Abstract
OBJECTIVE
This study investigated high-resolution magnetic resonance neurography (MRN) in distal symmetric diabetic polyneuropathy (dPNP).
RESEARCH DESIGN AND METHODS
MRN comprised high-resolution transaxial imaging of peripheral nerves of the lower limbs in 20 patients with type 2 diabetes (10 with dPNP, type 2/dPNP[+], and 10 without dPNP, type 2/dPNP[−]), seven patients with type 1 diabetes (two with dPNP, type 1/dPNP[+], five without dPNP, type 1/dPNP[−]), and 10 nondiabetic control subjects. Intraneural T2 lesions, as the main diagnostic criterion of MRN, were detected visually by two independent observers and quantitatively by analysis of T2 contrast ratios.
RESULTS
Multifocal fascicular, symmetric intraneural T2 lesions occurred in the proximal trunks of sciatic nerves in four patients (three with type 2/dPNP[+] and one with type 1/dPNP[+]) but not in control subjects (type 2/dPNP[−], type 1/dPNP[−], nondiabetic control subjects), which was confirmed by quantitative analysis. Clinical severity was higher in patients with T2 lesions (neuropathy deficit score: 10 vs. 7.8; P = 0.05).
CONCLUSIONS
For the first time, proximal neuropathic lesions of dPNP are reported in vivo. This supports that accumulation of proximal, multifocal fascicular injury may be important in disease progression.
Magnetic resonance neurography (MRN) at high clinical magnetic field strength of 3 Tesla and with the use of dedicated surface-array coils provides excellent microstructural anatomic detail of peripheral nerves. Moreover, MRN is an emerging tool for precise lesion localization exploiting the intraneural T2 contrast (1,2). In this pilot study, MRN was used for the first time in patients with distal symmetric diabetic polyneuropathy (dPNP) and revealed fascicular, symmetric lesions precisely located within proximal nerve trunks.
RESEARCH DESIGN AND METHODS
This study was approved by the local ethics committee (S-057/2009). Written informed consent was obtained from all participants. Ten patients with type 2 diabetes and dPNP (type 2/dPNP[+]; seven male, three female, mean age 71 ± 6.4 years), 10 patients with type 2 diabetes without dPNP (type 2/dPNP[−]; six male, four female, mean age 63.7 ± 9.5 years), two patients with type 1 diabetes and dPNP (type 1/dPNP[+]; one male, one female, mean age 61.5 ± 12.0 years), five patients with type 1 diabetes without dPNP (type 1/dPNP[−]; two male, three female, mean age 48.2 ± 13.2 years), and 10 nondiabetic control subjects (five male, five female, mean age 54 ± 5.0 years) were included. The study patients with dPNP presented with distal leg sensory or motor involvement manifested variably by pain, paresthesia, numbness, weakness, and hyporeflexia in the legs. None of the participants presented with clinical signs or a history of diabetic lumbosacral radiculoplexus neuropathy, which is typically characterized by pelvic-femoral pain and focal and unilateral weakness affecting the leg and thigh, and spreading to the contralateral limb in later stages (3). Clinical evaluation included specific items of the neuropathy deficit score (NDS) and neuropathy symptom score (NSS) (4) in all patients.
MRN was performed at 3 Tesla (Magnetom-VERIO, Siemens AG, Erlangen, Germany). Technical parameters of MRN and criteria for the determination of neuropathic lesions have been reviewed (1). In this investigation, lesion detection was performed in the axial plane with a high-resolution turbo spin echo T2-weighted sequence and fat suppression by inversion recovery: in-plane resolution 0.42 × 0.52 mm, slice thickness 5 mm, inversion time 180 ms, echo time 63 ms, and repetition time 6,210 ms. Coverage was from the infrapiriform foramen to the joint space of the knee.
To determine intraneural T2 lesions visually, a consensus between two observers, each with more than 5 years of training in diagnostic MRN (M.P., M.B.), was reached. Both were blinded to clinical and demographic patient data. Further confirmation of intraneural T2 lesions was achieved by quantitative analysis of contrast ratios between T2-weighted signal from a region of interest inside the sciatic nerve (intraneural) and another region of interest inside adjacent muscle. A lesion was classified if the cutoff contrast ratio value was 2 SDs above the group mean of nondiabetic control subjects.
RESULTS
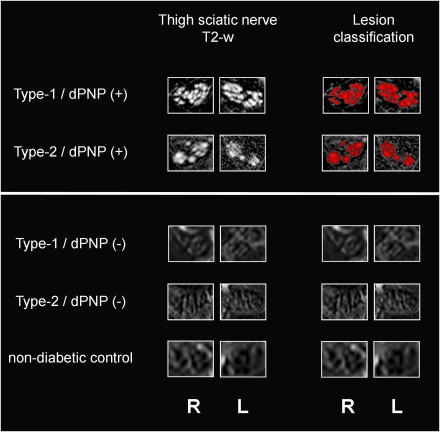
Intraneural T2 lesions were visually detected and quantitatively confirmed in three of 10 patients in the type 2/dPNP(+) group and in one of two patients in the type 1/dPNP(+) group. These intraneural T2 lesions could be characterized and exactly located as multifocal fascicular lesions within the proximal tibial and peroneal divisions of the sciatic nerve (Fig. 1). Lesion distribution was symmetric, in that no difference in the cross-sectional lesion area was observed between left and right. T2 lesions were not observed in the control groups: type 2/dPNP(−), type 1/dPNP(−), and nondiabetic control subjects. On quantitative analysis, the mean contrast ratio between nerve and adjacent muscle in the subgroup of four patients with dPNP exhibiting intraneural T2 lesions by visual evaluation was significantly higher (4.2 ± 0.9) when tested against 10 nondiabetic control subjects (1.9 ± 0.2, P = 0.003) or 15 diabetic control subjects without dPNP (2.1 ± 0.3, P = 0.004).
Figure 1.
Multifocal fascicular symmetric lesions within proximal sciatic nerve trunks in type 1 and type 2 diabetic patients with distal symmetric polyneuropathy (two upper rows). Fascicular symmetric lesions of one type 1 diabetic patient with dPNP (type 1/dPNP[+]) (top row) and of one type 2 diabetic patient with dPNP (type 2/dPNP[+]) (second row from top). Diagnostic conspicuity by visual evaluation arises from pathologically increased T2-weighted hyperintense/bright contrast (left column, “Thigh sciatic nerve T2-w”) and is confirmed by quantitative lesion classification from contrast ratios (red overlay on T2-weighted images in right column, “Lesion classification”). In the three control groups, intraneural T2 lesions were not observed (type 1/dPNP[−], type 2/dPNP[−], and nondiabetic control subjects). One representative subject from each control group is shown (third to fifth row below white horizontal bar). Right and left sciatic nerves are denoted at the bottom. L, left; R, right; T2-w, T2 weighted. (A high-quality digital representation of this figure is available in the online issue.)
Mean NDS and NSS were 8.3 and 6.9, respectively, in the type 2/dPNP(+) group and 10 and 7.5, respectively, in the type 1/dPNP(+) group. All patients in whom intraneural T2 lesions were detected presented with numbness as the predominant symptom and higher NDS than patients with dPNP in whom intraneural T2 lesions were not detected (10 vs. 7.8; P = 0.05). Significant differences in mean NSS (7 vs. 6.8), mean duration of disease (16.8 vs. 15.4 years), A1C levels (7.0 vs. 6.9), or the frequency of cardiovascular risk factors (arterial hypertension, hyperlipidemia, coronary heart disease, myocardial infarction, and smoking) were not observed.
CONCLUSIONS
For the first time, high-resolution MRN revealed multifocal fascicular, symmetric lesions of the proximal sciatic nerve trunks in type 1 and type 2 diabetic patients with severe dPNP. Patients with dPNP revealing intraneural T2 lesions had higher NDS than patients with dPNP in whom intraneural T2 lesions were not detected.
The pathomechanisms underlying dPNP have long been unclear and are still not fully understood (5–7). One important and likely predominant primary process in the development of dPNP is the loss of myelinated fibers, referred to as “axonal neuropathy” (8–10). It is being discussed whether axonal degeneration in dPNP begins distally and continuously proceeds proximally as a so-called dying-back axonopathy (11). Alternative explanations include proximal to distal axonal degeneration by multifocal injury at proximal levels presumably preceding distal fiber loss (12). The density of proximal lesions of this pattern could be shown to correlate with the severity of distal loss of myelinated nerve fibers (8,10). Our MRN imaging findings resemble this histologic pattern obtained from autopsies or sural nerve biopsies and may therefore support that the cumulative burden of proximal multifocal fascicular nerve lesions is associated with distal axonal degeneration and disease progression. It has to be clarified that the structural resolution of MRN as used in this pilot study was limited to the detection of fascicular pathology. Microstructural resolution beyond the fascicular level, for example, to contrast myelinated axons or the microcirculation, or to specifically delineate perineurial or endoneurial degeneration, was not achieved. Furthermore, the histopathologic alterations underlying diabetic intraneural T2 lesions may not be uniform. Endoneurial edema clearly would be expected to increase the T2 relaxation time (13,14). It is also conceivable that degenerative scarring in the endoneurial compartment or of perineurial layers contributes to T2 signal increase.
In summary, this first-time visualization of proximal, multifocal fascicular nerve lesions in dPNP may contribute to the understanding of pathomechanisms in dPNP. High-resolution in vivo nerve imaging by MRN accomplishes ample and continuous sampling. It may therefore become an important method to better characterize the spatial distribution and temporal evolution of diabetic nerve injury.
Acknowledgments
This study was supported by a Postdoctoral Fellowship granted to M.P. from the Medical Faculty of the University of Heidelberg and in part by the Dietmar-Hopp-Stiftung (to A.B., P.M.H., and P.P.N.).
No potential conflicts of interest relevant to this article were reported.
M.P., D.O., P.M.H, S.H., and M.B. designed the study. M.P., D.O., and P.B. acquired data. M.P. analyzed data. M.P., D.O., P.M.H., M.B., and P.P.N. interpreted data. M.P. and D.O. wrote the article. M.P., D.O., P.M.H., A.B., P.P.N., and M.B. reviewed and edited the article.
The authors thank Thorsten Kästel, MSc, from the Department of Neuroradiology, University of Heidelberg, for excellent technical assistance in performing MRN examinations.
References
- 1.Bendszus M, Stoll G. Technology insight: visualizing peripheral nerve injury using MRI. Nat Clin Pract Neurol 2005;1:45–53 [DOI] [PubMed] [Google Scholar]
- 2.Stoll G, Bendszus M, Perez J, Pham M. Magnetic resonance imaging of the peripheral nervous system. J Neurol 2009;256:1043–1051 [DOI] [PubMed]
- 3.Dyck PJ, Norell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral radiculoplexus neuropathy. Neurology 1999;53:2113–2121 [DOI] [PubMed] [Google Scholar]
- 4.Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993;36:150–154 [DOI] [PubMed] [Google Scholar]
- 5.Bierhaus A, Humpert PM, Rudofsky G, et al. New treatments for diabetic neuropathy: pathogenetically oriented treatment. Curr Diab Rep 2003;3:452–458 [DOI] [PubMed] [Google Scholar]
- 6.Ziegler D, Bierhaus A. Treatment of diabetic neuropathy. Dtsch Med Wochenschr 2007;132:1043–1047 [DOI] [PubMed] [Google Scholar]
- 7.Zochodne DW. Diabetic polyneuropathy: an update. Curr Opin Neurol 2008;21:527–533 [DOI] [PubMed] [Google Scholar]
- 8.Dyck PJ, Karnes JL, O’Brien P, Okazaki H, Lais A, Engelstad J. The spatial distribution of fiber loss in diabetic polyneuropathy suggests ischemia. Ann Neurol 1986;19:440–449 [DOI] [PubMed] [Google Scholar]
- 9.Dyck PJ, Lais A, Karnes JL, O’Brien P, Rizza R. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol 1986;19:425–439 [DOI] [PubMed] [Google Scholar]
- 10.Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Ann Neurol 1986;19:450–457 [DOI] [PubMed] [Google Scholar]
- 11.Thomas PK. Classification, differential diagnosis, and staging of diabetic peripheral neuropathy. Diabetes 1997;46(Suppl. 2):S54–S57 [DOI] [PubMed] [Google Scholar]
- 12.Sugimura K, Dyck PJ. Multifocal fiber loss in proximal sciatic nerve in symmetric distal diabetic neuropathy. J Neurol Sci 1982;53:501–509 [DOI] [PubMed] [Google Scholar]
- 13.Does MD, Snyder RE. Multiexponential T2 relaxation in degenerating peripheral nerve. Magn Reson Med 1996;35:207–213 [DOI] [PubMed] [Google Scholar]
- 14.Bendszus M, Wessig C, Solymosi L, Reiners K, Koltzenburg M. MRI of peripheral nerve degeneration and regeneration: correlation with electrophysiology and histology. Exp Neurol 2004;188:171–177 [DOI] [PubMed] [Google Scholar]