001), and time to peak exposure decreased from 49 to 30 min (P < 0.0001) and from 117 to 57 min (P = 0.002) for lispro and RHI, respectively. The fraction of total insulin exposure within the first hour following injection increased from 39% for lispro alone to 57% for lispro plus rHuPH20 (P = 0.0002) and from 16% with RHI alone to 39% for RHI plus rHuPH20 (P < 0.0001). The fraction of insulin exposure >2 h after injection was reduced from 22% for lispro alone to 13% for lispro plus rHuPH20 (P = 0.052) and from 57% for RHI alone to 23% for RHI plus rHuPH20 (P < 0.0001). Total insulin exposure increased slightly with the addition of rHuPH20 but not to a significant degree for either lispro (3%; P = 0.763) or RHI (9%; P = 0.379).
Figure 1.
Coadministration of rHuPH20 with lispro (A) or RHI (B) accelerates insulin pharmacokinetics as assessed by serum insulin concentrations, which were assayed using a standard insulin radioimmunoassay (Millipore, St. Charles, MO) validated for both lispro and RHI, and improved postprandial glycemic response (C and D) to a standardized liquid test meal (12 oz standard-formula Ensure [Abbott Laboratories, Abbott Park, IL]; 60 g carbohydrates). Blood glucose levels were determined using a YSI STAT2300 glucose analyzer (YSI Incorporated, Yellow Springs, OH). Data shown are from efficacy-evaluable populations.
The accelerated absorption of lispro and RHI with rHuPH20 reduced both peak and total postprandial glycemic excursions (Fig. 1C and D). Peak postprandial glucose levels decreased from 174 to 148 mg/dL (−26 mg/dL; P = 0.002) for lispro plus rHuPH20 and from 190 to 166 mg/dL (−24 mg/dL; P = 0.017) for RHI plus rHuPH20. Total hyperglycemic excursions (area under the curve >140 mg/dL) for the first 4 h after the study drug administration were reduced by 79% (P = 0.090) for lispro (from 526 to 111 min ⋅ mg−1 ⋅ mL−1) and 85% (P = 0.049) for RHI (from 1,238 to 181 min ⋅ mg−1 ⋅ mL−1) after coadministration with rHuPH20.
The experienced adverse events (AEs) were generally mild, regardless of the treatment administered. No serious or severe AEs were observed. The most common AE was hypoglycemia, which was generally mild or occasionally moderate and occurred in a majority of patients with each study drug (in 13 of 22 patients with lispro with or without rHuPH20, in 16 of 21 with RHI alone, and in 11 of 21 with RHI plus rHuPH20). The number of subjects administered glucose for the treatment of hypoglycemia was 6 of 21 for lispro alone, 9 of 21 for lispro plus rHuPH20, 12 of 21 for RHI alone, and 7 of 21 for RHI plus rHuPH20. Other AEs included mild injection-site erythema in two patients who received RHI alone. All AEs resolved without treatment and had no lasting effects.
CONCLUSIONS
The acceleration of insulin absorption with rHuPH20 observed in this study of patients with type 1 diabetes confirms previous results in healthy volunteers (5); both studies produced greater and earlier peak insulin exposure. In addition, in this study, the acceleration of insulin pharmacokinetics led to reductions in postprandial hyperglycemic excursions for identical doses of lispro or RHI plus rHuPH20. The coadministration of rHuPH20 with each insulin was well tolerated.
Limitations of this study include its single-blind design and the use of a liquid test meal, which, despite being standardized, is not representative of real-world food intake. In addition, because the matched insulin doses were individually optimized for making pharmacokinetic comparisons, they were not necessarily optimal for pharmacodynamic comparisons. Furthermore, hypoglycemia results should be interpreted cautiously because the study design allowed for hypoglycemic rescue (intravenous glucose infusion) at the investigator’s discretion.
The results of this study suggest that a coformulation of prandial insulins with rHuPH20 may provide benefits for treating patients with diabetes by decreasing postprandial hyperglycemic excursions without increasing the risk for late postprandial hypoglycemic events.
Acknowledgments
Halozyme Therapeutics, San Diego, CA, sponsored this work. M.H. and L.M. are employees of the Profil Institute for Clinical Research. D.E.V. and D.B.M. are employees of Halozyme Therapeutics, the sponsor of this work. No other potential conflicts of interest relevant to this article were reported.
M.H., D.B.M., L.M., and D.E.V. contributed to and made a scientific contribution to this manuscript as well as assisted with the drafting and revision of the manuscript.
Parts of this article were presented in abstract form at the 69th Scientific Sessions of the American Diabetes Association, New Orleans, Louisiana, 5–9 June 2009, and at the 45th Annual Meeting of the European Association for the Study of Diabetes, Vienna, Austria, 29 September to 2 October 2009.
Medical writing support for this manuscript was provided by Dennis A. Stancavish, MA, of Embryon, a division of Advanced Health Media. The authors thank Lutz Heinemann of the Profil Institute for Clinical Research for his critical evaluation of the manuscript.
Footnotes
Clinical trial reg. no. NCT00774800, clinicaltrials.gov.
References
- 1.American Diabetes Association Standards of medical care in diabetes: 2009. Diabetes Care 2009;32(Suppl. 1):S13–S61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabet Med 2008;25:1151–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plank J, Siebenhofer A, Berghold A, et al. Systematic review and meta-analysis of short-acting insulin analogues in patients with diabetes mellitus. Arch Intern Med 2005;165:1337–1344 [DOI] [PubMed] [Google Scholar]
- 4.Davies M. The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges. Int J Obes Relat Metab Disord 2004;28(Suppl. 2):S14–S22 [DOI] [PubMed] [Google Scholar]
- 5.Vaughn DE, Yocum RC, Muchmore DB, et al. Accelerated pharmacokinetics and glucodynamics of prandial insulins injected with recombinant human hyaluronidase. Diabetes Technol Ther 2009;11:345–352 [DOI] [PubMed] [Google Scholar]