Abstract
OBJECTIVE
Intrauterine exposure to high maternal glucose is associated with excess weight gain during childhood, but it is not clear whether the excess weight represents increased fat or lean mass. The purpose of this study was to examine the relationship between maternal glucose concentrations during pregnancy and offspring body composition. A secondary goal was to examine whether the association between maternal glucose and children’s body fat was independent of energy intake, energy expenditure, or physical activity.
RESEARCH DESIGN AND METHODS
Children aged 5–10 years and their biological mothers (n = 27) were recruited. Maternal glucose concentration 1 h after a 50-g oral glucose load, used to screen for gestational diabetes mellitus at 24–28 weeks gestation, was retrieved from medical records. Children underwent dual-energy X-ray absorptiometry to measure body composition, indirect calorimetry to measure resting energy expenditure (REE), accelerometry to measure physical activity, and three 24-h diet recalls to measure energy intake.
RESULTS
Maternal glucose concentration during pregnancy was positively associated with children’s lean mass (P < 0.05) and adiposity (fat mass adjusted for lean mass; P < 0.05). The association between maternal glucose and children’s adiposity was independent of children’s REE, percent of time spent physically active, and energy intake (P < 0.001).
CONCLUSIONS
Intrauterine exposure to relatively high maternal glucose is associated with greater lean mass and adiposity among prepubertal offspring. Further research is needed to examine the mechanisms by which maternal glucose concentrations during pregnancy influence children’s body composition.
Children born to diabetic women (1–3) or those with relatively high glucose concentrations during pregnancy (4,5) have greater birth weight and are at greater risk for obesity than are those born to nondiabetic women. These conclusions are based on measures of BMI percentiles or skin fold measures of subcutaneous fat, and so it is not clear whether the larger body size among offspring of diabetic women also reflects greater lean mass in addition to greater fat mass. Excess childhood weight gain among offspring of diabetic mothers is believed to be a result of prenatal exposure to increased fuel from the mother, and glucose has been specifically implicated (6,7). It would be of interest, therefore, to examine the association between maternal glucose concentrations during pregnancy across a range incorporating both diabetic and nondiabetic extremes and children’s fat and lean mass.
Despite the large body of literature supporting associations of maternal diabetes and glucose concentrations with offspring body weight, it is not known whether greater energy intake or reduced expenditure among offspring contributes to this effect. Evidence from animal models supports the hypothesis that regulation of appetite and energy balance may be impaired following prenatal exposure to high maternal glucose (rev. in 8), but in clinical studies, investigators have found no difference in energy expenditure, physical activity, or energy intake under controlled conditions among children with and without prenatal exposure to maternal diabetes (9,10). These studies did not consider maternal glucose concentration specifically, however, which may vary considerably in women with diabetes based on their degree of glycemic control.
The purpose of this study was to test the hypothesis that prepubertal children who were prenatally exposed to relatively high maternal glucose concentrations would have greater fat, but not lean, mass. A secondary hypothesis was to examine whether this association was independent of children’s energy intake, expenditure, or physical activity. These hypotheses were tested in prepubertal children for whom mothers’ medical records during pregnancy were available.
RESEARCH DESIGN AND METHODS
Children aged 5–10 years and their biological mothers were recruited. Mothers were eligible if they were ≥16 years old at delivery, and initiated prenatal care during the first trimester. Women who had developed gestational diabetes mellitus (GDM) during the target pregnancy were over-sampled relative to the 3–8% prevalence in the general population (11), in order to increase the range of gestational glucose concentrations represented. Children were eligible if they were singletons and were born at ≥37 weeks gestation. Children who were growth restricted in utero (<2,500 g at birth), had congenital defects, type 1 diabetes, or a current weight of <11 kg precluding adequate blood sampling, were excluded.
Procedure
Mother-child pairs attended two study related visits spaced ∼10 days apart. During the first visit, informed consent/assent was obtained from mothers and children, mothers provided permission for retrieval of medical records related to the target pregnancy, and children underwent a physical examination during which prepubertal status was confirmed. During the second visit, mothers brought their children to the Clinical Research Unit of the Center for Clinical and Translational Science at 6.30 a.m. after an overnight fast. Children’s resting energy expenditure (REE) was measured by indirect calorimetry, and body composition was measured by dual-energy X-ray absorptiometry (DXA). The Institutional Review Board for Human Use at the University of Alabama at Birmingham approved all study procedures.
Maternal medical records
Upon enrollment, mothers provided permission for study investigators to request prenatal care and delivery records from physicians and hospitals. Subsequently, records from two thirds of the participants were obtained, and the remaining one third of the sample was excluded from the final analyses. Data retrieved from the records included 1-h glucose concentration from the 50-g oral glucose tolerance test (OGTT) used to screen for GDM (12), GDM status and mode of treatment, fasting glucose concentrations recorded after the diagnosis of GDM if applicable, maternal age, gestational week at delivery, and offspring birth weight. Blood glucose from the OGTT was used as the major independent variable in the statistical analyses, representing maternal glucose during gestation. Consequently, if women were diagnosed with GDM but subsequently maintained fasting glucose within the normal range, they were excluded from the study to avoid confounding interpretation of the results. This resulted in the exclusion of three mother-child pairs.
Body composition
Body weight was measured to the nearest 0.1 kg (Scale-tronix 6702 W; Scale-tronix, Carol Stream, IL) in minimal street clothing without shoes. Height, to the nearest 0.01 cm, was measured with a digital stadiometer (Heightronic 235; Measurement Concepts, Snoqualmie, WA). Waist circumference at the level of the umbilicus and hip circumference at the widest part of the buttock were measured to the nearest 0.1 cm using a flexible tape measure (Gulick II; Country Technology, Gays Mills, WI). Waist and hip circumferences were measured at least twice, with the final recorded measurement being the one corroborated by at least two measurements. The same investigator (N.C.B.) performed all measures.
Body composition was measured by DXA (Lunar iDXA; GE Healthcare, General Electric Company, Madison, WI). Scans were analyzed for total fat and lean mass using the encore software package (version 1.33; GE Healthcare, General Electric Company, Madison, WI). The same investigator (N.C.B.) analyzed all scans.
REE
REE was obtained by indirect calorimetry using a Deltatrac Metabolic Monitor (Sensorimedics Corp., Yorba Linda, CA), and methods previously conducted at this facility (13). The time at which any large body movements, yawning, or coughing occurred was noted and that particular 1-min period subsequently removed from the data. The remaining values were averaged to provide REE.
Physical activity
Free-living physical activity was measured by uni-axial accelerometer (Actigraph GT1M; Actigraph, Pensacola, FL). Units were worn on elastic bands around the waist above the right front hip, under clothing. Children wore the units for at least 8 days, with removal only for bathing, swimming, and sleeping. Mothers recorded the times during which the units were worn on an activity log. The accelerometers were set to record activity in 60-s epochs. Data were uploaded using Actilife desktop software for the GT1M units (Actigraph, Pensacola, FL). The periods of nonuse noted by the mothers were verified by manual review of the raw data, and any other time interval when 0 counts were recorded for a period of 4 h or more was also presumed to be a period of nonuse. One investigator (P.C.C.-L.) cleaned all of the data files by removing these time periods. The Statistical Analysis System (SAS; SAS Institute, Cary, NC) was used to calculate the total counts and percent of time engaged in activity above sedentary levels, using cut-points previously described (14). The total percent of time engaged in physical activity was averaged across days with at least 8 h of recorded activity. Data from children with at least 3 8-h days of activity were included in the final analysis, thereby excluding one child.
Energy intake
A trained dietitian (N.C.B.) interviewed mothers and children together to obtain three 24-h diet recalls using the multipass method (15). Two recalls were conducted in person and one by telephone, and recalls included 2 weekdays and 1 weekend day. Data were entered into the Nutrition Data System for Research (NDSR version 2007; University of Minnesota, MN) and average daily total energy intake (kcal) was calculated for each participant.
Statistical analysis
Associations among maternal glucose concentrations and children’s outcomes (i.e., body composition, energy intake, REE, and physical activity) were explored with Pearson simple and partial correlation analyses. Multiple linear regression modeling was then used to examine whether maternal glucose was associated with children’s fat and lean mass, independent of expected confounders such as gender, ethnicity, and height, where appropriate. Subsequent exploratory linear regression analyses then were conducted to examine whether the association between maternal glucose and children’s body fat was independent of children’s energy intake, REE, and physical activity. Maternal glucose concentration, and children’s birth weight, lean and fat mass were log10 transformed prior to analysis. α was set at 0.05 for statistical significance, and all analyses were performed using the Statistical Package for the Social Sciences, version 18 (SPSS; SPSS, Chicago, IL).
RESULTS
Forty-eight mother-child pairs were enrolled in the study. Final analyses were conducted on 27 of these pairs with complete data. Reasons for exclusion from the final analyses included missing maternal OGTT results (16), fasting glucose concentrations <100 mg/dL following a diagnosis of GDM, indicating adequate glycemic control (3), outlier for maternal glucose concentration (1), and child use of attention deficit disorder medication (1). Characteristics of the mothers and children included in the analyses are shown in Table 1. Mothers ranged in age from 17 to 37 years at the time of delivery, and the children from 5.2 to 10.7 years at the time of testing. All children were at Tanner stage 1 of pubertal development (16). Eight of the mothers (30%) had been diagnosed with GDM, and of these, four were treated with insulin and the other four were diet-controlled.
Table 1.
Characteristics of the children and their mothers
| Children | |
|---|---|
| Ethnicity | 78% AA (6 EA, 21 AA) |
| Sex | 44% male (12 male, 15 female) |
| Age (years) | 7.3 ± 1.5 |
| Birth weight (g) | 3,362.7 ± 508.5 |
| BMI percentile at time of testing* | 74.2 ± 25.9 |
| Average daily total energy intake (kcals) | 1,567.5 ± 382.9 |
| REE (kcals) | 1,078.0 ± 212.9 |
| Time spent active (%)† | 32.5 ± 9.7 |
| Waist circumference (cm) | 61.5 ± 11.5 |
| Hip circumference (cm) | 68.7 ± 10.3 |
| Total fat mass (kg) | 9.5 ± 6.2 |
| Total lean mass (kg) | 20.4 ± 4.7 |
| Mothers | |
|---|---|
| Age at delivery | 25.7 ± 6.5 |
| GDM status | 30% GDM (8 GDM, 19 non–GDM) |
| 1-h glucose concentration | 130.5 ± 34.5 |
| Current BMI | 31.4 ± 7.4 |
*BMI percentiles are based reference data provided by the U.S. Centers for Disease Control and Prevention (20).
†Accelerometry available for 26 of 27 children.
AA, African American; EA, European American.
Maternal glucose concentrations during pregnancy were positively associated with children’s total fat mass (Table 2), and this association remained significant after adjusting for children’s lean mass (r = 0.443, P < 0.05). Maternal glucose was not associated with children’s lean mass in simple correlation analysis, but after adjustment for height and sex, a trend for a positive association was uncovered (r = 0.371, P = 0.07). Children’s birth weight tended to be associated with their lean mass at 5–10 years of age (r = 0.386, P = 0.051). However, this association was attributable to the greater height of children who were heavier at birth (birth weight × lean mass: r = 0.081, P = 0.70, after adjustment for children’s height).
Table 2.
Simple (except where noted) correlation coefficients of the association between maternal glucose during pregnancy and children’s characteristics at 5–10 years of age
| Children’s characteristics | Maternal glucose |
|---|---|
| Birth weight† | 0.306 |
| BMI percentile | 0.445* |
| Waist circumference | 0.469* |
| Hip circumference | 0.449* |
| Height | 0.078 |
| Total lean mass | 0.122 |
| Total fat mass | 0.418* |
| Average energy intake (kcals/day) | 0.001 |
| % kcals from fat | 0.276 |
| % kcals from carbohydrate | −0.086 |
| % kcals from protein | −0.319 |
| REE | 0.265 |
| % of time spent active | 0.226 |
†Adjusted for gestational age at delivery.
*P < 0.05.
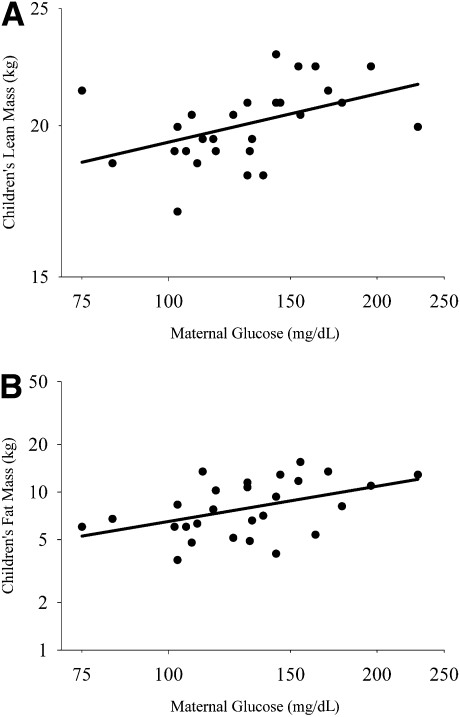
In multiple linear regression analysis, maternal glucose concentration during pregnancy was positively associated with children’s lean mass (standardized [Std] β = 0.146, P < 0.05), independent of children’s ethnicity, sex, birth weight, and current height (P < 0.05; Fig. 1A). Maternal glucose concentration was also independently and positively associated with children’s fat mass (Std β = 0.316, P < 0.05), after adjustment for ethnicity, sex, and lean mass (Fig. 1B).
Figure 1.
A: Partial regression plot showing that maternal glucose during pregnancy was associated with children’s lean mass independent of ethnicity, sex, height, and birth weight (overall model adjusted R2 = 0.893, P < 0.001; maternal glucose Std β = 0.146, partial r = 0.436, P < 0.05). B: Partial regression plot showing that maternal glucose during pregnancy was associated with children’s fat mass independent of ethnicity, sex, and lean mass (overall model adjusted R2 = 0.576, P < 0.001; maternal glucose Std β = 0.316, partial r = 0.462, P < 0.05).
With respect to ethnicity and sex, neither was found to be associated with fat mass in the linear regression models, and so they were not included in subsequent analyses. Sex, but not ethnicity, was associated with lean mass, such that boys had more lean mass than girls (Std β = −0.175, P < 0.05).
In an effort to secure some statistical evidence of the adequacy of this sample size, we performed power calculations using nQuery Advisor, version 7, for the multiple linear regression models. Assuming a two-sided test with an α of 0.05, analyses indicated that we had greater than 99% power to detect our R2 of 0.893 for Model 1, and an R2 of 0.576 for Model 2, where n = 27 for each model.
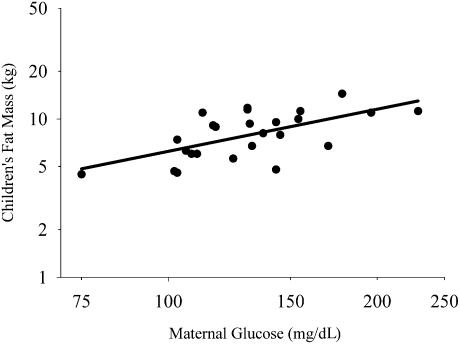
To examine whether the association between maternal glucose and children’s adiposity was independent of children’s energy intake, REE, or physical activity, these variables were included in exploratory models for the dependent variable children’s fat mass. The model that explained the most variance in children’s fat mass included maternal glucose concentration during pregnancy, children’s energy intake, REE, and time spent physically active (P < 0.001; Fig. 2). Maternal glucose remained positively associated with children’s fat mass, independent of children’s lean mass, energy intake, and time spent engaged in physical activity (Std β = 0.469, P < 0.001). Children’s energy intake was independently and inversely associated with their fat mass (P < 0.001; data not shown). Power calculations for this multiple linear regression model, assuming a two-sided test with an α of 0.05, indicated greater than 99% power to detect our R2 of 0.794, where n = 26.
Figure 2.
Partial regression plot showing that maternal glucose during pregnancy was associated with children’s fat mass independent of lean mass, REE, energy intake, and time spent physically active (overall model adjusted R2 = 0.794, P < 0.001; maternal glucose Std β = 0.469, partial r = 0.712, P < 0.001).
CONCLUSIONS
This study examined whether prenatal exposure to high maternal glucose is associated with greater childhood adiposity specifically, and whether this association was independent of children’s energy intake, REE, and time spent physically active. Results in this cohort suggest that maternal glucose during pregnancy had a long-term impact on both lean mass and adiposity, and that the effect of maternal glucose on the proportion of tissue that is fat was independent of children’s diet and lifestyle. This study therefore supports the hypothesis that maternal glucose is involved in programming offspring body composition (6,7).
The positive, albeit modest, association between maternal glucose and children’s lean mass is contradictory to a previous study showing no difference in lean mass among children with and without prenatal exposure to GDM (17). These discrepant results may be due to methodological differences; with the use of maternal glucose in the current study likely providing a more sensitive index of maternal metabolic status than would diabetes status per se. It was also notable that in the current study, the association of maternal glucose with children’s lean mass was independent of their birth weight. This implies that greater body mass among the children who were exposed to relatively high maternal glucose was not simply due to the children continuing on a higher growth trajectory that began prior to birth.
Children from mothers with higher glucose concentrations were also found to have a greater proportion of fat mass relative to lean mass. This finding extends previous research (4,5) by showing a continuous, positive relationship between maternal glucose during pregnancy and children’s adiposity. The association between maternal glucose and children’s adiposity was also found to be independent of children’s energy intake, REE, and physical activity, at the time of testing. Although data from animal models implicate altered central regulation of food intake in the development of obesity among offspring of hyperglycemic dams (rev. in 8), we did not observe an association between maternal glucose and children’s energy intake in this study. Our data are in agreement, however, with other clinical studies finding no association between maternal glucose concentrations or diabetes status and children’s energy intake (10,17). Similarly, findings in this cohort are consistent with another study showing no effect of GDM on children’s energy expenditure and physical activity (9).
There are several possible explanations for why the association between maternal glucose and children’s adiposity was independent of children’s diet and lifestyle. First, it is possible that although children exposed to relatively high glucose concentrations were not currently overeating or more sedentary than their peers at the time of testing, they may have engaged in these types of obesity-promoting behaviors at a younger age. Alternately, given that energy intake may be under-reported among overweight children (18,19), results of the current study may reflect a biased and inaccurate assessment of energy intake. The inverse association between children’s energy intake and fat mass found in this cohort is consistent with the possibility that under-reporting of energy intake among those with more adiposity may have confounded the results. It is also plausible that the results shown here reflect an effect of maternal glucose to prenatally “program” metabolism or anabolic growth, independent of children’s postnatal diet and lifestyle. Although the mechanism is not clear, prenatal exposure to relatively high maternal glucose may program children to store rather than use energy. Future research is warranted to examine synthesis, action, and target tissue responsiveness to endocrine/neuroendocrine factors affecting anabolic growth, such as growth hormone, insulin-like growth factor-1, and insulin in children differing with respect to the prenatal environment.
Strengths of this study included the retrieval of objectively-measured maternal glucose concentrations during routine prenatal GDM screening tests, and the robust measures of children’s body composition, REE, and physical activity. This study was limited by the small sample size, although statistical analyses indicated sufficient power to detect the effects shown here.
To conclude, evidence from this cohort suggests the existence of a dose response relationship between maternal glucose concentrations during pregnancy and the fat and lean mass of prepubertal offspring. Further, results support the hypothesis that prenatal metabolic programming may result in a long-lasting partitioning of energy toward anabolic growth. These findings support and extend the growing body of literature calling for the development of programs to optimize maternal glucose control during pregnancy as a means to reduce the risk for obesity among offspring.
Acknowledgments
This work was supported by the Thrasher Research Fund (NR-0025) and the National Institutes of Health (F32 DK-082028, UL-1RR025777, P30 DK-056336, P60 DK-079626).
No potential conflicts of interest relevant to this article were reported.
P.C.C.-L., N.C.B., D.J.R., and B.A.G. conceived of and designed the study. P.C.C.-L. collected and analyzed data and wrote the manuscript. N.C.B. collected and analyzed data and reviewed the manuscript. D.J.R. and M.S.M. provided medical oversight and advice, and reviewed the manuscript. B.A.G. analyzed data and reviewed the manuscript.
Parts of this study were presented in abstract form at the 27th Annual Meeting of the Obesity Society, Washington, D.C., 24–28 October 2009, and at the 92nd Annual Meeting of the Endocrine Society, San Diego, California, 19–22 June 2010.
Rachel Copper and Mickey Parks from the University of Alabama (UAB) Center for Women’s Reproductive Health provided administrative, nursing, and data collection support. Maryellen Williams and Cindy Zeng from the Metabolic Core laboratory of the UAB Diabetes Research and Training Center, the Nutrition Obesity Research Center, and the Center for Clinical and Translational Science, conducted laboratory analyses. The authors thank Dr. Robert Oster of the UAB Division of Preventive Medicine and the Center for Clinical and Translational Science for assistance with data analyses.
References
- 1.Vohr BR, Boney CM. Gestational diabetes: the forerunner for the development of maternal and childhood obesity and metabolic syndrome? J Matern Fetal Neonatal Med 2008;21:149–157 [DOI] [PubMed] [Google Scholar]
- 2.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–e226 [DOI] [PubMed] [Google Scholar]
- 3.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol 2007;50:972–979 [DOI] [PubMed] [Google Scholar]
- 4.Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood obesity and metabolic imprinting: the ongoing effects of maternal hyperglycemia. Diabetes Care 2007;30:2287–2292 [DOI] [PubMed] [Google Scholar]
- 5.Pettitt DJ, Bennett PH, Saad MF, Charles MA, Nelson RG, Knowler WC. Abnormal glucose tolerance during pregnancy in Pima Indian women: long-term effects on offspring. Diabetes 1991;40(Suppl. 2):126–130 [DOI] [PubMed] [Google Scholar]
- 6.Pedersen J. The pregnant diabetic and her newborn: problems and management. Baltimore, MD, Wilkens, 1967 [Google Scholar]
- 7.Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. J Pediatr 1998;132:768–776 [DOI] [PubMed] [Google Scholar]
- 8.Plagemann A. A matter of insulin: developmental programming of body weight regulation. J Matern Fetal Neonatal Med 2008;21:143–148 [DOI] [PubMed] [Google Scholar]
- 9.Salbe AD, Fontvieille AM, Pettitt DJ, Ravussin E. Maternal diabetes status does not influence energy expenditure or physical activity in 5-year-old Pima Indian children. Diabetologia 1998;41:1157–1162 [DOI] [PubMed] [Google Scholar]
- 10.Gluck ME, Venti CA, Lindsay RS, Knowler WC, Salbe AD, Krakoff J. Maternal influence, not diabetic intrauterine environment, predicts children’s energy intake. Obesity (Silver Spring) 2009;17:772–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metzger BE, Coustan DR, The Organizing Committee Summary and recommendations of the Fourth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 1998;21(Suppl. 2):B161–B167 [PubMed] [Google Scholar]
- 12.O’Sullivan JB, Mahan CM, Charles D, Dandrow RV. Screening criteria for high-risk gestational diabetic patients. Am J Obstet Gynecol 1973;116:895–900 [DOI] [PubMed] [Google Scholar]
- 13.Higgins PB, Fernández JR, Goran MI, Gower BA. Early ethnic difference in insulin-like growth factor-1 is associated with African genetic admixture. Pediatr Res 2005;58:850–854 [DOI] [PubMed] [Google Scholar]
- 14.Freedson P, Pober D, Janz KF. Calibration of accelerometer output for children. Med Sci Sports Exerc 2005;37(Suppl.):S523–S530 [DOI] [PubMed] [Google Scholar]
- 15.Johnson RK, Driscoll P, Goran MI. Comparison of multiple-pass 24-hour recall estimates of energy intake with total energy expenditure determined by the doubly labeled water method in young children. J Am Diet Assoc 1996;96:1140–1144 [DOI] [PubMed] [Google Scholar]
- 16.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catalano PM, Farrell K, Thomas A, et al. Perinatal risk factors for childhood obesity and metabolic dysregulation. Am J Clin Nutr 2009;90:1303–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fisher JO, Johnson RK, Lindquist C, Birch LL, Goran MI. Influence of body composition on the accuracy of reported energy intake in children. Obes Res 2000;8:597–603 [DOI] [PubMed] [Google Scholar]
- 19.Maffeis C, Schutz Y, Zaffanello M, Piccoli R, Pinelli L. Elevated energy expenditure and reduced energy intake in obese prepubertal children: paradox of poor dietary reliability in obesity? J Pediatr 1994;124:348–354 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Centers for Disease Control and Prevention, National Center for Health Statistics CDC growth charts for the United States [Internet], 2000. Available from http://www.cdc.gov/growthcharts. Accessed 22 September 20109742976 [Google Scholar]