Abstract
OBJECTIVE
The pathogenesis of painful diabetic neuropathy (DN) remains undetermined, with both central and peripheral mechanisms implicated. This study investigates whether thalamic perfusion abnormalities occur in painful DN.
RESEARCH DESIGN AND METHODS
Eighteen subjects with type 1 diabetes (no DN = 6, painful DN = 5, painless DN = 7) and six healthy volunteers (HV) were recruited. Microvascular perfusion characteristics (relative cerebral blood volume [rCBV], flow [rCBF], and transit time [ttFM]) of the thalamus and caudate nucleus were assessed using magnetic resonance perfusion imaging. The caudate nucleus was chosen to serve as an in vivo control region.
RESULTS
Subjects with painful DN had significantly greater thalamic rCBV (means [SD]; painful DN, 228.7 [19.5]; no DN, 202.3 [25.8]; painless DN, 216.5 [65.5]; HV, 181.9 [51.7]; P = 0.04) and the longest ttFM(s) (painful DN, 38.4 [3.6]; no DN, 35.3 [13.2]; painless DN, 35.9 [13.7]; HV, 33.7 [14.9]; P = 0.07). There was no significant difference in markers of caudate nucleus perfusion.
CONCLUSIONS
Painful DN is associated with increased thalamic vascularity. This may provide an important clue to the pathogenesis of pain in DN.
Diabetic neuropathy (DN) results in chronic painful symptoms that can affect quality of life immensely (1). We previously reported thalamic neuronal dysfunction in subjects with painless DN but not painful DN (2). The aim of this study was to assess thalamic microvascular perfusion characteristics in DN.
RESEARCH DESIGN AND METHODS
Eighteen right-handed men with type 1 diabetes were recruited. Exclusion criteria were as follows: clinically significant systemic diseases, alcohol consumption (>20 units/day), neuropathies other than DN, hypoglycemia in the preceding 24 h, and standard magnetic resonance (MR) exclusion criteria. Medications that could alter cerebrovascular perfusion were omitted. Subjects with painful DN (NTSS 6 Score >4 and <16) (3) had symptoms for at least 6 months and were on stable pain medications. Six age- and sex-matched nondiabetic healthy volunteers (HV) were also recruited. All subjects gave written informed consent, and the study had ethical approval.
Assessment of neuropathy
Clinical and neurophysiological assessments were undertaken (4,5,6) to provide a neuropathy composite score (NCS) based on the neuropathy impairment score of the lower limbs plus seven tests (NIS[LL]+7) as described previously (7). Subjects were divided into the following groups: 1) no DN (asymptomatic subjects with normal NCS, 2) painless DN (pain-free subjects with both clinical and at least two abnormalities of neurophysiologic assessment), and 3) painful DN (painful symptoms together with clinical and neurophysiological abnormalities).
MR perfusion protocol
Examinations were performed on a 1.5-T system (Eclipse, Philips Medical Systems). Cerebral perfusion was assessed using a multitime point, single shot T2* weighted echo-planar imaging (EPI) sequence (TEeff = 60 ms; TR = 1.4 s; acquisition matrix = 192 × 188, zero-filled before Fourier transformation to 256 × 256; field of view (FOV) = 25 cm). Exogenous perfusion contrast was provided by a 20-mL bolus of gadolinium diethylenetriamine pentaacetic acid (Gd-DTPA; Magnevist, Schering AG, Germany), which was followed by a 20-mL saline flush, administered intravenously at a rate of 5 mL/s.
The microvascular perfusion characteristics of the thalamus (plays a central role in modulating/processing nociceptive information) (8) and the caudate nucleus were assessed. The caudate nucleus was chosen as a control region since it is not involved in somotosensory perception (9).
The following hemodynamic markers of cerebral perfusion were calculated: 1) relative cerebral blood volume (rCBV), the volume of blood per unit time passing through a region of brain tissue relative proximal internal carotid artery flow; 2) first moment transit time (TTFM, in seconds), the average time for contrast bolus to pass through a region of brain tissue; and 3) relative cerebral blood flow (rCBF), the average volume of blood per unit time (rCBV:TTFM) (10).
Statistical analysis
Analyses were performed using SPSS 14.0. Appropriate tests for normality were conducted to guide subsequent analysis. Subgroup demographics were compared using one-way ANOVA and perfusion markers using nonparametric tests.
RESULTS
Subjects with painful DN (62.0 [3.9]) were significantly older than those with no DN (44.9 [7.1]) and HV (45.8 [14.7]; P = 0.03; painful DN vs. no DN, P = 0.005, 95% CI 5.7–28.5; painful DN vs. HV, P = 0.01, 95% CI 3.8–28.5). Subjects were matched for BMI (HV 26.7 [5.2], no DN 30.2 [3.9], painless DN 25.6 [2.3], painful DN 31.1 [5.1]; P = 0.08) and HbA1c (no DN 8.4 [0.2]), painless DN 8.9 [0.9], and painful DN 7.7 [0.9]; P = 0.71). Subjects with painful DN (NCS 31.0 [9.5]) and painless DN (21.8 [15.5]) had comparable severity of neuropathy, which were greater than those with no DN (1.0 [1.1]). There was no difference in the presence of microvascular complications (diabetic retinopathy data from retinal screening database; painful DN [n = 3], painless DN [n = 2], no DN [n = 2], and diabetic nephropathy based on albumin:creatinine ratio; painful DN [n = 3], painless DN [n = 3], no DN [n = 1]) between subjects.
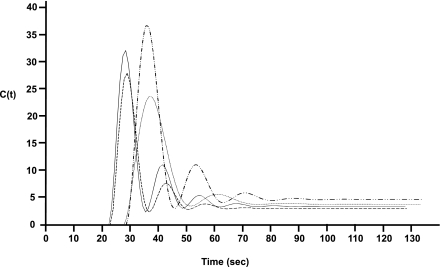
Figure 1 is a composite time profile of thalamic perfusion of the study groups. The bolus arrival time (in seconds) was delayed in both neuropathy subgroups (painful DN 28.6 [1.6] and painless DN 27.3 [2.4]) compared with HV 23.6 (6.3) and no DN 24.2 (5.9), P = 0.7, χ2 = 1.3. Overall group comparison showed that subjects with painful DN (rCBV 228.7 [19.5]) have the tallest peak concentration of Gd-DTPA and significantly greater mean thalamic rCBV compared with HV (181.9 [51.7]), no DN (202.3 [25.8]), and painless DN (216.5 [65.5]); P = 0.04, χ2 = 8.3). Subjects with painful DN (TTFM 38.4 [3.6]) had the longest thalamic TTFM (in seconds) compared with the other study groups (HV 33.7 [14.9]), no DN 35.3 [13.2], painless DN 35.9 [13.7]; P = 0.07, χ2 = 6.9). Caudate nucleus perfusion markers were not significantly different between groups.
Figure 1.
Composite concentration time profiles of the bolus passage of exogenous contrast agent (Gd-DTPA) though the thalamus in each subgroup: HV, no DN, painless DN, and painful DN.
CONCLUSIONS
Painful DN is the most distressing complication of diabetes (11), but unfortunately current treatments are often ineffective (12). This may be as a result of our poor understanding of the pathophysiological processes involved (13). Using established MR perfusion techniques, we demonstrated increased thalamic vascularity (increased rCBV) with sluggish flow (prolonged TTFM) in painful DN, possibly reflecting underlying vasodilatation. Delay in bolus arrival time in both neuropathy subgroups reflects the burden of underlying vascular disease. Similar perfusion abnormalities have been described in the sural nerve (14). Despite this, there remains clear difference in the perfusion profiles of both painful and painless DN. There were no significant differences in the microvascular perfusion characteristics of the caudate nucleus. Unlike the caudate, the thalamus plays a central role in modulating/processing somatosensory information that is relayed to the cerebral cortex (8).
We have previously reported that preservation of thalamic neuronal function may be a prerequisite for the perception of pain in DN (2). Hyperexcitable thalamic neurons have since been reported to contribute to neuropathic pain in experimental diabetes (15). Thus thalamic neurons can act as central generators or amplifiers of pain in diabetes. Our finding of elevated thalamic perfusion may be related to increased neuronal activity.
Limitations of the current study include an age spread of several years between cohorts, and age is a factor in cerebral hypoperfusion. Paradoxically, however, subjects with painful DN comprised the oldest cohort but possessed the greatest thalamic rCBV. This would suggest comparative hyperperfusion rather than hypoperfusion. Interestingly, the difference in thalamic microvascular perfusion between painful and painless DN is not reflected by microvascular disease burden elsewhere with comparable prevalence of minimal retinopathy and nephropathy in both groups.
Our goal was to assess whether thalamic perfusion abnormalities are present in DN. The data presented here at least preliminarily support this view. A larger study with sample sizes of 12 from each of the four groups would achieve 91% power to detect significant differences among the groups. Future MR perfusion studies may lead to identification of objective hemodynamic correlates of painful DN enabling the targeting of specific components of the pain matrix pharmacologically, hopefully resulting in the development of more effective and better tolerated drugs.
Acknowledgments
This research was supported by a Diabetes UK grant.
No potential conflicts of interest relevant to this article were reported.
D.S. researched data and wrote the article. I.D.W. researched data, contributed to discussion, and reviewed and edited the article. R.G. researched data and contributed to discussion. P.D.G. contributed to discussion. S.T. researched data, contributed to discussion, and reviewed and edited the article.
Parts of this study were presented in abstract form at the American Diabetes Association 69th Scientific Sessions, New Orleans, Louisiana, 5–9 June 2009.
The authors would like to acknowledge the invaluable contributions of the radiographers in this study.
References
- 1.Vileikyte L, Leventhal H, Gonzalez JS, et al. Diabetic peripheral neuropathy and depressive symptoms: the association revisited. Diabetes Care 2005;28:2378–2383 [DOI] [PubMed] [Google Scholar]
- 2.Gandhi R, Selvarajah D, Emery CJ, Griffiths PD, Wilkinson ID, Tesfaye S. Thalamic neuronal dysfunction in diabetic peripheral neuropathy. Diabetes 2007;56:A0002 [Google Scholar]
- 3.Bastyr EJ, 3rd, Price KL, Bril V, MBBQ Study Group Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther 2005;27:1278–1294 [DOI] [PubMed] [Google Scholar]
- 4.Dyck PJ, Thomas PK. Diabetic Neuropathy. Dyck PJ, Thomas PK, Eds. London, W.B. Saunders, 1999 [Google Scholar]
- 5.Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 1993;43:1508–1512 [DOI] [PubMed] [Google Scholar]
- 6.O’Brien IA, O’Hare P, Corrall RJ. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J 1986;55:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyck PJ, Litchy WJ, Daube JR, et al. Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 2003;27:202–210 [DOI] [PubMed] [Google Scholar]
- 8.Wilson P, Kitchener PD, Snow PJ. Cutaneous receptive field organization in the ventral posterior nucleus of the thalamus in the common marmoset. J Neurophysiol 1999;82:1865–1875 [DOI] [PubMed] [Google Scholar]
- 9.Grahn JA, Parkinson JA, Owen AM. The cognitive functions of the caudate nucleus. Prog Neurobiol 2008;86:141–155 [DOI] [PubMed] [Google Scholar]
- 10.Griffiths PD, Pandya H, Wilkinson ID, Hoggard N. Sequential dynamic gadolinium magnetic resonance perfusion-weighted imaging: effects on transit time and cerebral blood volume measurements. Acta Radiol 2006;47:1079–1084 [DOI] [PubMed] [Google Scholar]
- 11.Gore M, Brandenburg NA, Dukes E, Hoffman DL, Tai KS, Stacey B. Pain severity in diabetic peripheral neuropathy is associated with patient functioning, symptom levels of anxiety and depression, and sleep. J Pain Symptom Manage 2005;30:374–385 [DOI] [PubMed] [Google Scholar]
- 12.Ziegler D. Treatment of diabetic neuropathy and neuropathic pain: how far have we come? Diabetes Care 2008;31(Suppl. 2):S255–S261 [DOI] [PubMed] [Google Scholar]
- 13.Malik RA. The pathology of human diabetic neuropathy. Diabetes 1997;46(Suppl. 2):S50–S53 [DOI] [PubMed] [Google Scholar]
- 14.Eaton SE, Harris ND, Ibrahim S, et al. Increased sural nerve epineurial blood flow in human subjects with painful diabetic neuropathy. Diabetologia 2003;46:934–939 [DOI] [PubMed] [Google Scholar]
- 15.Fischer TZ, Waxman SG. Neuropathic pain in diabetes—evidence for a central mechanism. Nat Rev Neurol 2010;6:462–466 [DOI] [PubMed] [Google Scholar]