Abstract
Numerous plant species have been known for decades that respond to herbivore attacks by systemically synthesizing defensive chemicals to protect themselves from predators. The nature of systemic wound signals remained obscure until 1991, when an 18-aa peptide called systemin was isolated from tomato leaves and shown to be a primary signal for systemic defense. More recently, two new hydroxyproline-rich, glycosylated peptide defense signals have been isolated from tobacco leaves, and three from tomato leaves. Because of their origins in plants, small sizes, hydroxyproline contents (tomato systemin is proline-rich), and defense-signaling activities, the new peptides are included in a functionally defined family of signals collectively called systemins. Here, we review structural and biological properties of the systemin family, and discuss their possible roles in systemic wound signaling.
Keywords: prosystemin, systemin receptor, plant defense
Systemin, the initial peptide signal found in plants, is an intracellular signaling molecule that is synthesized within the amino acid sequence of a 200-aa precursor, called prosystemin (1, 2). Systemin induces proteinase inhibitor protein synthesis in leaves of young tomato plants when supplied for a few minutes through their cut stems at nanomolar concentrations (1). Radioactively labeled systemin, when placed on wound sites on leaves, is found in the phloem (1, 3). A key role for systemin in systemic signaling was established by showing that tomato plants expressing an antisense prosystemin gene become deficient in long-distance wound signaling and are more susceptible to insect attacks than wild-type plants (4).
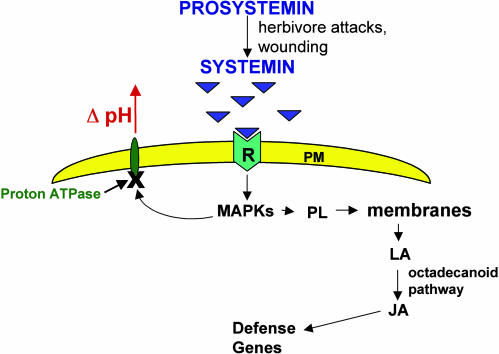
In contrast to animal peptide hormones, the systemin precursor protein lacks a leader or signal sequence that is required for synthesis and processing through the secretory pathway (2). Immunolocalization techniques revealed that prosystemin is localized in parenchyma cells of vascular bundles (5). This localization in the vicinity of the sieve tubes of the phloem may facilitate transport of systemin and the oxylipins it induces in response to wounding to distal cells. Systemin activates defensive genes by interacting with a cell-surface receptor, called SR160, a 160-kDa transmembrane protein with an extracellular leucinerich domain, and an intracellular receptor kinase domain (6, 7). The interaction of systemin with the receptor is the first step of a complex intracellular signaling pathway that involves the activation of a mitogen-activated protein kinase (MAPK) (8), the rapid alkalinization of the extracellular medium (7, 9), the activation of a phospholipase (10, 11), and the release of linolenic acid that is converted into oxylipins such as phytodienoic acid and jasmonic acid that are powerful signals for defense genes (Fig. 1) (12, 13). The pathway exhibits analogies to the inflammatory response in animals (14) in which wounding activates MAPKs, phospholipases, the release of arachidonic acid from membranes, and its conversion to prostaglandins, which are analogs of phytodienoic acid and jasmonic acid
Fig. 1.
A simplified diagram of the systemin signaling pathway. The pathway shows several key steps of the signaling pathway, and in particular the steps leading to the blockage of a proton ATPase that leads to the alkalinization of the extracellular medium, which is the basis of the assay developed to identify signaling peptides.
The early alkalinization in response to systemin in tomato suspension cultures was the basis for the development of an assay system that led to the identification and characterization of the systemin receptor, SR160 (7). SR160 is homologous to the BRI1 receptor from Arabidopsis (15), with a high percentage of amino acid identity. This was the first indication that the systemin receptor may be a close relative of the BRI1 receptor. This possibility was confirmed by the identification and cloning of the tomato brassinolide receptor, BRI1 (16), which was found to be identical to the tomato SR160 receptor. The identity of a receptor with two functions, i.e., defense and development, was unique in plants, but examples are known in the animal kingdom. The dual function of the SR160/BRI1 receptor was supported by experiments in which the tomato SR160/BRI1 receptor cDNA was expressed in tobacco, which does not express a prosystemin gene and therefore does not produce systemin as a defense signal (17). Transformed tobacco suspension-cultured cells synthesized the receptor and targeted it to the cell surface membranes of tobacco, where it displayed the identical binding characteristics with systemin as SR160 in tomato cells. The systemin–receptor interaction in tobacco cells induced the alkalinization response, indicating that signaling components for the early steps in the systemin signaling pathway were present in tobacco and could be activated by the tomato SR160 receptor when it interacted with systemin. Additionally, a tomato mutant cu-3, which was caused by a mutation in the BRI1 receptor and led to the isolation of the BRI1 gene (16), is severely impaired in systemin signaling (17).
Because tobacco does not produce systemin, the presence of components in tobacco cells that react to the systemin–receptor interaction indicated that the BRI1 receptor may have, or may have had in the past, a defensive role in plants that was co-opted by systemin as the prosystemin gene evolved in species of the Solaneae subtribe of the Solanaceae family. Tobacco does exhibit a fairly strong systemic defense response to wounding in young plants, but it is much weaker in older plants. Wounded tobacco plants synthesize a trypsin inhibitor (TTI) that is a paralog of tomato inhibitor II (18), which is induced in tomato leaves in response to wounding. The induction of TTI in tobacco leaves in response to wounding indicates a genetic link between the wound-signaling systems of tomato and tobacco, despite the absence of systemin in tobacco. The synthesis of TTI in young tobacco plants is strongly induced by jasmonic acid (18), indicative of the early steps of signaling that result in the release of linolenic acid from membranes, similar to tomato plants. The roles of both systemin and jasmonate in systemic signaling have been the subject of considerable speculation (19, 20). We hypothesize here that the evolution of the prosystemin gene in species of the Solaneae subtribe resulted in the production of systemin, a strong systemic signal that is not found in other plants, that amplifies the jasmonate signaling pathway. Prosystemin released from cells at the wound site is likely processed to systemin by proteinases also released from damaged cells. This would allow diffusion of systemin to the apoplast of nearby unwounded vascular cells to interact with its receptor and induce the synthesis of jasmonates. As jasmonates move through the plant, it would induce more prosystemin along with proteolytic enzymes that are known to be induced by jasmonate (14) that could process the nascent prosystemin to systemin to continue to amplify the jasmonate signal in nearby cells.
A major source of jasmonates at wound sites is from linolenic acid that is produced by the degradation of membrane lipids within the cellular debris. This source of jasmonates would likely provide an important “kick-start” for defense signaling, as the oxylipins diffuse into the vascular system and are transported to parenchyma cells (5) to up-regulate signaling pathway genes, including the prosystemin gene (in Solaneae species). This hypothetical scenario led us to suspect that other peptide signals that were not systemic may be present in tobacco and tomato plants that might help amplify wound signaling. Such peptides in tobacco, which lacks a systemic peptide signal, might contribute to a localized amplification of the synthesis of jasmonates in response to wounding, and to amplification of jasmonate synthesis in the absence of systemin.
Tobacco Systemins I and II
The search for peptide signals in tobacco was facilitated by the development of a biological assay that is based on the alkalinization of the medium of tomato suspension-cultured cells in response to systemin, which is characterized by an increase in pH (up to 1 pH unit per 10 min) in the culture medium (9). Suspension cultured tobacco cells do not exhibit an alkalinization response when supplied with tomato systemin, but do so in response to a crude peptide fraction obtained from tobacco leaves, suggesting that a peptide–receptor interaction may be occurring that is coupled to an intracellular response. The alkalinization of 1 ml of suspension cultured tobacco cells in response to 1-μl aliquots from fractions eluting from HPLC or other columns revealed the presence of two peptides that, when purified and characterized, were found to be 18-aa glycopeptides that contained multiple hydroxyproline residues (21). The two peptides are active in the alkalinization assay with tobacco suspension cultures at nM concentrations, and both cause a rapid activation of a 48-kDa MAPK, similar to the 48-kDa MAPK activated by tomato systemin in tomato cells (8). These peptides, supplied to young excised tobacco plants through their cut stems at nM concentrations, induce the synthesis of TTI in leaves.
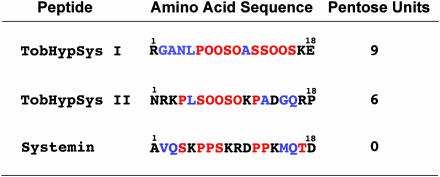
Because of their similarities to tomato systemin in signaling properties, the two peptides were called tobacco systemin I and II (21). However, because of their hydroxyproline (O) contents, they are now named tobacco hydroxyproline-rich systemin (TobHypSys) I and II to identify them as members of a functionally related systemin family (22). Their amino acid sequences are shown in Fig. 2. Neither peptide exhibits homology with tomato systemin, but -OOS-motifs found in the tobacco peptides are posttranslational modifications of the primary translation motif -PPS-that is found in tomato systemin (1). The two new peptides are rich in P/O residues, and in S and T residues as well. These three amino acids make up 50% of each peptide and are likely involved in their recognition as defense signals.
Fig. 2.
The amino acid sequences of tobacco hydroxyproline-rich systemins, TobHypSys I and TobHypSys II, are shown compared with the sequence of tomato systemin. Hydroxyproline (O), proline (P), threonine (T), and serine (S) residues are in red, charged amino acids are in black, and neutral amino acids are in blue. The ranges of pentose units attached to each peptide, determined by mass spectrometry, are shown at the right.
Mass spectroscopy of the two peptides revealed that the attached carbohydrate moieties consist of pentose residues; nine in TobHypSys I and six in TobHypSys II. The structural properties of TobHypSys I and II (leader sequence, hydroxylation of -P-residues, and carbohydrate decorations) indicate that they are synthesized through the secretory system, unlike tomato systemin, which is not glycosylated, whose prolines are not hydroxylated, and whose precursor has no signal sequence (1, 2). Both TobHypSys peptides originate from a single 165-aa-long preproprotein, including a signal sequence, with the TobHypSys I sequence near the N terminus and the TobHypSys II sequence near the C terminus (21). The presence of multiple signaling peptides contained in a single preproprecursor is a characteristic of many animal peptide hormones, but the two tobacco systemins provide the first example in plants of a peptide hormone precursor harboring multiple peptide signals.
Although tobacco does not use a tomato systemin homolog for systemic wound signaling, TobHypSys I and II appear to serve roles in defense signaling. Because proTobHypSys is hydroxylated and glycosylated, like well characterized hydroxyproline-rich glycoproteins (23), it may be associated with cell walls, and may be processed from the precursor at wound sites to provide signals to amplifiy the synthesis of oxylipins during long distance wound signaling. Zhang and Baldwin (24) have elegantly shown that wounding of tobacco causes the synthesis of jasmonic acid that acts as a systemic signal from leaves to roots. It may be that the TobHypSys peptides help generate jasmonic acid that is targeted to the roots of the plant in response to wounds.
Tomato Leaf Hydroxyproline-Containing Systemin Peptides
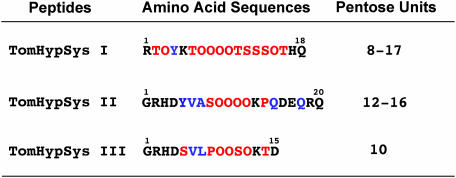
The isolation of 18-aa, glycosylated, hydroxyproline-containing tobacco systemins led to an investigation of the possibility that tomato plants may also have peptide defense signals similar to the tobacco systemins. The alkalinization assay used to identify and isolate the two tobacco systemins was used to analyze tomato leaf extracts for peptide signals in addition to systemin. The assay identified several components from tomato leaf extracts that caused an alkalinization response. Purification and characterization of these components confirmed that one peptide was tomato systemin and identified three new peptides (22). The novel peptides exhibit several properties similar to TobHypSys I and II, being hydroxyproline-rich glycopeptides, and ranging in size from 15 to 20 aa. Each of the peptides contains an internal continuous sequence of from 5 to 11 aa variously composed of O, P, S, or T residues, and all are flanked by various charged residues (Fig. 3). The peptides are decorated with variable numbers of pentose residues, but their identities and locations on the peptides have not been determined. The amino acid sequences of tomato hydroxyproline-rich systemin (TomHypSys) II and III indicated that they shared limited amino acid sequence homology and were likely products of gene duplication-elongation events. The three tomato peptides exhibit similar biological activities as tomato systemin, indicating that they are defense signals (22). They all exhibit similar specific activities in the alkalinization assays, and all are effective inducers of proteinase inhibitors I and II synthesis when supplied to young tomato plants. The tomato peptides were therefore included in the functionally defined systemin family and named tomato hydroxyproline-rich systemins, i.e., TomHypSys I (18 amino acid residues), TomHypSys II (20 amino acid residues), and TomHypSys III (15 amino acid residues). Although the three TomHypSys peptides are powerful inducers of defense genes when supplied to excised tomato plants, they do not serve as primary systemic signals, because tomato plants transformed with an antisense prosystemin gene were incapable of systemic signaling in response to wounding (4).
Fig. 3.
The amino acid sequences of tomato hydroxyproline-rich systemins, TomHypSys I, proline (P), TomHypSys II, and TomHypSys III, are shown. Hydroxyproline (O), threonine (T), and serine (S) residues are colored as in Fig. 2. The number of pentose units associated to each peptide are shown in the right column.
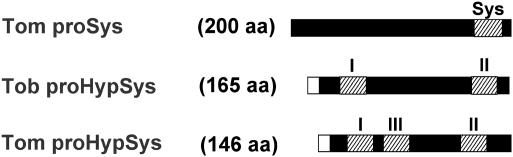
Isolation and characterization of cDNAs coding for the tomato peptides revealed that all three were derived from the same 146-aa preproprotein precursor that includes a signal sequence. This precursor, along with the precursor of the TobHypSys peptides, provides the only examples in plants of polyprotein hormone precursors. Box diagrams of the three precursor proteins that harbor the six members of the systemin family are compared in Fig. 4. A comparison of the amino acid sequences of the TomHypSys precursor with the TobHypSys precursor revealed a 10-aa sequence at their N termini that were identical at eight residues. The nucleotide sequence identity of this sequence was 90%. The significance of this identity is not clear, but does suggest that the two precursor genes may have a common ancient precursor, and that this sequence may have an important function that has been conserved. No homology was evident between prosystemin and either of the two preproprecursor proteins. However, it is of interest that the sequence of tomato systemin contains 7 of 18 residues that are P, S, and T (1). Because prosystemin has been found only in species of the Solaneae subtribe of the Solanaceae family, we speculate that prosystemin may have been a member of the TomHypSys family and that some mutational event may have caused the loss of the leader sequence that resulted in the synthesis of the nascent precursor peptide to shift from a secretory pathway origin to a cytoplasmic origin, providing a powerful systemic defense signal (systemin) that was retained in the evolving species of the Solaneae subtribe.
Fig. 4.
Box diagrams of the precursors of tobacco and tomato systemin peptides. The open boxes represent the leader sequences of the newly translated proteins. The HypSys peptides are shown as hatched boxes, with their numeral identities above each peptide. The systemin peptide is shown as Sys. The length of each preproprecursor is shown in parentheses.
Perspectives
The multiple P, O, S, and T residues in all six members of the systemin family in tomato and tobacco plants suggest that these residues have important structural roles for interacting with receptors. The P residues confer polyproline II structures (PP II) that have distinct kinks that may be the key to receptor recognition (25, 26). PP II structures are commonly found in peptide ligands of animals, where they appear to be important for recognition by receptors (27). In all five HypSys peptides, the central P and O residues are flanked by basic or acidic amino acids, either internally or near both the N and C termini.
The discovery of the HypSys defense signals in tomato and tobacco raise many questions about wound signaling in these and other plant species. The relationship of the systemins to local and systemic signaling and whether the HypSys peptides interact with homologs of the systemin receptor or have entirely different receptors for each peptide remain to be determined. Also of interest is whether the different peptides in tomato plants can activate the same complement of defense genes as systemin in response to wounding. The presence of a family of functionally similar HypSys defense signaling peptides in tomato and tobacco that are derived from parologous precursors introduces the possibility that, in other plant families, related defense signaling peptides may be present that share a common ancestral origin. A search for such signals is now possible by using the same strategies that led to the discovery of the defense signaling peptides and their genes in tomato and tobacco that are described herein.
Acknowledgments
We thank S. Vogtman for plant growth and maintenance of plants, G. Munske for amino acid sequence analyses, and W. Seims for matrix-assisted laser desorption ionization mass spectrometric analyses. This research was supported by Washington State University College of Agriculture and Home Economics Project 1791 and by National Science Foundation Grant IBN 0090766.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Chemical Communication in a Post-Genomic World,” held January 17–19, 2003, at the Arnold and Mabel Beckman Center of the National Academies of Science and Engineering in Irvine, CA.
Abbreviations: MAPK, mitogen-activated protein kinase; TobHypSys, tobacco hydroxyproline-rich systemin; TomHypSys, tomato hydroxyproline-rich systemin.
References
- 1.Pearce, G., Strydom, D., Johnson, S. & Ryan, C. A. (1991) Science 253, 895-897. [DOI] [PubMed] [Google Scholar]
- 2.McGurl, B., Pearce, G., Orozco-Cardenas, M. & Ryan, C. A. (1992) Science 255, 1570-1573. [DOI] [PubMed] [Google Scholar]
- 3.Narvaez-Vasquez, J., Pearce, G., Orozco-Cardenas, M. L., Franceschi, V. R. & Ryan, C. A. (1995) Planta 195, 593-600. [Google Scholar]
- 4.Orozco-Cardenas, M., McGurl, B. & Ryan, C. A. (1993) Proc. Natl. Acad. Sci. USA 90, 8273-8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Narvaez-Vasquez, J. & Ryan, C. A. (2003) Planta, in press.
- 6.Scheer, J. M. & Ryan, C. A. (1999) Plant Cell 11, 1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer, J. M. & Ryan, C. A. (2002) Proc. Natl. Acad. Sci. USA 99, 9585-9590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stratmann, J., Scheer, J. & Ryan, C. A. (2000) Proc. Natl. Acad. Sci. USA 97, 8862-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meindl, T., Boller, T. & Felix, G. (1998) Plant Cell 10, 1561-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Narvaez-Vasquez, J., Florin-Christensen, J. & Ryan, C. A. (1999) Plant Cell 11, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiguro, S., Kawai-Oda, A., Weda, K., Nishida, L. & Okada, K. (2001) Plant Cell 13, 2191-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farmer, E. E. & Ryan, C. A. (1991) Plant Cell 4, 129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wasternack, C. & Hause, B. (2002) Prog. Nucleic Acid Res. Mol. Biol. 72, 165-221. [DOI] [PubMed] [Google Scholar]
- 14.Bergey, D., Howe, G. & Ryan, C. A. (1996) Proc. Natl. Acad. Sci. USA 93, 12053-12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, J. & Chory, J. (1997) Cell 90, 926-938. [DOI] [PubMed] [Google Scholar]
- 16.Montoya, T., Nomura, T., Farrar, K., Kaneta, T., Yokota, T. & Bishop, G. (2002) Plant Cell 14, 3163-3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheer, J. M., Pearce, G. & Ryan, C. A. (2003) Proc. Natl. Acad. Sci. USA 100, 10114-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearce, G., Johnson, S. & Ryan, C. A. (1993) Plant Physiol. 102, 639-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li, L., Li, C., Gyu, I. L. & Howe, G. (2002) Proc. Natl. Acad. Sci. USA 99, 6416-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stratmann, J. (2003) Trends Plant Sci. 8, 247-250. [DOI] [PubMed] [Google Scholar]
- 21.Pearce, G., Moura, D. S., Stratmann, J. & Ryan, C. A. (2001) Nature 411, 817-820. [DOI] [PubMed] [Google Scholar]
- 22.Pearce, G. & Ryan, C. A. (2003) J. Biol. Chem. 278, 30044-30050. [DOI] [PubMed] [Google Scholar]
- 23.Sommer-Knudson, J., Bacic, A. & Clarke, A. E. (1998) Phytochemistry 47, 483-497. [Google Scholar]
- 24.Zhang, Z.-P. & Baldwin, I. T. (1997) Planta 203, 436-441. [Google Scholar]
- 25.Toumadje, A. & Johnson, C., Jr. (1995) J. Am. Chem. Soc. 117, 7023-7024. [Google Scholar]
- 26.Vanhoof, G., Goossens, F., De Meester, I., Hendriks, D. & Scharpe, S. (1995) FASEB J. 9, 736-744. [PubMed] [Google Scholar]
- 27.Ferriss, P. J., Woessner, J. P., Waffenschmidt, S., Kilz, S., Drees, J. & Goodenough, U. W. (2001) Biochemistry 40, 2978-2987. [DOI] [PubMed] [Google Scholar]