Abstract
OBJECTIVE
Many guidelines recommend that patients with type 2 diabetes should aim to reduce their intake of salt. However, the precise relationship between dietary salt intake and mortality in patients with type 2 diabetes has not been previously explored.
RESEARCH DESIGN AND METHODS
Six hundred and thirty-eight patients attending a single diabetes clinic were followed in a prospective cohort study. Baseline sodium excretion was estimated from 24-h urinary collections (24hUNa). The predictors of all-cause and cardiovascular mortality were determined by Cox regression and competing risk modeling, respectively.
RESULTS
The mean baseline 24hUNa was 184 ± 73 mmol/24 h, which remained consistent throughout the follow-up (intraindividual coefficient of variation [CV] 23 ± 11%). Over a median of 9.9 years, there were 175 deaths, 75 (43%) of which were secondary to cardiovascular events. All-cause mortality was inversely associated with 24hUNa, after adjusting for other baseline risk factors (P < 0.001). For every 100 mmol rise in 24hUNa, all-cause mortality was 28% lower (95% CI 6–45%, P = 0.02). After adjusting for the competing risk of noncardiovascular death and other predictors, 24hUNa was also significantly associated with cardiovascular mortality (sub-hazard ratio 0.65 [95% CI 0.44–0.95]; P = 0.03).
CONCLUSIONS
In patients with type 2 diabetes, lower 24-h urinary sodium excretion was paradoxically associated with increased all-cause and cardiovascular mortality. Interventional studies are necessary to determine if dietary salt has a causative role in determining adverse outcomes in patients with type 2 diabetes and the appropriateness of guidelines advocating salt restriction in this setting.
In patients with type 2 diabetes, hypertension is associated with a range of adverse outcomes including cardiovascular disease (CVD) and premature mortality. Consequently, clinical guidelines recommend that patients with type 2 diabetes undertake measures to maintain a blood pressure at or below target levels. Among the interventions advocated to assist in achieving these targets, most guidelines recommend a reduced intake of salt, as dietary sodium intake is positively correlated with blood pressure levels in the general population (1). Moreover, in patients with type 2 diabetes, salt restriction confers a modest reduction in blood pressure (2), and salt supplementation reduces the antihypertensive efficacy of blood pressure–lowering agents in the short term (3). However, the precise relationship between salt intake and mortality in patients with type 2 diabetes has not been previously explored. It is widely assumed that any blood pressure lowering associated with reduced dietary salt intake may be translated into protection from end-organ damage in the context of diabetes. However, there is also evidence that reduced sodium intake is associated with activation of metabolic and neurohormonal pathways, including the sympathetic nervous system (4) and the renin-angiotensin-aldosterone system (RAAS) (4), as well as increases in total and LDL cholesterol (4) and reduced peripheral insulin sensitivity (5). In the context of type 2 diabetes, each of these factors may offset or even outweigh gains achieved from blood pressure lowering. Hence, in this study we explored the association between dietary salt intake, the best estimate of which is 24-h urine collection as ∼90% of dietary sodium intake is renally excreted (6), and all-cause and cardiovascular mortality in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Patient recruitment
This study was initiated in July 2000 as a prospective survey of patients with type 2 diabetes who were in long-term follow-up in a single diabetes clinic at Austin Health, Melbourne, Australia. Austin Health is a major university teaching hospital and a tertiary referral center, serving a population of approximately 700,000. Long-term follow-up was defined by the patients having at least three previous estimations of urinary albumin excretion rate (AER) performed on 24-h urine collections with at least one AER having been performed within the year 2000. Patients with type 1 diabetes or diabetes secondary to medication or pancreatitis were specifically excluded from the current study. Using these criteria, 665 patients with type 2 diabetes were eligible to participate in the survey. Informed consent was obtained from participating patients as approved by the Austin Health Human Research Ethics Committee.
Measurement of baseline characteristics
Baseline data collection was performed during routine clinical visits between January and December 2001. Baseline clinical and biochemical characteristics of all participants were ascertained including a full clinical history, medication use, anthropometric data, and smoking habits. The presence of preexisting CVD was defined on the basis of a clinical history of myocardial infarction, unstable angina requiring hospitalization, coronary revascularization (including coronary artery bypass grafting, angioplasty, or coronary stenting), heart failure, stroke, carotid artery surgery, peripheral revascularization (including bypass grafting, angioplasty, or stenting), and amputation for critical limb ischemia. Fasting blood samples were taken for glucose, HbA1c, lipid profiles, and creatinine. The glomerular filtration rate was estimated (eGFR) using the chronic kidney disease-epidemiology collaboration (CKD-EPI) formula.
All regularly attending patients at the Austin Health diabetes clinics perform a 24-h urine collection before each visit for the determination of urinary sodium, potassium, and albumin excretion using standard methods in the Department of Laboratory Medicine at Austin Health. Baseline levels for each participant were determined as the mean excretion rates across all urine samples performed during 2001. Data on 24hUNa were available in 638 participants (96%) who formed the study cohort (Supplementary Figure 1). The clinical characteristics of these patients were not different from those in whom 24hUNa was not measured.
Patients were given general dietary advice as part of their routine care at an initial assessment by a dietitian. However detailed assessment of dietary salt intake was not performed. During follow-up, all patients continued to have standard medical care including antihypertensive, lipid-lowering, and antidiabetic medications according to recommended guidelines.
Mortality outcomes
The primary outcome was death from any cause until the 31 March 2010. Timing and cause of death were identified from a search of hospital patients’ records and the Australian Institute of Health and Welfare Death Registry. To evaluate the independent predictors of all-cause mortality in patients with type 2 diabetes, we used Cox proportional hazards models. The final model variables were determined by sequential penalized likelihood (Akaike information criterion) (7). The functional form (in particular, nonlinearity) of continuous variables in the final model was explored by both fractional polynomials and restricted cubic splines (7). Categorical variables were parameterized as simple indicator variables; the potential for multicollinearity was assessed using the variance inflation factor and condition number. Overall Cox model fit was assessed by approximation of cumulative Cox-Snell residuals to (-log) Kaplan-Meier estimates, residual plots, and testing of the proportional hazards assumption and goodness-of-fit test (8). The cumulative hazard of all-cause mortality was graphically displayed using Nelson-Aalen estimates stratified by percentiles of covariate(s) of interest (9).
To identify the independent predictors of the cumulative incidence of cardiovascular mortality, we used the Fine and Gray model, which extends the Cox proportional hazards model to competing risks data (10) by considering the subdistribution hazard while adjusting for the competing risk of noncardiovascular deaths. The strength of the association between each variable and the outcome was assessed using the sub-hazard ratio, which is the ratio of hazards associated with the cumulative incidence function in the presence and absence of a prognostic factor. The Fine and Gray model was implemented in Stata statistical software (V11, 2009; College Station, TX) using the stcrreg module.
RESULTS
Cohort characteristics
The mean age of participants was 64 years, 56% were men, the median duration of diabetes was 11 years, and 47% were obese (BMI >30 kg/m2). Complications of diabetes were common with 45% of participants having previously experienced a cardiovascular event, including 35% who had a myocardial infarction. One-third had background retinopathy on ophthalmological review (33%), 30% had an eGFR <60 mL/min/1.73 m2, and 45% had elevated urinary albumin excretion (>20 μg/min), including 13% with macroalbuminuria (>200 μg/min). Eighty-five percent of patients had hypertension (defined by the use of antihypertensives and/or blood pressure >140/90). Achievement of therapeutic targets was low, reflecting the tertiary referral nature of the clinic, with two-thirds of patients having an HbA1c >7.0%, 55% with an LDL cholesterol level >2.5 mM, and 44% with a systolic blood pressure >140 mmHg.
24-h urinary sodium excretion
Baseline urinary sodium excretion was estimated in 638 patients from a median of two collections performed during the year 2001 (range 1–5). The mean urinary sodium excretion was 184 mmol/24 h, similar to previous global population surveys (11). One-third of participants had a sodium excretion <150 mmol/24 h (lowest tertile), and one-third had an excretion >208 mmol/24 h (highest tertile) with the middle tertile falling between 150 and 208 mmol/24 h. Patients with urinary sodium excretion in the highest tertile were younger, with a shorter duration of diabetes, more likely to be obese, but with better renal function, and hemoglobin, when compared with patients in tertile 2 (Table 1). Sex was also a strong predictor of sodium excretion, consistent with the higher sodium intake described in men in global population surveys (11). On multivariate analysis, 24hUNa (as a continuous variable) was positively correlated with eGFR, BMI, male sex, and the use of ACE inhibitors and was negatively correlated with age (full regression model [Supplementary Table 1]). The presence or absence of heart failure, atrial fibrillation, and the use of diuretics or β-blockers was not associated with 24hUNa after adjusting for age and sex.
Table 1.
Baseline characteristics of patients with type 2 diabetes, stratified according to tertiles (T) of 24-h urinary sodium excretion
| Baseline parameter | T1 |
T2 |
T3 |
|---|---|---|---|
| <150 mmol/24 h | 150–208 mmol/24 h | >208 mmol/24 h | |
| Age (years) | 67 ± 12* | 64 ± 11 | 61 ± 12* |
| Sex (% male) | 42* | 56 | 70* |
| Diabetes duration (years) | 14 ± 9* | 12 ± 8 | 11 ± 8 |
| Obese (BMI >30 kg/m2) (%) | 41 | 45 | 55* |
| Macrovascular disease (%) | 49 | 43 | 44 |
| Coronary heart disease (%) | 34 | 32 | 37 |
| Atrial fibrillation (%) | 20 | 20 | 12* |
| Congestive cardiac failure (%) | 17 | 11 | 15 |
| C-reactive protein (geometric mean; IU/mL) | 2.4 | 2.1 | 2.6 |
| Systolic blood pressure (mmHg) | 141 ± 17 | 140 ± 17 | 140 ± 16 |
| Diastolic blood pressure (mmHg) | 77 ± 10* | 80 ± 9 | 78 ± 10* |
| Antihypertensive therapy (%) | 78 | 78 | 76 |
| ACE inhibitor (%) | 45* | 56 | 57 |
| Angiotensin receptor blocker (%) | 13 | 13 | 10 |
| Diuretic (%) | 38 | 32 | 42 |
| β-Blocker (%) | 23* | 15 | 20 |
| Calcium channel blocker (%) | 34 | 28 | 34 |
| α-Blocker (%) | 5 | 5 | 5 |
| HbA1c (%) | 7.8 ± 1.7 | 7.8 ± 1.3 | 7.7 ± 1.5 |
| Fasting plasma glucose (mmol/L) | 9.6 ± 4.0 | 9.3 ± 3.2 | 9.3 ± 3.7 |
| Metformin (%) | 50 | 57 | 58 |
| Sulfonylurea (%) | 37* | 51 | 40* |
| Insulin (%) | 49%* | 36% | 39% |
| LDL cholesterol (mmol/L) | 2.6 ± 0.8 | 2.7 ± 0.8 | 2.6 ± 0.8 |
| Triglycerides (mmol/L) | 2.0 ± 1.8 | 2.0 ± 1.4 | 2.2 ± 1.6 |
| HDL cholesterol (mmol/L) | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.1 ± 0.3 |
| Statin (%) | 53 | 52 | 49 |
| eGFR (mL/min/1.73 m2) | 67 ± 26* | 73 ± 25 | 78 ± 24* |
| Albuminuria (micro and macro, %) | 42 | 42 | 50 |
| Hemoglobin (g/dL) | 13.2 ± 1.5 | 13.4 ± 1.5 | 13.7 ± 1.5* |
Data are mean ± SD unless otherwise indicated.
*P < 0.01 vs. middle tertile (T2).
Variability in sodium excretion
Baseline 24hUNa excretion was strongly correlated with the yearly mean 24hUNa during follow-up. Over two-thirds of individuals with 24hUNa in the highest tertile at baseline remained in the highest tertile in subsequent years. Similarly, over two-thirds of individuals with 24hUNa in the lowest tertile remained in the lowest tertile in subsequent years (data not shown). Less than 5% of individuals changed from the lowest to the highest tertile or the highest to the lowest tertile on an annual basis. Overall, the intraindividual CV of mean annual 24hUNa was 23 ± 11% over 8 years.
Mortality and 24-h urinary sodium excretion
Vital status was determined in 620 patients with the remaining 18 (3%) lost to follow-up. The median follow-up for survival analysis was 9.9 years (5,475 patient-years) during which 175 deaths were recorded (28%). CVD was listed as a major contributing cause in 75 deaths (43% of all deaths) with the other major causes of death including cancer (n = 36) and infection (n = 19). In 17 patients, although death was verified, no cause of death could be ascertained.
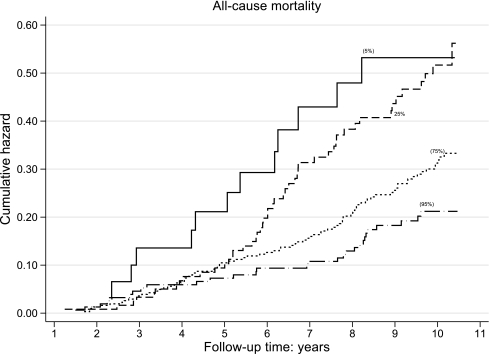
All-cause mortality was inversely associated with 24hUNa such that individuals with the lowest 24hUNa had the highest cumulative hazard for mortality (Fig. 1). After adjusting for parameters associated with 24hUNa, as well as other factors independently associated with all-cause mortality (including age, sex, duration of diabetes, atrial fibrillation, the presence and severity of CKD (eGFR and log AER) for every 100 mmol rise in 24hUNa, all-cause mortality was 28% lower (95% CI 6–45%, P = 0.02) (Table 2). Overall, 24hUNa explained 7% of the variability (R2 = 0.07, 95% CI 0.02–0.14) in all-cause mortality in this cohort. No significant interactions between categorical variables or between categorical and continuous variables were demonstrated in the model implying the relative hazard associated with sodium intake was uniform regardless of the presence and severity of hypertension, CVD, CKD, and other risk factors. Moreover, its effect was stronger than for other reversible risk factors including LDL cholesterol and HbA1c, and was equivalent to that of systolic blood pressure (Table 2). Interestingly, blood pressure levels were inversely correlated with all-cause mortality with no convincing evidence of nonlinear effects including J-curve associations.
Figure 1.
Cumulative hazard (Nelson-Aalen) of all-cause mortality, stratified by percentiles (5th, 25th, 75th, and 95th) of 24-h urinary sodium excretion. All-cause mortality was inversely associated with 24-h urinary sodium excretion.
Table 2.
Independent associations with all-cause mortality and cumulative incidence of cardiovascular mortality in individuals with type 2 diabetes
| All-cause mortality | |||
|---|---|---|---|
| Baseline parameter | Hazard ratio | P | 95% CI |
| 24-h urinary sodium excretion (per 100 mmol/day) | 0.72 | 0.017 | 0.55–0.94 |
| Age (per decade) | 1.05 | <0.001 | 1.03–1.07 |
| Male sex (yes/no) | 1.51 | 0.013 | 1.09–2.09 |
| Pre-existing CVD (yes/no) | 1.85 | 0.001 | 1.30–2.64 |
| eGFR (per 10 mL/min/1.73 m2) | 0.988 | 0.002 | 0.980–0.996 |
| Atrial fibrillation (yes/no) | 1.97 | <0.001 | 1.39–2.81 |
| Log10 AER | 1.71 | <0.001 | 1.38–2.12 |
| Systolic blood pressure (mmHg) | 0.986 | 0.015 | 0.974–0.997 |
| Diabetes duration (decades) |
1.02 |
0.010 |
1.01–1.04 |
| Cardiovascular mortality | |||
| Baseline parameter |
Sub-hazard ratio |
P |
95% CI |
| 24-h urinary sodium excretion (per 100 mmol/day) | 0.65 | 0.026 | 0.44–0.95 |
| Male sex (yes/no) | 1.93 | 0.011 | 1.17–3.20 |
| Pre-existing CVD (yes/no) | 1.88 | 0.014 | 1.14–3.11 |
| eGFR (per 10 mL/min/1.73 m2) | 0.985 | 0.001 | 0.98–0.99 |
| Atrial fibrillation (yes/no) | 2.78 | <0.001 | 1.71–4.53 |
| Log10 AER | 1.76 | <0.001 | 1.28–2.42 |
| Systolic blood pressure (mmHg) | 0.97 | <0.001 | 0.96–0.99 |
| Diabetes duration (decades) | 1.05 | <0.001 | 1.02–1.08 |
All-cause mortality: independent associations with all-cause mortality in individuals with type 2 diabetes in a multivariate Cox model. The model explained 52% of the variation in all-cause mortality (95% CI 0.42– 0.64) and was well specified (Harrell’s C: 0.79; PH test: P = 0.136; goodness-of-fit test: P ≥ 0.37). PH, proportional hazard. Cardiovascular mortality: independent associations with the cumulative incidence of cardiovascular mortality in individuals with type 2 diabetes in the Fine and Gray (proportional hazards) model after accounting for the competing risk of noncardiovascular death.
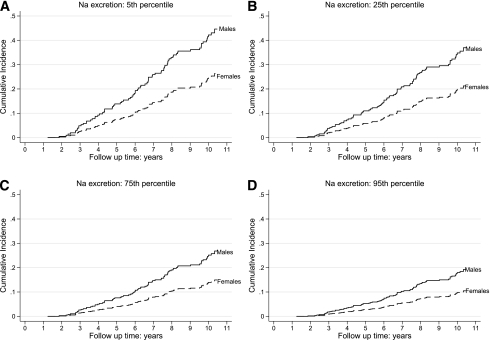
Cardiovascular mortality was also significantly associated with 24hUNa (Fig. 2) after adjusting for the competing risk of noncardiovascular death and other predictors (Table 2). No association was observed between 24hUNa and noncardiac deaths (data not shown). When modeled as a continuous variable, the association between 24hUNa and all-cause and cardiovascular mortality was linear. No convincing evidence of nonlinear effects, including J-shaped associations, was demonstrated as judged by residual-by-time analysis, fractional polynomials, and (cubic) regression splines (7). Again, no significant interactions between categorical variables or between categorical and continuous variables were demonstrated, implying the relationship between 24hUNa and mortality was independent of BMI, age, sex, and the type of antihypertensive therapy or achieved level of blood pressure control. Furthermore, the association between 24hUNa and mortality was independent of 24-h potassium excretion (data not shown).
Figure 2.
The cumulative incidence (Fine and Gray) of cardiovascular mortality over the 5th, 25th, 75th, and 95th percentile (A–D, respectively) of 24-h urinary sodium excretion in men and women (solid line and dotted line, respectively), adjusted for other covariate predictors (Table 2) and accounting for noncardiovascular mortality as the competing risk. The other predictors are set at: eGFR = 76.6 mL/min/1.73 m2 (median); atrial fibrillation = yes; preexisting cardiovascular disease = yes; Log10 AER = 1.2 (median); systolic blood pressure = 140 mmHg (mean); diabetes duration = 10.4 years (median).
CONCLUSIONS
Advice to reduce dietary salt intake is a key element of many lifestyle intervention programs for type 2 diabetes. However, the association between dietary salt intake and mortality outcomes has not been previously studied specifically in the context of type 2 diabetes. In this article, we show that 24hUNa was independently associated with all-cause and cardiovascular mortality in patients with type 2 diabetes such that the highest mortality risks were observed in individuals with the lowest sodium intake and vice versa.
Although such data may seem contrary to established dogma, in fact previous observational studies, both in the general population and in hypertensive patients, have failed to give consistent results regarding the association of salt and mortality. In some studies, higher salt intake has been associated with an increased risk of cardiovascular events (12–15), while in others—including the National Health and Nutrition Examination Surveys (NHANES) I, II, and III—lower salt intake has been associated with an increased risk of cardiovascular events and or mortality (16,17). Other studies in the general community have failed to demonstrate any association of dietary salt intake with cardiovascular events (18–20). Although hypertension and CVD are common in patients with type 2 diabetes, the issue of whether dietary salt intake influences mortality and morbidity has not been studied specifically in the context of type 2 diabetes, and any inference from outcomes in other populations should be viewed with caution.
The major strength of the current study is the use of multiple 24-h urine collections to estimate dietary sodium intake. With few exceptions (13,16), the majority of previous studies that have examined the association between salt and mortality, including NHANES (17), have relied on dietary recall, which can underestimate dietary salt intake by up to half (21). All patients were trained in urine collection and were experienced in performing 24-h urine collections at the start of the study. Moreover, in this population, the annual intraindividual CV of 24hUNa was low (23% over an 8-year period). In addition, the urinary sodium excretion observed was very similar to previous global population surveys (11), confirming that the patients were not sodium-restricted, which may otherwise confound analysis by indication.
The chief limitations of our data are that they are context-specific. The findings of our study specifically detail outcomes in a high-risk subpopulation of patients with longstanding type 2 diabetes attending a tertiary referral center. Nonetheless, these are precisely the patients in whom more aggressive lifestyle interventions are often applied. Our patients were mostly obese, hypertensive, with a history of poor metabolic control, and multiple comorbidities, including a significant burden of renal impairment and preexisting CVD at baseline. In all patients, cardiac risk factors were treated aggressively. It is possible that in this clinical setting, nontraditional risk factors have a stronger relationship with outcomes or that paradoxical associations are observed (22). For example, most of our patients were hypertensive despite treatment, and it has been previously shown in treated hypertensive men that low 24hUNa is associated with an increased risk of cardiovascular morbidity and mortality (16) and that tight blood pressure control in diabetic patients is associated with increased all-cause mortality (23). However, in our study, the relationship between urinary sodium excretion and mortality was independent of the presence and severity of hypertension.
In our statistical analysis, we specifically assessed the nonlinear effects of predictive variables modeled for potential interactions and, in the case of cardiovascular mortality, modeled within the paradigm of a formal competing risks (Fine and Gray) model, which estimated the cumulative incidence of cardiovascular deaths while taking into consideration the competing risk of other causes of death, which may otherwise confound results (24). This strategy is especially important in patients with diabetes, as diabetes is also associated with increased noncardiovascular mortality (25), which may potentially confound cause-specific analysis. Although such strategies have a number of advantages, it is nevertheless possible that associations demonstrated in this study may be because of confounding by unmeasured factors or those that are difficult to quantify. Variability in dietary sodium intake may be associated with a range of differences in diet composition, processing, and preparation that may themselves impact on adverse outcomes in diabetic individuals. For example, sodium intake is dominated by sodium added in manufactured foods in Western diets, (∼75% of intake) (11). In addition, we cannot exclude the possibility that salt appetite and cardiovascular risk are linked to a common unidentified etiological factor.
Any pathophysiological mechanisms that may underlie our observed association between mortality and salt intake in patients with type 2 diabetes remain to be established. Certainly, RAAS activity (4), insulin resistance (5), catecholamine levels (4), and lipids (4) may be influenced by sodium intake, and each of these potential mediators may be of particular relevance in the setting of type 2 diabetes and/or established atherosclerosis. For example, dietary sodium restriction leads to increased levels of angiotensin II and aldosterone, chiefly via an increase in plasma renin activity. Given the primacy of RAAS in the development and progression of diabetes complications, as adjudged by the efficacy of RAAS blockade, it is perhaps not surprising that activation of RAAS by reducing sodium intake may also be associated with adverse outcomes. The same may also be said for increased sympathetic activity, insulin resistance, and dyslipidemia associated with sodium restriction.
In summary, we show that 24hUNa, the best marker of dietary sodium intake, was negatively associated with all-cause mortality specifically in the setting of type 2 diabetes, after adjusting for baseline risk factors. This may reflect the special status of dietary salt and the pathways it regulates in diabetic pathophysiology. Such data call into question universal recommendations that all adults should endeavor to reduce their salt intake (12). Ultimately, the explanation for our findings needs to be tested experimentally in clinical trials performed specifically in diabetic patients.
Supplementary Material
Acknowledgments
E.I.E. was supported by a National Health and Medical Research Council (NHMRC) medical postgraduate scholarship and by the Austin Hospital Medical Research Fund (AHMRF). E.I.E. and S.C. were both recipients of the Cardiovascular Lipid Research Grant from Pfizer for unconditional use of funding for research projects. S.C. was supported by AHMRF during data collection. M.C.T. is supported by the KHA Bootle bequest and a NHMRC senior research fellowship.
No other potential conflicts of interest relevant to this article were reported.
E.I.E., S.C., M.C.T., R.J.M., and G.J. designed the study. E.I.E. wrote the manuscript and researched data. S.C. researched data. M.C.T. researched data, performed statistics, and edited the manuscript. J.L.M. performed statistics and edited the manuscript. K.C. researched data. R.J.M. and G.J. edited the manuscript.
Parts of this study were presented in abstract and poster form at the 70th Scientific Sessions of the American Diabetes Association, Orlando, Florida, 25–29 June 2010.
The authors thank the Australian Institute of Health and Welfare for assistance during data collection for this project.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1723/-/DC1.
References
- 1.Elliott P, Stamler J, Nichols R, et al. Intersalt Cooperative Research Group Intersalt revisited: further analyses of 24 hour sodium excretion and blood pressure within and across populations. BMJ 1996;312:1249–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houlihan CA, Allen TJ, Baxter AL, et al. A low-sodium diet potentiates the effects of losartan in type 2 diabetes. Diabetes Care 2002;25:663–671 10.2337/diacare.25.4.663 [DOI] [PubMed] [Google Scholar]
- 3.Ekinci EI, Thomas G, MacIsaac RJ, et al. Salt supplementation blunts the blood pressure response to telmisartan with or without hydrochlorothiazide in hypertensive patients with type 2 diabetes. Diabetologia 2010;53:1295–1303 10.1007/s00125-010-1711-2 [DOI] [PubMed] [Google Scholar]
- 4.Graudal NA, Galløe AM, Garred P. Effects of sodium restriction on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride: a meta-analysis. JAMA 1998;279:1383–1391 10.1001/jama.279.17.1383 [DOI] [PubMed] [Google Scholar]
- 5.Petrie JR, Morris AD, Minamisawa K, et al. Dietary sodium restriction impairs insulin sensitivity in noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 1998;83:1552–1557 10.1210/jc.83.5.1552 [DOI] [PubMed] [Google Scholar]
- 6.Holbrook JT, Patterson KY, Bodner JE, et al. Sodium and potassium intake and balance in adults consuming self-selected diets. Am J Clin Nutr 1984;40:786–793 [DOI] [PubMed] [Google Scholar]
- 7.Royston P, Sauerbrei W. Multivariable Model-Building: A Pragmatic Approach to Regression Analysis Based on Fractional Polynomials for Modelling Continuous Variables. Chichester, West Sussex, John Wiley and Sons Ltd., 2008 [Google Scholar]
- 8.Therneau TM, Grambsh PM. Modelling Survival Data: Extending the Cox Model. New York, Springer-Verlag, 2000 [Google Scholar]
- 9.Moeschberger JPKML. Survival Analysis: Techniques for Censored and Truncated Data. Moeschberger JPKML, Ed. New York, Springer-Verlag, 2003 [Google Scholar]
- 10.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509 10.2307/2670170 [DOI] [Google Scholar]
- 11.Brown IJ, Tzoulaki I, Candeias V, Elliott P. Salt intakes around the world: implications for public health. Int J Epidemiol 2009;38:791–813 10.1093/ije/dyp139 [DOI] [PubMed] [Google Scholar]
- 12.He FJ, MacGregor GA. How far should salt intake be reduced? Hypertension 2003;42:1093–1099 10.1161/01.HYP.0000102864.05174.E8 [DOI] [PubMed] [Google Scholar]
- 13.Tuomilehto J, Jousilahti P, Rastenyte D, et al. Urinary sodium excretion and cardiovascular mortality in Finland: a prospective study. Lancet 2001;357:848–851 10.1016/S0140-6736(00)04199-4 [DOI] [PubMed] [Google Scholar]
- 14.Cook NR, Cutler JA, Obarzanek E, et al. Long term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the trials of hypertension prevention (TOHP). BMJ 2007;334:885–888 10.1136/bmj.39147.604896.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umesawa M, Iso H, Date C, et al. JACC Study Group Relations between dietary sodium and potassium intakes and mortality from cardiovascular disease: the Japan Collaborative Cohort Study for Evaluation of Cancer Risks. Am J Clin Nutr 2008;88:195–202 [DOI] [PubMed] [Google Scholar]
- 16.Alderman MH, Madhavan S, Cohen H, Sealey JE, Laragh JH. Low urinary sodium is associated with greater risk of myocardial infarction among treated hypertensive men. Hypertension 1995;25:1144–1152 [DOI] [PubMed] [Google Scholar]
- 17.Cohen HW, Hailpern SM, Alderman MH. Sodium intake and mortality follow-up in the Third National Health and Nutrition Examination Survey (NHANES III). J Gen Intern Med 2008;23:1297–1302 10.1007/s11606-008-0645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tunstall-Pedoe H, Woodward M, Tavendale R, A’Brook R, McCluskey MK. Comparison of the prediction by 27 different factors of coronary heart disease and death in men and women of the Scottish Heart Health Study: cohort study. BMJ 1997;315:722–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kagan A, Popper JS, Rhoads GG, Yano K. Dietary and other risk factors for stroke in Hawaiian Japanese men. Stroke 1985;16:390–396 [DOI] [PubMed] [Google Scholar]
- 20.Geleijnse JM, Witteman JC, Stijnen T, Kloos MW, Hofman A, Grobbee DE. Sodium and potassium intake and risk of cardiovascular events and all-cause mortality: the Rotterdam Study. Eur J Epidemiol 2007;22:763–770 10.1007/s10654-007-9186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiba A, Vald A, Peleg E, Shamiss A, Grossman E. Does dietary recall adequately assess sodium, potassium, and calcium intake in hypertensive patients? Nutrition 2005;21:462–466 10.1016/j.nut.2004.08.021 [DOI] [PubMed] [Google Scholar]
- 22.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int 2003;63:793–808 10.1046/j.1523-1755.2003.00803.x [DOI] [PubMed] [Google Scholar]
- 23.Cooper-DeHoff RM, Gong Y, Handberg EM, Bavry AA, Denardo SJ, Bakris GL, Pepine CJ. Tight blood pressure control and cardiovascular outcomes among hypertensive patients with diabetes and coronary artery disease. JAMA 2010;304:61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pintilie M. Competing Risks: A Practical Perspective. Chichester, UK, John Wiley & Sons Ltd., 2006 [Google Scholar]
- 25.Barr EL, Zimmet PZ, Welborn TA, et al. Risk of cardiovascular and all-cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation 2007;116:151–157 10.1161/CIRCULATIONAHA.106.685628 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.