Abstract
OBJECTIVE
Variation in the fat mass and obesity-associated (FTO) gene is associated with obesity. The extent to which separate and combined effects of physical activity and caloric intake modify this association remains unclear.
RESEARCH DESIGN AND METHODS
FTO polymorphism rs8050136 was measured, and physical activity, caloric intake, and anthropometrics were self-reported in 21,675 apparently healthy Caucasian women.
RESULTS
The effect of the risk allele (A) on BMI was larger among inactive or higher intake women, with additive effects of inactivity and high intake on the associated genetic risk. Specifically, each A allele was associated with mean BMI difference of +0.73 (SE 0.08) kg/m2 among inactive women (≤median, 8.8 MET-hours/week), compared with +0.31 (0.06) kg/m2, P < 0.0001, among active women (>8.8 MET-hours/week). Similarly, each A allele was associated with mean BMI difference of +0.65 (0.07) among high intake women (>median, 1,679 kcals/day), compared with +0.38 (0.07) kg/m2, P = 0.005, among low intake women (≤1,679 kcals/day). Among inactive/high intake women, each A allele was associated with mean BMI difference of +0.97 (0.11) kg/m2 vs. +0.22 (0.08) kg/m2 among inactive/low intake women, P < 0.0001. Among inactive/high intake women, each A allele carried increased risk of obesity (odds ratio 1.39, 95% CI 1.27–1.52) and diabetes (odds ratio 1.36, 95% CI 1.07–1.73).
CONCLUSIONS
In this study, lifestyle factors modified the genetic risk of FTO on obesity phenotypes, particularly among women who were both inactive and had high intake. Healthier lifestyle patterns blunted but did not completely eliminate the associated genetic risk.
There has been a steady rise in the prevalence of obesity such that almost one in three U.S. adults is now obese (1). The major environmental determinants of obesity are excessive caloric intake and low physical activity (1). Recently, high-throughput approaches to finding the genetic determinants of obesity and type 2 diabetes have led to the discovery and confirmation of a cluster of highly correlated single-nucleotide polymorphisms (SNPs) in intron 1 of the fat mass and obesity-associated (FTO) gene in strong association with both type 2 diabetes and obesity (2–4).The FTO-diabetes association is abolished by adjustment for BMI, suggesting that the association of FTO with type 2 diabetes is mediated through its effect on adiposity (2,4). In Caucasian populations, FTO heterozygotes have ~1.75 kg greater body weight and 20–30% higher prevalence of overweight/obesity (3,4). The population attributable risk for obesity because of genetic variation in FTO is at least 20% (2).
The extent to which lifestyle factors may modify this genetic risk is unclear. Some studies suggested that the obesogenic effects of FTO may be accentuated by lower physical activity (5) or blunted by higher physical activity (6), whereas other studies found no interaction between FTO and activity (7). One recent study reported that high-fat and low-carbohydrate intake accentuated the FTO-associated susceptibility to obesity (8). These findings are highly suggestive for a role of lifestyle in modifying the association between FTO and obesity. Despite this, the interaction between FTO and combinations of physical activity and caloric intake has not been well studied.
Therefore, we evaluated the separate and combined effects of two key lifestyle factors (physical activity and caloric intake) (9,10) on modifying the inherited predisposition to obesity that is carried by a common variant in the FTO gene (rs8050136). This variant, located in the intron 1 SNP cluster of the FTO gene, has been significantly associated with both BMI and type 2 diabetes and is in complete linkage disequilibrium with the other commonly associated FTO polymorphism, rs9939609, also located in this cluster.
RESEARCH DESIGN AND METHODS
Study participants
Study participants were members of the Women’s Genome Health Study (WGHS), a prospective genetic evaluation study in initially healthy U.S. women (11). Study participants were health professionals who were age 45 years and older and free of major chronic disease including cancer and cardiovascular disease at study entry (1992–1995). Information on baseline variables including medical history and dietary and lifestyle factors was self-reported on questionnaires. Participants provided a baseline blood sample and consented to ongoing analyses linking blood-derived observations with risk factor profiles and disease. The study was approved by the institutional review board of Brigham and Women’s Hospital (Boston, MA).
Genotype data were available for 22,054 women.We excluded women with missing rs8050136 genotype (N = 3) or BMI (N = 376), resulting in 21,675 women of European ancestry for this analysis. Caloric information was missing for 555 women, and physical activity information was missing for one woman; these women were included in other analyses.
Assessment of obesity, physical activity, and caloric intake
Obesity-related phenotypes.
Weight and height were self-reported on baseline questionnaires. BMI was calculated as weight divided by the square of the height (kg/m2) and used to define categories of BMI (normal <25 kg/m2, overweight 25–29.9 kg/m2, and obese ≥30 kg/m2). Waist-to-hip ratio and waist-circumference were self-reported and available only at 72 months. Participants were identified as having diabetes and hypertension as previously described (12,13). Specifically, hypertension was defined as self-reported history of hypertension, antihypertensive treatment, or blood pressure of at least 140 mmHg for systolic or 90 mmHg for diastolic. Diabetes status was indicated at baseline by reported physician-diagnosed diabetes.
Physical activity.
Physical activity was assessed using a questionnaire that has been shown previously to be both valid and reliable, with test-retest correlation of 0.59 (14). The correlation of activity reported on the questionnaires as compared with activity diaries kept for four weeks over a year was 0.62 (14). Participants were asked on the questionnaire to estimate the average time per week over the past year spent on eight groups of recreational activities and to report the number of flights of stairs climbed daily (15). A MET score was assigned to each activity based on the energy cost of that activity. The energy expended on each activity was estimated by multiplying its MET score with hours/week and summed across all activities, providing an estimate that is independent of body weight.
Caloric intake.
Participants completed a 131-item semiquantitative food frequency questionnaire previously validated in a similar population (Nurses Health Study) (16). Briefly, participants were asked to estimate average consumption over the past year of each food item and allowed nine responses, ranging from “never” to “six or more times per day.” The average daily intake for each food item was calculated by multiplying the intake frequency by portion size. Nutrient intake was computed by multiplying the intake frequency of each unit of food by the nutrient content of the specified portion size according to food composition tables from the Harvard School of Public Health (Boston, MA) (17).
Genotyping.
DNA samples were genotyped with the Infinium II technology from Illumina (Human HAP300 panel) (11). Either the HumanHap300 Duo-Plus chip or the combination of the HumanHap300 Duo and I-Select chips was used. The rs9939609 polymorphism in FTO was not on our chip. Another previously validated polymorphism (3,4), rs8050136, which is in complete linkage disequilibrium with rs9939609 (r2 = 1 and D’=1 in the CEU sample) was used for this study. Both SNPs are in intron 1 of FTO and are 4,252 base pairs apart. The call rate for rs8050136 was >99%.
Statistical analysis
All analyses were performed using SAS/Genetics 9.1 package (SAS Institute, Cary, NC). We calculated allele frequencies at rs8050136 and performed Hardy-Weinberg equilibrium tests using χ2 tests. We compared baseline variables based on means or medians using χ2 tests for categorical variables, ANOVA for normally distributed variables, and Kruskal-Wallis tests for nonnormally distributed variables. We performed linear regression to test associations of the risk allele with BMI, physical activity, and caloric intake. We performed stratified analysis to test for associations of the risk allele with obesity, using study median levels or U.S. federal guideline-based recommended levels of physical activity and caloric intake (9,10) with linear regression for BMI as a continuous variable and logistic regression for dichotomously defined BMI (<30 vs. ≥30 kg/m2 [obese]). Similar logistic regression models were also performed for type 2 diabetes as the outcome.
Statistical tests for interaction were performed using regression models (linear or logistic) that included age, genotype, physical activity or caloric intake, and an interaction term (e.g., physical activity*genotype). In these models, physical activity and caloric intake were categorized either by median levels or prespecified percentile cut-points (<10th, ≥10–25th, >25–50th, >50–75th, >75–90th, and >90th percentiles) to evaluate the effect of a range of values of physical activity and caloric intake. Statistical tests for interaction were obtained using likelihood-ratio tests that compared models with and without the interaction term.
Associations and interactions were also similarly examined in four combinations of physical activity and caloric intake levels in order to examine the joint effects of physical activity and caloric intake on the FTO gene–related risk.
RESULTS
The frequency of the FTO risk-allele (A) was 0.40, and Hardy-Weinberg equilibrium was met (χ2 = 0.02, d.f. = 1, P = 0.88). The mean age of participants was 54.2 years, with mean BMI of 25.9 (SD 5.0) kg/m2. Each FTO A allele was significantly associated with a mean BMI difference of +0.52 kg/m2 (SE 0.05 kg/m2, P ≤ 0.001).
Characteristics of the study population according to FTO genotype are shown in Table 1. There were statistically significant genotype associations with all the reported obesity phenotypes; A/A homozygotes had higher values for all obesity phenotypes compared with heterozygotes, consistent with an additive mode of inheritance. There were statistically significant associations for FTO with obesity-related traits such as hypertension and type 2 diabetes (P ≤ 0.001). There were no genotype differences in the total MET-hours/week or number of hours spent walking. There were no associations with total caloric intake or intake from specific nutritional components of food (fats, carbohydrates, legumes, or fruits). Associations were found for higher intake of two nutritional components (protein and cereal) with the A allele.
Table 1.
Characteristics of the study population by FTO genotype
| A/A (high risk) | A/C | C/C (low risk) | P | |
|---|---|---|---|---|
| N | 3,495 | 10,404 | 7,776 | |
| Age, years | 54.1 (7.1) | 54.2 (7.1) | 54.2 (7.1) | 0.90 |
| Height, m | 1.64 (0.06) | 1.64 (0.06) | 1.64 (0.06) | 0.34 |
| Weight, kg | 72.1 (15.6) | 69.9 (14.1) | 69.1 (13.4) | <0.0001 |
| BMI, kg/m2 | 26.7 (5.4) | 25.9 (5.0) | 25.6 (4.7) | <0.0001 |
| BMI categories, % | ||||
| Normal | 45.9 | 51.6 | 54.5 | |
| Overweight | 31.7 | 31.2 | 29.8 | <0.0001 |
| Obese | 22.5 | 17.1 | 15.7 | |
| Waist-to-hip ratio | 0.843 (0.229) | 0.834 (0.084) | 0.833 (0.116) | <0.001 |
| Waist circumference, cm | 90.43 (15) | 88.86 (14.4) | 88.08 (13.6) | <0.0001 |
| Hypertension, % | 27.3 | 24.5 | 23.1 | <0.0001 |
| Diabetes, % | 3.1 | 2.7 | 2.1 | 0.001 |
| MET-hours/wk | 8.86 (2.85–20.37) | 8.70 (2.85–20.47) | 8.91 (2.95–20.89) | 0.51 |
| Exercise frequency, % | ||||
| Rare/never | 38 | 37 | 37 | |
| <1 time/wk | 20 | 19 | 20 | 0.49 |
| 1–3 times/wk | 31 | 32 | 32 | |
| 4 times/wk | 12 | 11 | 12 | |
| Total caloric intake, kcal/day | 1,680 (1,346–2,082) | 1,678 (1,355–2,049) | 1,680 (1,361–2,046) | 0.81 |
| Saturated fat, gm/day | 19 (14–24) | 19 (14–24) | 19 (14–24) | 0.93 |
| Carbohydrates, gm/day | 221 (199–242) | 221 (199–242) | 221 (198–244) | 0.82 |
| Proteins, gm/day | 82 (73–90) | 81 (72–90) | 80 (72–90) | 0.01 |
| Cereal, kcal/day | 441 (321–582) | 435 (316–577) | 432 (314–568) | 0.03 |
| Fruit, kcal/day | 153 (94–223) | 155 (96–224) | 153 (94–225) | 0.91 |
| Legumes, kcal/day | 33 (19–62) | 31 (18–59) | 30 (13–62) | 0.32 |
| Soluble fiber, gm/day | 6 (4–7) | 6 (4–7) | 6 (4–7) | 0.18 |
| Vegetables, kcal/day | 105 (72–150) | 105 (74–147) | 104 (75–147) | 0.85 |
Values shown for variables are means (SD) or median (interquartile range: 25th to 75th percentile) and percentage. BMI categories are as follows: normal weight (BMI <25 kg/m2), overweight (BMI 25–29.9 kg/m2), and obese (BMI >30 kg/m2). A/A, homozygous carriers of the A allele; A/C, heterozygous carriers; C/C, noncarriers.
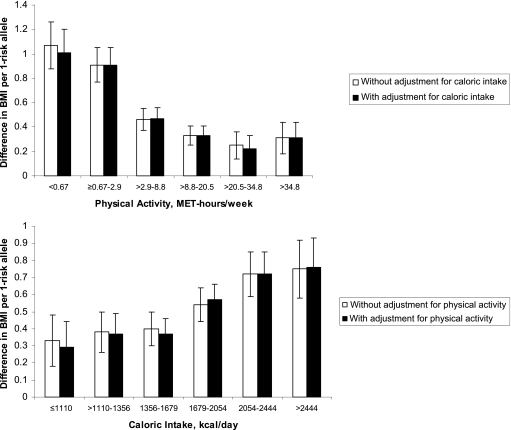
There were statistically significant effects of the FTO A allele within all prespecified percentile categories of physical activity and caloric intake (Fig. 1). Although there was no direct relationship between genotype and either physical activity or caloric intake (as shown in Table 1), both activity and intake modified the association of genotype with obesity (significant interaction terms). Specifically, larger effects of the A allele on BMI were noted in women who were less active or had higher intake (Fig. 1). Furthermore, the effects of activity and intake on modifying the association of FTO with BMI were independent of each other, since the associations remained essentially unchanged after mutual adjustment. The statistical tests for these interactions were significant before and after adjusting for the other lifestyle factor (P before adjustment: <0.001 and <0.001; after mutual adjustment: <0.001 and 0.003, for activity and intake, respectively).
Figure 1.
The difference in mean BMI per FTO A allele at prespecified percentiles of physical activity and caloric intake, with and without adjustment. The values for MET-hours per week and kilocalories per day represent the following percentile groups: <10%, ≥10–25%, >25–50%, >50–75%, >75–90%, and >90%. The bars represent means and SE. For physical activity, the P values within each percentile category for the association of the A allele with mean BMI (starting with <10% and ending with >90%) were <0.0001, <0.0001, <0.0001, 0.0001, 0.02, and 0.01, respectively. For caloric intake, the respective P values were 0.03, 0.002, <0.0001, <0.0001, <0.0001, and <0.0001. Tests for these interactions were significant (P before adjustment: <0.001 and <0.001; after mutual adjustment: <0.001 and 0.003, for physical activity and caloric intake, respectively).
When the analyses were performed according to median levels of activity, each copy of the A allele was associated with a mean BMI difference of +0.73 (SE 0.08) kg/m2 among inactive women compared with +0.31 (0.06) kg/m2, P < 0.0001, among active women. Similarly, among women stratified by median levels of intake, each A allele was associated with a mean BMI difference of +0.65 (0.07) among women with high intake compared with +0.38 (0.07) kg/m2, P = 0.005, among women with low intake.
The joint effects of combinations of physical activity and caloric intake are shown in Table 2. We found an additive effect of activity and intake in modifying the effect of the A allele on BMI according to these combined strata. Specifically, among inactive/high intake women, each A allele was associated with a mean BMI difference of +0.97 (0.11) kg/m2 vs. +0.22 (0.08) kg/m2 among inactive/low intake women, P < 0.0001. Interestingly, BMI differences between A allele carriers and noncarriers were blunted, but not eliminated, in the active/low intake group (Table 2).
Table 2.
Difference in BMI and risk of obesity per FTO A allele according to combinations of physical activity and caloric intake
| Inactive |
Active |
P* | |||
|---|---|---|---|---|---|
| High intake | Low intake | High intake | Low intake | ||
| N | 5,145 | 5,400 | 5,416 | 5,158 | |
| Mean BMI, kg/m2 (SD) | 27.1 (5.5) | 26.5 (5.2) | 25.2 (4.3) | 24.8 (4.2) | |
| A/A (high risk) | 28.2 (6.0) | 27.2 (5.5) | 25.8 (4.8) | 25.3 (4.6) | |
| A/C | 27.1 (5.4) | 26.4 (5.1) | 25.1 (4.2) | 24.6 (4.1) | |
| C/C (low risk) | 26.3 (4.9) | 26.1 (4.8) | 24.9 (4.3) | 24.6 (4.1) | |
| Difference in BMI (SE) per A allele, kg/m2 | +0.97 (0.11) | +0.49 (0.10) | +0.38 (0.08) | +0.22 (0.08) | <0.0001 |
| P† | <0.0001 | <0.0001 | <0.0001 | 0.009 | |
| OR (95% CI) of obesity per A allele‡ | 1.39 (1.27–1.52) | 1.21 (1.11–1.33) | 1.15 (1.02–1.28) | 1.13 (0.99–1.28) | 0.003 |
| P‡ | <0.0001 | <0.0001 | 0.02 | 0.07 | |
High intake, >1,679 kcal/day; low intake, ≤1,679 kcal/day; inactive, ≤8.8 MET-hours/week; active, >8.8 MET-hours/week.
*P indicates the significance of the interaction across the four categories defined by caloric intake and physical activity.
†P value indicates the significance of the association for having one copy of the FTO A allele with BMI (continuous variable, kg/m2) within each category of caloric intake and physical activity.
‡Odds ratio (OR), 95% CI, and associated P value for likelihood of obesity (categorical variable, BMI ≥30 kg/m2) per A allele within each category of caloric intake and physical activity.
When BMI was categorized according to obesity status (BMI ≥30 kg/m2), the A allele was associated with greater odds of obesity in all categories except the active/low intake participants, which was borderline significant (P = 0.07). When compared with noncarriers of the A allele, inactive/high intake women had 39% greater likelihood of obesity associated with each copy of the A allele, whereas active/low intake carriers had only 13% greater likelihood (P = 0.003).
The prevalence of diabetes was low in this study (2.6%), and the overall results were very similar to the results among the subgroup of women without diabetes. Among women with diabetes, significant association for FTO with BMI and obesity were only seen among inactive/high intake women, with each A allele associated with a mean BMI difference of +1.67 kg/m2, P = 0.04, and an increased risk of obesity (odds ratio 1.61, 95% CI 0.96–2.69; P = 0.07). Statistically significant interactions were noted for physical activity and caloric intake in relation to BMI and obesity among the subgroups of women with and without diabetes.
Generally similar results were obtained when physical activity and caloric intake were categorized according to U.S. federal guideline-based recommended levels of physical activity and caloric intake (9,10) instead of medians.
Table 3 shows odds ratios per A allele of type 2 diabetes among women stratified by combinations of activity and intake. Among inactive/high intake women, each copy of the A allele was associated with significantly greater likelihood of type 2 diabetes (odds ratio 1.36, 95% CI 1.07–1.73) compared with noncarriers. To test whether the association of FTO with diabetes may be mediated through its effect on BMI, we further adjusted the analyses in Table 3 for BMI and found that the associations with diabetes became attenuated and nonsignificant.
Table 3.
Difference in risk of type 2 diabetes per FTO A allele according to combinations of physical activity and caloric intake
| Inactive |
Active |
P* | |||
|---|---|---|---|---|---|
| High intake | Low intake | High intake | Low intake | ||
| N | 5,145 | 5,400 | 5,416 | 5,158 | __ |
| Type 2 diabetes, N (%) | 137 (2.7) | 175 (3.2) | 113 (2.1) | 108 (2.1) | |
| OR (95% CI) of type 2 diabetes per A allele† | 1.36 (1.07–1.73) | 1.20 (0.97–1.48) | 1.14 (0.87–1.48) | 1.33 (1.01–1.75) | 0.78 |
| P† | 0.01 | 0.10 | 0.35 | 0.04 | |
High intake, >1,679 kcal/day; low intake, ≤1,679 kcal/day; inactive, ≤8.8 MET-hours/week; active, >8.8 MET-hours/week.
*P indicates the significance of the interaction across the four categories defined by caloric intake and physical activity.
†Odds ratio (OR), 95% CI, and associated P value for likelihood of type 2 diabetes per A allele within each category of caloric intake and physical activity.
CONCLUSIONS
In this study of healthy U.S. Caucasian middle-aged women, variation in the rs8050136 FTO genotype was associated with higher BMI, obesity phenotypes, and obesity-related conditions including hypertension and type 2 diabetes. There was statistically significant modification of this risk by lifestyle, with the largest magnitude of effect noted in women with both low levels of physical activity and high caloric intake. Healthier lifestyle blunted but did not completely eliminate this associated FTO-related genetic risk.
The allele frequency and the modest strength of association with obesity in this study was similar to previously reported studies in populations of European descent (4,18). Each A allele in this study was associated with an increase in mean BMI of +0.52 kg/m2, consistent with previously reported increases in the range of 0.40 to 0.66 kg/m2 (2). Importantly, we found that the effect of the risk alleles ranged from as much as 1.18 kg/m2 per allele in inactive/high intake women to as little as 0.24 kg/m2 per allele in active/low intake women.
The main finding of this study is that modifiable behaviors that define healthy or unhealthy lifestyles may amplify or blunt the genetic risk of increased weight associated with FTO gentoype. We found interactions between FTO, physical activity, and caloric intake, and combinations of these lifestyle choices. Prior studies have been inconsistent in reporting associations or interactions between physical activity, caloric intake, and FTO risk alleles and have not examined the combined effects of activity and intake on FTO-related risk. Several studies have found that the effects of FTO risk alleles are accentuated by lower levels of physical activity (5,8) and blunted by physical activity (6). However, one recent study in a large cohort showed no interaction between FTO risk alleles and physical activity (7). Studies have been unclear as to whether increased caloric intake may be a mechanism by which FTO predisposes to obesity (19–21), and a recent study reported that dietary fat and carbohydrate intake modified the obesogenic effect of FTO (8). The current study extends our knowledge of this topic by reporting an interaction across a combination of lifestyle choices (activity and intake) and FTO genotype.
The function of FTO is incompletely understood. The rs8050136 polymorphism is likely not causal, since other polymorphisms in the first intron of the FTO gene that are in linkage disequilibrium with rs8050136 have been found to be associated with obesity phenotypes (3). It is unclear whether these changes influence FTO expression or splicing, or tag another genetically mechanistic region.
It should be noted that the effect of FTO on BMI and type 2 diabetes is modest, and the clinical applications, if any, of genetic testing remain to be determined. At this time, lifestyle interventions that emphasize both increased physical activity and dietary interventions that help individuals obtain and maintain an ideal body weight should be recommended to all individuals (9,10,22).
Several limitations of this study warrant consideration. Physical activity, caloric intake, and obesity variables were assessed by self report. However, the effect of the FTO A allele on BMI in this study was similar to studies with more objective measurements of adiposity. Under-reporting of energy intake and overestimation of physical activity (23,24) is a documented phenomenon among obese individuals, and misclassification of this nature would be expected to bias our results toward the null. Data on energy intake were obtained from food frequency questionnaires, which may underestimate absolute energy intake. By contrast, the relative ordering of subjects (ranked by medians or percentile cut-points) has good reproducibility, as assessed by correlation coefficients of 0.6 or greater (16). We only had data on Caucasian women, which may limit the generalizability of these findings. Although our study is large and had detailed phenotyping and genotyping, it is cross-sectional in design. Finally, the BMI variance explained by the FTO allele is modest and the missing heritability is possibly explained by other common polymorphisms and rare variants.
Strengths of this study include the combined information on activity, dietary intake, and other risk factors in a large cohort of women that allowed for a comprehensive look at the lifestyle by gene interactions at FTO with obesity. Given the large number of participants in our study, we were able to examine the effect of FTO across a wide range of values of activity, intake, and obesity phenotypes.
In this study, we found that carriers of the rs8050136 A allele in the FTO gene have a greater risk of both obesity and its related conditions including type 2 diabetes. Physical activity and caloric intake had separate and additive effects on the FTO-associated genetic risk. Finally, this study shows that healthier lifestyle behaviors relating to caloric intake and physical activity may blunt the deleterious effects of this common genetic variant.
Acknowledgments
T.A. was supported by the National Heart, Lung, and Blood Institute (NHLBI) Grant T32HL07575. S.M. was supported by NHLBI Grant K08 HL094375. The WGHS is supported by Grants HL43851 and CA47988 from the NHLBI and the National Cancer Institute and the Donald W. Reynolds Foundation, Leducq Foundation, and the Doris Duke Charitable Foundation. Genotyping was provided by Amgen (Cambridge, MA). No other potential conflicts of interest relevant to this article were reported.
The funding agencies played no role in the design, conduct, data management, analysis, or manuscript preparation related to this article.
T.A. designed the study, planned the analyses, performed statistical analyses, and wrote the first draft. I.-M.L., G.P., and D.I.C. gathered data. L.R. performed statistical analyses. P.M.R. gathered data. S.M. designed the study, planned the analyses, and wrote the first draft. All authors reviewed and edited the article.
The authors thank the investigators, staff, and participants of the Women’s Health Study for their valuable contributions.
References
- 1.Friedman JM. Obesity: causes and control of excess body fat. Nature 2009;459:340–342 [DOI] [PubMed] [Google Scholar]
- 2.Li S, Loos RJ. Progress in the genetics of common obesity: size matters. Curr Opin Lipidol 2008;19:113–121 [DOI] [PubMed] [Google Scholar]
- 3.Loos RJ, Bouchard C. FTO: the first gene contributing to common forms of human obesity. Obes Rev 2008;9:246–250 [DOI] [PubMed] [Google Scholar]
- 4.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasen CH, Stender-Petersen KL, Mogensen MS, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes 2008;57:95–101 [DOI] [PubMed] [Google Scholar]
- 6.Rampersaud E, Mitchell BD, Pollin TI, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Arch Intern Med 2008;168:1791–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonsson A, Renström F, Lyssenko V, et al. Assessing the effect of interaction between an FTO variant (rs9939609) and physical activity on obesity in 15,925 Swedish and 2,511 Finnish adults. Diabetologia 2009;52:1334–1338 [DOI] [PubMed] [Google Scholar]
- 8.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr 2009;90:1418–1425 [DOI] [PubMed] [Google Scholar]
- 9.Physical Activity Guidelines Advisory Committee Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC, U.S. Department of Health and Human Services, 2008 [Google Scholar]
- 10.U.S. Department of Health and Human Services; U.S. Department of Agriculture Dietary Guidelines for Americans, 2005. 6th ed. Washington, DC, U.S. Govt. Printing Office, 2005 [Google Scholar]
- 11.Ridker PM, Chasman DI, Zee RY, et al. Women’s Genome Health Study Working Group Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem 2008;54:249–255 [DOI] [PubMed] [Google Scholar]
- 12.Conen D, Ridker PM, Mora S, Buring JE, Glynn RJ. Blood pressure and risk of developing type 2 diabetes mellitus: the Women’s Health Study. Eur Heart J 2007;28:2937–2943 [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Lee I-M, Song Y, et al. Vitamin E and risk of type 2 diabetes in the women’s health study randomized controlled trial. Diabetes 2006;55:2856–2862 [DOI] [PubMed] [Google Scholar]
- 14.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs. body mass index with type 2 diabetes in women. JAMA 2004;292:1188–1194 [DOI] [PubMed] [Google Scholar]
- 15.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA 2006;295:1412–1419 [DOI] [PubMed] [Google Scholar]
- 16.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol 1985;122:51–65 [DOI] [PubMed] [Google Scholar]
- 17.Watt B, Merrill A. Composition of Foods: Raw, Processed, Prepared, 1963-1992. Agricultural Handbook No. 8. Washington, DC, U.S. Govt. Printing Office, 1993 [Google Scholar]
- 18.Song Y, You NC, Hsu YH, et al. FTO polymorphisms are associated with obesity but not diabetes risk in postmenopausal women. Obesity (Silver Spring) 2008;16:2472–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haupt A, Thamer C, Staiger H, et al. Variation in the FTO gene influences food intake but not energy expenditure. Exp Clin Endocrinol Diabetes 2009;117:194–197 [DOI] [PubMed] [Google Scholar]
- 20.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–2566 [DOI] [PubMed] [Google Scholar]
- 21.Hakanen M, Raitakari OT, Lehtimäki T, et al. FTO genotype is associated with body mass index after the age of seven years but not with energy intake or leisure-time physical activity. J Clin Endocrinol Metab 2009;94:1281–1287 [DOI] [PubMed] [Google Scholar]
- 22.Eckel RH, Kahn R, Robertson RM, Rizza RA. Preventing cardiovascular disease and diabetes: a call to action from the American Diabetes Association and the American Heart Association. Circulation 2006;113:2943–2946 [DOI] [PubMed] [Google Scholar]
- 23.Mahabir S, Baer DJ, Giffen C, et al. Comparison of energy expenditure estimates from 4 physical activity questionnaires with doubly labeled water estimates in postmenopausal women. Am J Clin Nutr 2006;84:230–236 [DOI] [PubMed] [Google Scholar]
- 24.Lichtman SW, Pisarska K, Berman ER, et al. Discrepancy between self-reported and actual caloric intake and exercise in obese subjects. N Engl J Med 1992;327:1893–1898 [DOI] [PubMed] [Google Scholar]