Abstract
OBJECTIVE
Glucagon-like peptide 1 (GLP-1) stimulates insulin secretion. However, GLP-1 also improves endothelial function in diabetes.
RESEARCH DESIGN AND METHODS
Sixteen type 2 diabetic patients and 12 control subjects received a meal, an oral glucose tolerance test (OGTT), and two hyperglycemic clamps, with or without GLP-1. The clamps were repeated in diabetic patients after 2 months of strict glycemic control.
RESULTS
During the meal, glycemia, nitrotyrosine, and plasma 8-iso prostaglandin F2α (8-iso-PGF2a) remained unchanged in the control subjects, whereas they increased in diabetic patients. Flow-mediated vasodilation (FMD) decreased in diabetes, whereas GLP-1 increased in both groups. During the OGTT, an increase in glycemia, nitrotyrosine, and 8-iso-PGF2a and a decrease in FMD were observed at 1 h in the control subjects and at 1 and 2 h in the diabetic patients. In the same way, GLP-1 increased in both groups at the same levels of the meal. During the clamps, in both the control subjects and the diabetic patients, a significant increase in nitrotyrosine and 8-iso-PGF2a and a decrease in FMD were observed, effects that were significantly reduced by GLP-1. After improved glycemic control, hyperglycemia during the clamps was less effective in producing oxidative stress and endothelial dysfunction and the GLP-1 administration was most effective in reducing these effects.
CONCLUSIONS
Our data suggest that during the meal GLP-1 can simultaneously exert an incretin effect on insulin secretion and a protective effect on endothelial function, reasonably controlling oxidative stress generation. The ability of GLP-1 in protecting endothelial function seems to depend on the level of glycemia, a phenomenon already described for insulin secretion.
Oral administration of glucose is a more potent secretory stimulus for insulin than its intravenous infusion (1). This observation gave rise to the “incretin effect” concept, i.e., stimulation of insulin secretion as a response to food before an increase in blood glucose levels. An incretin hormone is the glucagon-like peptide 1 (GLP-1).
Type 2 diabetes mellitus is increasing all over the world. Patients with diabetes have an increased risk of cardiovascular disease. Recently, much attention has been paid to evidence that abnormalities of the postprandial state are important contributing factors to the development of atherosclerosis, even in diabetes (2). In diabetic subjects, the combination of postprandial hyperglycemia and postprandial hypertriglyceridemia has been recently proposed as an independent risk factor for cardiovascular disease (2).
The response-to-injury hypothesis of atherosclerosis states that the initial damage affects the arterial endothelium, leading to endothelial dysfunction (3). Indeed, endothelial dysfunction has been demonstrated in patients with diabetes, and hyperglycemia has been implicated as a cause of endothelial dysfunction in normal and diabetic subjects (2). It has been suggested that hyperglycemia induces an endothelial dysfunction through the production of an oxidative stress (2).
GLP-1 is now being used in clinics to enhance insulin secretion and reduce body weight in patients with type 2 diabetes mellitus (4), in whom a defect of GLP-1 secretion/action in response to the meal has often been reported (5). GLP-1 has been shown to lower postprandial and fasting glucose and HbA1c, to suppress the elevated glucagon level, and to stimulate glucose-dependent insulin synthesis and secretion (4).
Apart from the well-documented incretin effect of GLP-1, its role in the cardiovascular system also arouses interest. GLP-1 effects on the cardiovascular system may include a direct action on the endothelium, where the presence of specific receptors for GLP-1 has been demonstrated (6). GLP-1 has been demonstrated to improve endothelial function in diabetes (7). However, the explanation of why GLP-1 may have such a relevant physiologic role on cardiovascular system still remains unknown. A possible explanation would be to consider GLP-1 as an endogenous protective factor for the vascular system when this protection is especially needed: during a meal. As pointed out by Zilversmit (8) many years ago, atherosclerosis could be considered to be a prandial phenomenon. Therefore, it is clearly plausible that GLP-1, on the one hand, can help during a meal (glucose homeostasis, appetite control, fat metabolism), and on the other, can protect the endothelium against the possible damaging effect of the meal. This protective effect should be exerted improving the antioxidant defenses of the endothelium (9), thereby protecting the vascular system against the oxidative stress that increases after ingesting a meal (2).
The aim of this study is to prove that GLP-1 physiologically protects the endothelial function during a meal and, more specifically, protects the endothelial function from the hyperglycemia-induced alterations, and that this effect is mediated by lowering oxidative stress. Moreover, a further aim is to explore this aspect in diabetes.
RESEARCH DESIGN AND METHODS
Sixteen type 2 diabetic patients and 12 matched healthy control subjects participated in the study. Baseline characteristics of the study groups are shown in Table 1.
Table 1.
Baseline characteristics of the control and type 2 diabetic subjects
| Control subjects (n = 12) | Type 2 diabetic subjects (n = 16) | |
|---|---|---|
| Sex | 6M/6F | 9M/7F |
| Age, years | 50.5 ± 2.5 | 51.3 ± 2.6 |
| BMI, kg/m2 | 28.5 ± 3.1 | 29.5 ± 3.3 |
| Duration of diabetes, years | 5.5 ± 1.3 | |
| Fasting plasma glucose, mmol/L | 4.5 ± 0.3 | 7.8 ± 2.2* |
| HbA1c, % | 4.8 ± 0.2 | 8.4 ± 0.3* |
| Resting systolic blood pressure, mmHg | 117.3 ± 5.5 | 123.4 ± 6.4 |
| Resting diastolic blood pressure, mmHg | 77.5 ± 2.2 | 80.2 ± 3.6 |
| Total cholesterol, mmol/L | 4.5 ± 0.6 | 5.1 ± 0.8 |
| Triglycerides, mmol/L | 0.9 ± 0.2 | 1.2 ± 0.4 |
| HDL cholesterol, mmol/L | 1.4 ± 0.2 | 1.2 ± 0.3 |
| LDL cholesterol, mmol/L | 2.5 ± 0.3 | 2.6 ± 0.4 |
| FMD, % | 11.7 ± 0.7 | 5.9 ± 0.6* |
| Nitrotyrosine, μmol/L | 0.24 ± 0.05 | 0.52 ± 0.03* |
| 8-iso-PGF2a, pg/mL | 32.6 ± 4.6 | 65.0 ± 4.5* |
| Fasting insulin, pmol/L | 73.3 ± 4.4 | 107.3 ± 15.2* |
Data are expressed as means ± SE.
*P < 0.001 vs. control subjects.
The study was approved by the ethics committee of Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), and written consent from the study subjects was obtained.
Ten patients were on diet alone, and the other six patients were on metformin, which was discontinued at least 4 weeks before the study. None of the type 2 diabetic patients had retinopathy, nephropathy, or neuropathy. Five patients had hypertension treated with an angiotensin-converting enzyme inhibitor, which was withheld on the study days. None of the subjects were receiving statin or antioxidant supplementation.
Synthetic GLP-1 (7–36) amide was purchased from PolyPeptide Laboratories (Wolfenbuttel, Germany), and the same lot number was used in all studies.
Study design
Both healthy control subjects and type 2 diabetic patients underwent the following studies: a standard meal according to Vollmer et al. (10) and an oral glucose tolerance test (OGTT; 75 g glucose in 300 mL water) in randomized order, on different days. These tests were followed in a randomized order and on different days by two hyperglycemic clamps (11) with or without GLP-1. GLP-1 was infused at a rate aiming to have the same plasma concentration reached during the OGTT (0.4 pmol ⋅ kg−1 ⋅ min−1) according to Nauck et al. (12). During the hyperglycemic clamp, the level of glycemia was settled at the same level as that of mean glycemia reached at 1 h (control subjects 8.5 mmol/L; diabetic patients 15 mmol/L) and 2 h (control subjects 5 mmol/L; diabetic patients 12.8 mmol/L) during the OGTT.
At the end of these studies, diabetic patients were treated intensively with insulin for 2 months to improve glycemic control. The clamp studies were then repeated randomly with the same levels of glycemia and GLP-1 infusion rate.
At baseline and at 1 and 2 h, during the meal test and the OGTT, and during each clamp, glycemia, insulin, endothelial function (flow-mediated vasodilation [FMD]), plasma nitrotyrosine and plasma 8-iso prostaglandin F2α (8-iso-PGF2a) (both markers of oxidative stress), and GLP-1 (active 7–36) plasma levels were measured.
Biochemical measurements
Cholesterol and triglycerides were measured enzymatically (Roche Diagnostics, Basel, Switzerland). HDL cholesterol was estimated after the precipitation of apolipoprotein B with phosphotungstate/magnesium (13). LDL cholesterol was calculated after lipoprotein separation (13). Plasma glucose was measured by the glucose-oxidase method, HbA1c was measured by high-performance liquid chromatography, and insulin was measured by microparticle enzyme immunoassay (Abbott Laboratories, Wiesbaden, Germany). Nitrotyrosine plasma concentration was assayed by enzyme-linked immunosorbent assay, recently validated by our laboratory (13).
A commercially available kit was used to measure 8-iso-PGF2a (Cayman Chemical, Ann Arbor, MI). GLP-1 (active 7–36) was measured by a radioimmunoassay kit (Peninsula Laboratories, Belmont, CA). The detection limit is 10 pg/mL, and the intra- and interassay coefficient of variation are 8 and 18% at 50 pg/mL and 5 and 13% at 300 pg/mL, respectively.
Endothelial function
FMD was evaluated (14). At the end of each test, sublingual nitroglycerin (0.3 mg) was administered, and 3 min later the last measurements were performed to measure endothelium-independent vasodilation.
Statistical analysis
Data are expressed as mean ± SE. The sample size was selected according to previous studies (7,10). The Kolmogorov–Smirnov algorithm was used to determine whether each variable had a normal distribution. Comparisons of baseline data among the groups were performed using unpaired Student t test or Mann-Whitney U test, where indicated. The changes in variables during the tests were assessed by two-way ANOVA with repeated measures or Kolmogorov–Smirnov test, where indicated. If differences reached statistical significance, post hoc analyses with paired, two-tailed t test or Wilcoxon signed rank test for paired comparisons were used to assess differences at individual time periods in the study. Statistical significance was defined as P < 0.05.
RESULTS
As expected, basal glycemia, insulin, HbA1c, nitrotyrosine, and 8-iso-PGF2a were increased in diabetes, and FMD was decreased (Table 1). Basal, fasting level of GLP-1 was not different between control subjects and diabetic patients (Table 1).
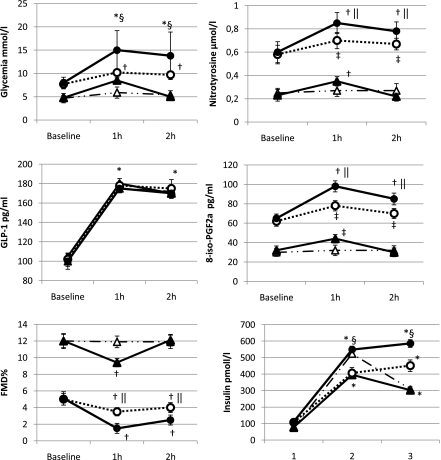
During the meal, all test parameters, except those for GLP-1 and insulin, remained unchanged in the control subjects, whereas a significant increase at 1 and 2 h of glycemia, insulin, nitrotyrosine, and 8-iso-PGF2a and a decrease in FMD were observed in type 2 diabetic patients compared with their basal values. In both control subjects and diabetic patients, GLP-1 increased at 1 and 2 h in a similar manner (Fig. 1).
Figure 1.
Changes of glycemia, GLP-1, FMD, plasma nitrotyrosine, 8-iso-PGF2a, and insulin during the meal and the OGTT in normal healthy control subjects and type 2 diabetic patients. Data are expressed as mean ± SE; △, meal test controls; ▲, OGTT controls; ○, meal test type 2 diabetes; ●, OGTT type 2 diabetes; *P < 0.001 vs. basal; †P < 0.01 vs. basal; ‡P < 0.05 vs. basal; §P < 0.01 vs. OGTT; ||P < 0.05 vs. OGTT.
In the control subjects, during the OGTT, an increase of glycemia, insulin, nitrotyrosine, and 8-iso-PGF2a and a decrease of FMD were observed at 1 h, whereas at 2 h all the parameters returned to the basal values (Fig. 1). In the diabetic patients, at both 1 and 2 h, a significant increase of glycemia, insulin, nitrotyrosine, and 8-iso-PGF2a and a decrease of FMD were observed compared with the basal values (Fig. 1). In both control subjects and diabetic patients, during the OGTT, GLP-1 increased at 1 and 2 h in a similar manner (Fig. 1), and the values were not different from those reached during the meal test (Fig. 1).
In diabetic patients, at both 1 and 2 h, a significant increase in glycemia (P < 0.01), insulin (P < 0.01), nitrotyrosine (P < 0.05), and 8-iso-PGF2a (P < 0.05), and a decrease in FMD (P < 0.05) were observed compared with the values reached during the OGTT, whereas no difference was found for GLP-1 (Fig. 1).
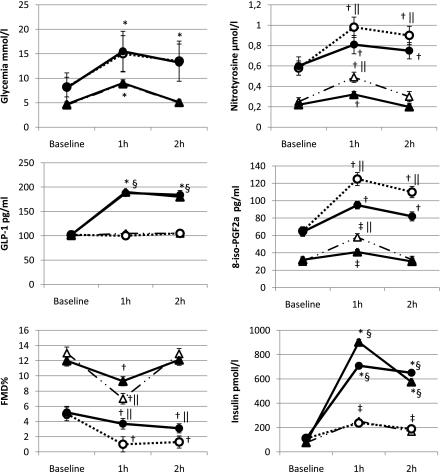
According to the values observed during the OGTT, during the clamps glycemia was maintained for the first hour at 8.5 mmol/L in control subjects and at 15 mmol/L 1 h in diabetic patients, whereas for the second hour glycemia was maintained at 5 mmol/L in control subjects and at 13 mmol/L in diabetic patients. During the clamps, performed with placebo, GLP-1 concentration remained unchanged during the study period in both control subjects and diabetic patients, whereas its concentration was almost equivalent to that observed during the meal test and the OGTT when it was constantly infused (Fig. 2). Insulin concentration increased in both control subjects and diabetic patients during the hyperglycemic clamp, and its increase was significantly higher during GLP-1 infusion (Fig. 2). During both the clamps, with or without GLP-1, in the control subjects, an increase in nitrotyrosine and 8-iso-PGF2a and a decrease in FMD were observed at 1 h, whereas at 2 h, all the parameters returned to their basal values (Fig. 2). Similarly, in diabetic patients, at both 1 and 2 h during both the clamps, a significant increase in nitrotyrosine and 8-iso-PGF2a and a decrease in FMD were observed compared with the basal values (Fig. 2). However, in the control subjects, at 1 h, the values of nitrotyrosine and 8-iso-PGF2a significantly increased, and the values of FMD significantly decreased in the clamp with placebo compared with the values observed during the clamp with GLP-1 (Fig. 2). Similarly, in diabetic patients at both 1 and 2 h, the values of nitrotyrosine and 8-iso-PGF2a significantly increased, and the values of FMD significantly decreased in the clamp with placebo compared with the values observed during the clamp with GLP-1 (Fig. 2).
Figure 2.
Changes of glycemia, GLP-1, FMD, plasma nitrotyrosine, 8-iso-PGF2a, and insulin during the hyperglycemic clamp with or without GLP-1 infusion in normal healthy control subjects and type 2 diabetic patients. Data are expressed as mean ± SE; △, hyperglycemic clamp + placebo control subjects; ▲, hyperglycemic clamp + GLP-1 control subjects; ○, hyperglycemic clamp + placebo type 2 diabetes; ●, hyperglycemic clamp + GLP-1 type 2 diabetes; *P < 0.001 vs. basal; †P < 0.01 vs. basal; ‡P < 0.05 vs. basal; §P < 0.01 vs. placebo; ||P < 0.05 vs. placebo.
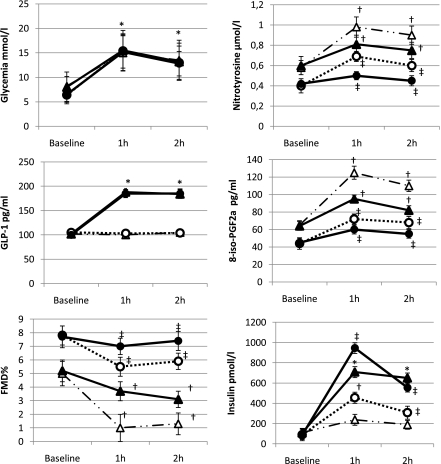
Two months of insulin treatment resulted in a significant decrease in HbA1c (8.4 ± 0.3 vs. 7.2 ± 0.4%, P < 0.01) and an improvement of fasting glycemia (8.2 ± 2.0 vs. 6.4 ± 1.8 mmol/L, P < 0.01), insulin (110.3 ± 17.2 vs. 86.2 ± 13.2 pmol/L, P < 0.01), nitrotyrosine (0.52 ± 0.03 vs. 0.39 ± 0.06 μmol/L, P < 0.05), 8-iso-PGF2a (65.0 ± 4.5 vs. 44.2 ± 2.5 pg/mL, P < 0.05), and FMD (5.9 ± 0.6 vs. 7.8 ± 0.7%, P < 0.05) in diabetic patients.
During the two clamps, in diabetic patients, at 1 and 2 h, a significant increase in nitrotyrosine and 8-iso-PGF2a and a decrease in FMD (P < 0.01) were observed compared with the basal values (Fig. 3). As before the improvement of glycemic control, at both 1 and 2 h, the values of nitrotyrosine and 8-iso-PGF2a significantly increased and the values of FMD significantly decreased in the clamp with placebo compared with the values observed during the clamp with GLP-1 (Fig. 3). However, the same values of glycemia were less effective in producing oxidative stress and endothelial dysfunction after 2 months of improved glycemic control. Because the basal values before and after tight glycemic control were significantly different, we have compared the Δ between the basal value and the value at 1 and 2 h, during each clamp, respectively: Δnitrotyrosine 1 h 0.42 ± 0.04 vs. 0.20 ± 0.05, P < 0.01; Δnitrotyrosine 2 h 0.32 ± 0.03 vs. 0.15 ± 0.05, P < 0.01; Δ 8-iso-PGF2a 1 h 68.9 ± 4.1 vs. 30.3 ± 3.2, P < 0.05; Δ 8-iso-PGF2a 2 h 50.5 ± 3.1 vs. 20.3 ± 1.2, P < 0.01; Δ FMD 1 h 4.1 ± 0.5 vs. 2.3 ± 0.2, FMD, P < 0.05; Δ FMD 2 h 3.7 ± 0.5 vs. 2.2 ± 0.2, FMD, P < 0.05 in the study performed with placebo, compared with that in the previous clamp. Of particular interest is that GLP-1 administration was most effective in this condition of improved metabolic control than in previous experiments: Δnitrotyrosine 1 h 0.21 ± 0.02 vs. 0.08 ± 0.02, P < 0.01; Δnitrotyrosine 2 h 0.15 ± 0.04 vs. 0.03 ± 0.02, P < 0.01; Δ 8-iso-PGF2a 1 h 31.5 ± 4.1 vs. 15.3 ± 3.7, P < 0.05; Δ 8-iso-PGF2a 2 h 20.3 ± 2.1 vs. 10.2 ± 1.5, P < 0.05; Δ FMD 1 h 1.5 ± 0.4 vs. 0.6 ± 0.2, FMD, P < 0.05; Δ FMD 2 h 2.1 ± 0.3 vs. 0.3 ± 0.2, FMD, P < 0.05 in the study performed with placebo, compared with the previous clamp. GLP-1 infusion after optimized glycemic control was accompanied by a significant increase in insulin secretion at both 1 and 2 h (Fig. 3). No difference was found in endothelium-independent vasodilatation in all the studies.
Figure 3.
Changes of glycemia, GLP-1, FMD, plasma nitrotyrosine, 8-iso-PGF2a, and insulin during the hyperglycemic clamp with or without GLP-1 infusion in normal healthy control subjects and type 2 diabetic patients at baseline and after 2 months of optimized glycemic control. For the comparisons between baseline and after 2 months of optimized glycemic control, see the results section. Data are expressed as mean ± SE; △, hyperglycemic clamp + placebo; ○, hyperglycemic clamp + placebo after 2 months of optimized glycemic control; ▲, hyperglycemic clamp + GLP-1; ●, hyperglycemic clamp + GLP-1 after 2 months of optimized glycemic control; *P < 0.001 vs. basal; †P < 0.01 vs. basal; ‡P < 0.05 vs. basal.
CONCLUSIONS
This study demonstrated that the presence of GLP-1 during hyperglycemia significantly protects endothelial function and decreases hyperglycemia-induced oxidative stress generation. This evidence clearly emerges when we compare the effect of hyperglycemia during the clamps in both normal and diabetic patients. In the absence of GLP-1, hyperglycemia induces endothelial dysfunction and oxidative stress, whereas the concomitant infusion of GLP-1 significantly prevents this effect. It has already been largely demonstrated that hyperglycemia induces endothelial dysfunction through the generation of an oxidative stress (2) and that GLP-1 can reduce oxidative stress (9). GLP-1 also improves endothelial dysfunction in diabetes (7). Therefore, our data suggest that GLP-1 may protect endothelia function during hyperglycemia, reducing oxidative stress generation.
Our data also support a possible physiologic protective role of GLP-1 on endothelial function. However, the effect of GLP-1 on endothelial function seems to be dependent on the level of hyperglycemia. The same plasma levels of GLP-1 have a different effect on the endothelial function and oxidative stress in normal subjects during the test meal and the OGTT. Consistent with a previous study, in normal control subjects the levels of GLP-1 are almost superimposable during the meal and the OGTT (9). However, during the meal test, when glycemia remains constantly in the normal range, endothelial function and oxidative stress remain unaltered. However, at 1 h, during the OGTT, as already reported (15), when hyperglycemia appears, endothelial function and oxidative stress also appear. These data suggest that in the presence of hyperglycemia, GLP-1 partly loses its protective effect on endothelial function and oxidative stress.
This finding is also supported by the data in diabetic patients. As previously reported (10), the plasma levels of GLP-1 were not different between the meal test and the OGTT. As expected, the levels of glycemia were significantly different between the meal test and the OGTT, and this was accompanied by a parallel worsening of both oxidative stress and endothelial function.
The possibility that hyperglycemia may condition the protective effects of GLP-1 on endothelial function and oxidative stress is also supported by the data with clamps in type 2 diabetic patients after a period of improved glycemic control.
As previously reported, improving glycemic control partly restored basal endothelial function and oxidative stress (16), as well as the response to the same level of glycemia during the clamp in terms of oxidative stress and endothelial function. However, the protective effect of infused GLP-1 was more pronounced in this situation of improved glycemic control compared with the previous study.
These data together support the hypothesis that the endothelium became less sensitive to GLP-1 in hyperglycemia, more than GLP-1 itself loses its activity.
This concept has already been proposed for insulin secretion, where evidence suggests that the impaired GLP-1 incretin effect is mostly related to an impairment of β-cell function than to an impairment of incretin secretion (5). Højberg et al. (17) reported that a near-normalization of blood glucose has no effect on postprandial GLP-1 secretion, but it augments β-cell responsiveness, similar to our data for endothelial function.
A possible direct influence of insulin concentration on our results cannot be excluded. Insulin by itself has antioxidant and vasodilatory effects (18), and GLP-1 infusion during any clamp was accompanied by a significant increase of insulin concentration compared with the placebo. However, it has been reported that acute hyperglycemia during a clamp, as in our case, blunts the vasodilatory effect of insulin (19). Moreover, at 2 h during the clamps the effect of GLP-1 on FMD was almost similar to that at 1 h; even at 2 h, insulin concentration was significantly reduced compared with 1 h (Fig. 3). In addition, insulin can induce an endothelial dysfunction (20,21), evidence that supports the possibility that GLP-1 has a direct beneficial effect on endothelial function.
In conclusion, our data suggest that during the meal, GLP-1 can simultaneously exert an incretin effect on insulin secretion and have a protective effect on the endothelial function, reasonably controlling oxidative stress generation. As for insulin secretion, the ability of GLP-1 in protecting endothelial function seems to be dependent on the level of glycemia (5). Hyperglycemia may induce at the endothelial level, as well as at the level of the β-cells, a resistance to the GLP-1 action.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.C. and K.E. researched data, wrote the article, and researched and reviewed the article. R.T. researched and reviewed the article. A.R.B. and M.M. reviewed the article. D.G. researched data, wrote the article, and researched and reviewed the article.
References
- 1.Drucker DJ. Minireview: the glucagon-like peptides. Endocrinology 2001;142:521–527 [DOI] [PubMed] [Google Scholar]
- 2.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005;54:1–7 [DOI] [PubMed] [Google Scholar]
- 3.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 1993;362:801–809 [DOI] [PubMed] [Google Scholar]
- 4.Peters A. Incretin-based therapies: review of current clinical trial data. Am J Med 2010;123(Suppl.):S28–S37 [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Nauck MA. Is the diminished incretin effect in type 2 diabetes just an epi-phenomenon of impaired beta-cell function? Diabetes 2010;59:1117–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mudaliar S, Henry RR. Effects of incretin hormones on beta-cell mass and function, body weight, and hepatic and myocardial function. Am J Med 2010;123(Suppl.):S19–S27 [DOI] [PubMed] [Google Scholar]
- 7.Nyström T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab 2004;287:E1209–E1215 [DOI] [PubMed] [Google Scholar]
- 8.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation 1979;60:473–485 [DOI] [PubMed] [Google Scholar]
- 9.Oeseburg H, de Boer RA, Buikema H, van der Harst P, van Gilst WH, Silljé HH. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler Thromb Vasc Biol 2010;30:1407–1414 [DOI] [PubMed] [Google Scholar]
- 10.Vollmer K, Holst JJ, Baller B, et al. Predictors of incretin concentrations in subjects with normal, impaired, and diabetic glucose tolerance. Diabetes 2008;57:678–687 [DOI] [PubMed] [Google Scholar]
- 11.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 1979;237:E214–E223 [DOI] [PubMed] [Google Scholar]
- 12.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest 1993;91:301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia 2001;44:834–838 [DOI] [PubMed] [Google Scholar]
- 14.Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265 [DOI] [PubMed] [Google Scholar]
- 15.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. J Am Coll Cardiol 1999;34:146–154 [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Kumar S, Piconi L, Esposito K, Giugliano D. Simultaneous control of hyperglycemia and oxidative stress normalizes endothelial function in type 1 diabetes. Diabetes Care 2007;30:649–654 [DOI] [PubMed] [Google Scholar]
- 17.Højberg PV, Vilsbøll T, Zander M, et al. Four weeks of near-normalization of blood glucose has no effect on postprandial GLP-1 and GIP secretion, but augments pancreatic B-cell responsiveness to a meal in patients with type 2 diabetes. Diabet Med 2008;25:1268–1275 [DOI] [PubMed] [Google Scholar]
- 18.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol 2009;53(Suppl.):S14–S20 [DOI] [PubMed] [Google Scholar]
- 19.Srinivasan M, Herrero P, McGill JB, et al. The effects of plasma insulin and glucose on myocardial blood flow in patients with type 1 diabetes mellitus. J Am Coll Cardiol 2005;46:42–48 [DOI] [PubMed] [Google Scholar]
- 20.Arcaro G, Cretti A, Balzano S, et al. Insulin causes endothelial dysfunction in humans: sites and mechanisms. Circulation 2002;105:576–582 [DOI] [PubMed] [Google Scholar]
- 21.Campia U, Sullivan G, Bryant MB, Waclawiw MA, Quon MJ, Panza JA. Insulin impairs endothelium-dependent vasodilation independent of insulin sensitivity or lipid profile. Am J Physiol Heart Circ Physiol 2004;286:H76–H82 [DOI] [PubMed] [Google Scholar]