Abstract
OBJECTIVE
Meta-analyses have shown that the risk for depression is elevated in type 2 diabetes. Whether this risk in individuals with impaired glucose metabolism (IGM) or undiagnosed diabetes (UDD) is elevated relative to normal glucose metabolism (NGM) or decreased relative to previously diagnosed type 2 diabetes (PDD) has not been the subject of a systematic review/meta-analysis. This study examined the prevalence of depression in IGM and UDD subjects relative to each other and to NGM and PDD subjects by reviewing the literature and conducting a meta-analysis of studies on this topic.
RESEARCH DESIGN AND METHODS
EMBASE and MEDLINE databases were searched for articles published up to May 2010. All studies that compared the prevalence of depression in subjects with IGM and UDD were included. Odds ratios (ORs) were calculated using fixed and random-effects models.
RESULTS
The meta-analysis showed that the risk for depression was not increased in IGM versus NGM subjects (OR 0.96, 95% CI 0.85–1.08). Risk for depression did not differ between individuals with UDD and individuals with either NGM (OR 0.94, 95% CI 0.71–1.25) or IGM (OR 1.16, 95% CI 0.88–1.54). Finally, individuals with IGM or UDD both had a significantly lower risk of depression than individuals with PDD (OR 0.59, 95% CI 0.48–0.73, and OR 0.57, 95% CI 0.45–0.74, respectively).
CONCLUSIONS
Results of this meta-analysis show that the risk of depression is similar for NGM, IGM, and UDD subjects. PDD subjects have an increased risk of depression relative to IGM and UDD subjects.
Several meta-analyses have shown that the risk of elevated levels of depression and the risk of incident depression are increased in people diagnosed with type 2 diabetes compared with nondiabetic control subjects (1,2). Comorbid depression in people with diabetes forms a serious threat to quality of life (3). Moreover, people with both depression and diabetes have been found to be at increased risk for the development of cardiovascular complications of diabetes and to have increased mortality rates and higher health care costs (4–6).
The reasons for the high prevalence of depression in type 2 diabetes remain unclear, although it is likely that the burden resulting from having a chronic disease and/or its associated complications plays an important role (7,8). It is also possible that increased levels of blood glucose are implicated, although the exact nature of the relationship between hyperglycemia and depression remains unclear (9).
Hyperglycemia (and insulin resistance) may contribute to depression by two mechanisms: 1) through its impact on symptoms, such as fatigue and difficulty concentrating (10), complications, and fear of complications (11), and 2) through physiological pathways, including inflammatory processes, and reductions in neurotrophic function (12–14), which in turn may lead to reduced plasticity of neuronal networks and subsequently depression (15).
It is worth noting that depression is a common disorder in the general population and is not only increased in people with diabetes (1,2), but also in people with other physical health problems, such as chronic pain, asthma, and heart disease (16). This would suggest that elevated blood glucose levels are not a necessary condition for developing depression.
One way to study whether elevated blood glucose levels affect mood is to investigate the prevalence of depression in people with impaired glucose metabolism (IGM) or undetected type 2 diabetes (UDD) and compare these to prevalence rates of depression in people with normal glucose metabolism (NGM) and people with previously diagnosed type 2 diabetes (PDD). Although people with IGM and people with UDD have, by definition, elevated blood glucose levels compared with individuals with NGM, the level of glucose impairment varies, ranging from NGM to IGM to full blown type 2 diabetes. Moreover, people with IGM or UDD differ from people with PDD, because they do not have the psychological burden of being diagnosed with the condition or having to self-manage it.
As the prevalence of depression in people with UDD or IGM has not been evaluated in a systematic review and meta-analysis, this report examines the relationship between these categories of glycemic dysregulation and risk of depression. We examined the combined prevalence of depression in samples of people with NGM, IGM, UDD, or PDD by conducting a meta-analysis of studies published on this subject in the peer-reviewed literature.
RESEARCH DESIGN AND METHODS
Retrieval of studies
To identify the studies of interest, MEDLINE (1950 to May 2010) and EMBASE (1947 to May 2010) databases were searched. The search terms are shown in Supplementary Table 1. Titles and abstracts of the retrieved studies were scanned by two authors (A.N. reviewed all abstracts, and the second reviews were divided among coauthors) to exclude studies that were clearly irrelevant. The full text of the remaining studies was then read by three authors (A.N., G.N., and F.P.) to determine whether the studies met our inclusion criteria. Furthermore, the reference lists of articles that studied our topic of interest were scanned to check for additional publications.
Inclusion/exclusion criteria
In this systematic review and meta-analysis, we included all studies that examined the prevalence of depression either in UDD or IGM subjects, also defined as impaired glucose tolerance, impaired fasting glucose, and impaired glucose resistance, comparing these rates to those in NGM and/or PDD subjects.
Data extraction
Three authors (A.N., G.N., and F.P.) independently extracted data from the studies, in particular regarding 1) name of first author, 2) publication year, 3) study design, 4) number of participants by category of glycemic (dys)regulation, 5) sex of participants, 6) age of participants, 7) method and criteria for depression assessment, 8) methods of diabetes and/or prediabetic state assessment, 9) number and percentage of case subjects with depression, 10) fasting plasma glucose, and 11) unadjusted and adjusted odds ratios (ORs) and 95% CIs. This allowed comparison of IGM with NGM and PDD and comparison of UDD with NGM, IGM, and PDD.
In the included studies, method of depression assessment could be either 1) a diagnosis of depression assessed by a diagnostic psychiatric interview, 2) assessment of depressive symptoms by a self-report questionnaire, 3) a diagnosis by a physician, or 4) in combination with a prescription of antidepressant medication.
Statistical analysis
The odds of depression in each category (UDD or IGM) were compared with the odds of depression in NGM or PDD to calculate the unadjusted OR. From these, pooled ORs were estimated. Both the fixed-effects model and the random-effects model were used. The fixed-effects model assumes that variability between studies is exclusively due to random variation, and individual studies are simply weighted by their precision. The random-effects model assumes that a different true effect size exists for at least one study and takes this into consideration as an additional source of variation. A random-effects meta-analysis is more conservative than a fixed-effects meta-analysis, since it may give wider CIs around the point estimate and is recommended when it is suspected that individual studies may not be estimating the same true effect size (17). A forest plot was made to show the OR and 95% CI of each study and the pooled OR and 95% CI.
We followed recommendations in the Cochrane Handbook (17) for investigating reporting bias; these recommendations advise that Harbord’s statistical test for small study bias is appropriate when the outcome statistic is an OR and the number of studies is at least 10 to avoid false-positive results in the presence of study heterogeneity. Forest plots were used to visually assess homogeneity of the studies. This was also tested with the Cochran’s Q test and the I-squared statistic. All statistical analyses were performed using STATA 10.0 (STATA Corporation, College Station, TX).
RESULTS
The MEDLINE search (1950 to May 2010) identified 3,357 articles, of which 12 met our inclusion criteria and were subsequently included in the systematic review and meta-analysis. The search in EMBASE (1947 to May 2010, total number of studies 4,337, excluding duplicates) identified one additional study meeting the selection criteria. Thus, a total of 13 studies were included in the meta-analysis. The characteristics and the extracted data of the 13 included studies are presented in Table 1 and, for the (un)adjusted odds ratios, in Supplementary Table 2.
Table 1.
Characteristics and results of the 13 studies included in the meta-analysis
| Study | Design | Number of participants | Age (years) |
n Male (%) |
Criteria for depression |
Assessment of DM2/IGM | Fasting plasma glucose (mmol/L) (SD) | Cases of depression | Methodological issues | |
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Range | |||||||||
| US: Palinkas et al., 1991 (29) | Population-based study | NGM: 1,284 (81%) | NGM: 69 (10) | ≥50 | NGM: 584 (46%) | Beck Depression Inventory ≥13 | WHO 1985 (OGTT, FPG) | NGM: 4.8 (0.5) UDD: 5.9 (1.5)PDD: 7.1 (2.6) | NGM: 58 (5%) | Excluded participants: -IGT and no personal diabetes history or current use of diabetes medication (n = 559) -Using insulin (n = 6) - Failed to fast for 12 h or missing glucose results (n = 106) |
| UDD: 209 (13%)PDD: 93 (6%) | UDD: 74 (8)PDD: 72 (9) | UDD: 101 (48%)PDD: 56 (60%) | Self-reported diabetes diagnosis by physician | UDD: 8 (4%)PDD: 10 (11%) | ||||||
| Current use of diabetes medication | ||||||||||
| Finland: Hiltunen et al., 1996 (19) | Population-based study | NGT: 149 (40%) | NGT: M 74; W 75 | 70–92 | NGT: 63 (42%) | Short Zung SDS score ≥28 | WHO 1985 (OGTT) | NA | NGT: 26 (17%) | Small sample |
| IGT: 122 (33%)UDD: 33 (9%)PDD: 65 (18%) | IGT: M 76; W 76UDD: M 74; W 78PDD: M 73; W 79(Median) | IGT: 43 (35%)UDD: 12 (36%)PDD: 19 (29%) | Previous diabetes diagnosisReceiving oral drug, insulin, or diet treatment | IGT: 19 (15%)UDD: 6 (18%)PDD: 9 (14%)a | ||||||
| Finland: Rajala et al., 1997 (18) | Population-based study | NGM: 480 (65%)IGT: 192 (26%)UDD: 26 (4%)PDD: 36 (5%) | NGM: 55IGT: 55UDD: 55PDD: 55 | 55 | NGM: 199 (41%)IGT: 85 (44%)UDD: 13 (50%)PDD: 24 (67%) | Zung SDS score ≥45 | WHO 1985 (OGTT)Self-reported diabetes diagnosis by physicianIn patients with random blood glucose value ≥8 2× FG or two FG values ≥6.7 mmol/L | NA | NGM: 56 (12%)IGT: 24 (13%)UDD: 3 (12%)PDD: 9 (25%) | Small samplePDD treatment: diet only, 22 (54%); oral agents, 11 (27%); oral agents and insulin, three (7%); insulin, five (12%)Median diabetes duration 5 years (range 1–40) |
| U.S.: Yaffe et al., 2004 (20) | Intervention multisite (n = 180) multicountry (n = 25) study | NGM: 6,463 (92%)IFG: 297 (4%)PDD: 267 (4%) | NGM: 66 (7)IFG: 68 (7)PDD: 68 (6) | NA | 0 (0%) | 15-item Geriatric Depression Scale ≥6 | FG Self-reported preexisting diabetesCurrently using hypoglycemic medication | NGM: 5.0 (0.4)IFG: 6.4 (0.2)PDD: 8.5 (2.7) | NGM: 142 (2%)IFG: 10 (3%)PDD: 11 (4%)b | Postmenopausal women only Of 267 women with diabetes, 184 (69%) reported having diabetes by history, 198 (74%) had FG level ≥7 mmol/L, and 111 (42%) reported using hypoglycemic medication |
| The Netherlands: Knol et al., 2007 (21) | Population-based study | NFG: 3,499 (81%)IFG: 671 (16%)UDD: 55 (1%)PDD: 102 (2%)c | NGM: 38 (11)IFG: 47 (14)UDD: 57 (13)PDD: 56 (14)c | ≥18 | NGG: 1,414 (40%)IFG: 427 (64%)UDD: 24 (44%)PDD: 54 (53%)c | Symptom Checklist-90depression subscale ≥25 and/or self-reported antidepressant use CES-D ≥16 and/or self-reported use of antidepressant medication | ADA 2005 (FPG)Self-reported diabetes diagnosis by physician | NFG: 4.9 (0.4)IFG: 5.9 (0.3)UDD: 8.3 (2.1)PDD: 8.3 (3.3)c | NFG: 667 (19%)IFG: 115 (18%)UDD: 11 (20%)PDD: 30 (30%)c | Patients with diagnosed diabetes who used insulin and no oral hypoglycemic agents were defined as having type 1 diabetes and were excluded (n = 14) |
| U.S.: Golden et al., 2007 (22) | Population-based study | NGM: 3,911 (58%)IFG: 1,879 (28%)UDD: 292 (4%)PDD: 672 (10%) | Depressed: 61 (10)Nondepressed: 62 (10) | 45–84 | 3,186 (47%) | CES-D ≥16 and/or self-reported use of antidepressant medication | ADA 2003 (FG)Use of hypoglycemic medication (oral agents and/or insulin) | NGM: 5.0 (0.3)IFG: 5.9 (0.3)UDD: 9.0 (3.0)PDD: 8.5 (3.1)d,e | NGM: 721 (18%)IFG: 304 (16%)UDD: 46 (16%)PDD: 153 (23%) | UDD defined as “untreated diabetes”PDD defined as “treated diabetes”Among 292 individuals with untreated diabetes, 50 (17%) were aware of diagnosis. CES-D scores of two groups were not significantly different (P = 0.28) |
| Germany: Icks et al., 2008 (30) | Population-based study | NGM: 3,995 (87%)UDD: 248 (5%)PDD: 352 (8%) | NGM: M: 59 (8) W: 59 (8)UDD: M: 60 (7) W: 62 (8)PDD: M: 63 (7) W: 64 (7) | 45–75 | NGM: 1,905 (48%)UDD: 175 (71%)PDD: 214 (61%) | CES-D short form ≥15 | FG, random blood glucoseSelf-reported diabetes diagnosis by physicianTaking glucose-lowering medication | NGM: 5.8 (0.5)UDD: 8.0 (1.9)PDD: 8.8 (2.5)e | NGM: 557 (14%)UDD: 15 (6%)PDD: 47 (13%) | |
| U.S.: Rhee et al., 2008 (24) | Adult volunteer subjects who were not known to have diabetes | NGT: 642 (61%)Pre-PDD: 352 (34%)UDD: 53 (5%) | NGT: 46 (12)Pre-PDD: 52 (10)UDD: 55 (10) | NA | NGT: 186 (29%)Pre-PDD: 180 (51%)UDD: 24 (45%) | Patient Health Questionnaire-9 ≥10 | ADA 2007 (OGTT, FPG) | NGT: 4.9 (0.4)Pre-PDD: 5.7 (0.1)UDD: 6.9 (0.9)d,e | NGT: 45 (7%)Pre-PDD: 25 (7%)UDD: 6 (11%) | Pre-PDD: IFG and/or IGTEthnicity (% black): NGT 55%; pre-PDD 51%; UDD 62%Past/current depression treatment: NGT: 11/10% Pre-PDD: 11/10% UDD: 4/13% |
| The Netherlands: Adriaanse et al., 2008 (23) | Population-based study | NGM: 260 (47%)IGM: 164 (30%)PDD: 126 (23%) | NGM: 69 (6)IGM: 70 (6)PDD: 71 (7) | 60–87 | NGM: 130 (50%)IGM: 86 (52%)PDD: 60 (48%) | CES-D ≥16 | WHO 1999 (OGTT, FPG) | NGM: 5.4 (0.4)IGM: 6.1 (0.5)PDD: 7.9 (2.1)e | NGM: 20 (8%)IGM: 24 (15%)PDD: 22 (17%) | IGM: IGT or IFG |
| U.K.: Holt et al., 2009 (25) | Population-based study | NGM: 1,568 (52%)IFG: 298 (10%)IGT: 698 (23%)UDD: 249 (8%)PDD: 182 (6%) | Total: 66 M: 66 (3)W: 67 (3) | 59–73 | NGM: 830 (53%)IFG: 207 (69%)IGT: 309 (44%)UDD: 124 (50%)PDD: 108 (59%) | Hospital Anxiety and Depression Scale depression subscale ≥11: probable depression | WHO (year not specified) (OGTT, FPG)Self-reported previous diabetes diagnosis | NGM: NAIGT: 5.9 (1.1)UDD: 7.2 (1.2)ePDD: NA | NGM: 19 (1%)IGT/IGF: 8 (1%)UDD: 6 (2%)PDD: 4 (2%) | Exact number of participants with NGM is unknown |
| U.K.: Aujla et al., 2009 (26) | Population-based study | NGT: 4,956 (82%)IGR: 855 (14%)UDD: 198 (3%) | 58 (10) | 40–75 | 2,849 (47%) | WHO-5 ≤13 | WHO 2006 (OGTT, FG) | NGT: 5.0 (0.4)IGR: 5.7 (0.7)UDD: 8.1 (3.0)e | NGT: 1,035 (25%)IGR: 167 (26%)UDD: 29 (21%) | IGR: IFG and/or IGTWHO-5 is not a measure of depression2% of participants had preexisting history of depressionOverall sample: 4,682 (78%) white European; 1,327 (22%) South Asian |
| U.S.: Gale et al., 2010 (27) | Ex-military personnel randomly drawn from records of U.S. veterans | NFG: 3,573 (83%)IFG: 492 (11%)UDD: 182 (4%)PDD: 46 (1%) | NFG: 38 (3)IFG: 39 (2)UDD: 39 (2)PDD: 40 (2) | 4,293 (100%) | Diagnostic Interview Schedule major depression | Fasting serum glucoseSelf-reported diabetes diagnosis by physicianUse of diabetes medication | NFG: 5.0 (0.3)IFG: 5.8 (0.1)UDD: 7.0 (2.2)PDD: 9.4 (4.3)e,f | NFG: 227 (6%)IFG: 25 (5%)UDD: 16 (9%)PDD: 8 (17%) | Random sample of 15,288 veterans of telephone surveyDiagnosis of depressionRelatively young sampleUDD | |
| The Netherlands: Bouwman et al., 2010 (28) | Population-based study | NGM: 2,061 (77%)IGM: 425 (16%)PDD: 181 (7%) | NGM: 53 (7)IGM: 55 (6)PDD: 56 (6) | 40–65 | NGM: 923 (45%)IGM: 241 (57%)PDD: 97 (54%) | CES-D ≥16 | WHO 2006 (OGTT, FPG) | NGM: 5.3 (0.4)IGM: 6.0 (0.5)PDD: 7.8 (2.2) | NGM: 258 (13%)IGM: 52 (12%)PDD: 38 (21%) | |
All values are rounded to the nearest integer. ADA, American Diabetes Association; CES-D, Center for Epidemiologic Studies Depression Scale; FG, fasting glucose; FPG, fasting plasma glucose; IFG, impaired fasting glucose; IGR, impaired glucose regulation; IGT, impaired glucose tolerance; M, men; NFG, normal fasting glucose; NGT, normal glucose tolerance; OGTT, oral glucose tolerance test; W, women; WHO, World Health Organization; WHO-5, World Health Organization-5 Wellbeing Index; Zung SDS, Zung Self-Rating Depression Scale.
a% unadjusted for age and sex.
b% differ from reported % in article; presence of missing values or calculation errors unclear.
cValues reported before multiple imputation.
dConverted from mg/dL to mmol/L.
eData obtained through correspondence with the authors.
fThe FG differed significantly between the UDD and PDD groups (P < 0.001).
In total, the included studies identified 1,483 case subjects with UDD, 6,236 case subjects with IGM, and 2,121 case subjects with PDD. However, because the number of studies varied for each comparison, numbers of cases may differ.
Depression in individuals with impaired glucose metabolism
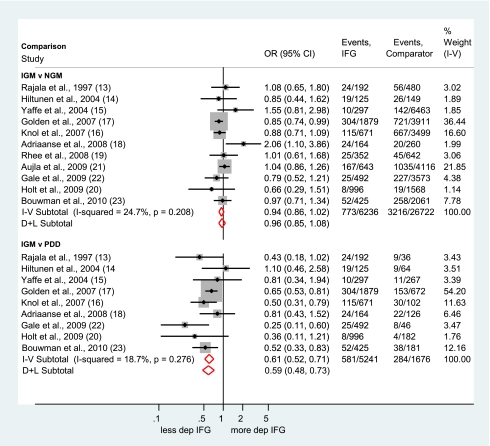
Eleven studies (18–28) compared the prevalence of depression in individuals with IGM relative to individuals with NGM. The forest plot (Fig. 1) clearly shows that, compared with people with normal glucose regulation, the prevalence of depression is not increased in patients with IGM (fixed-effects OR 0.94, 95% CI 0.86–1.02; random-effects OR 0.96, 0.85–1.08). Harbord’s test for reporting bias was negative (P = 0.2). Nine studies reported data on the prevalence of depression in individuals with IGM compared with individuals with PDD (18–23,25,27,28). Compared with individuals with PDD, people with IGM had an almost 40% lower risk of depression (fixed-effects OR 0.61, 95% CI 0.52–0.71; random-effects OR 0.59, 0.48–0.73). In both analyses, the I-squared value was low, suggesting that the results of the fixed-effects model may be appropriate for these comparisons. Because of ambiguities in two studies, we conducted sensitivity analyses to determine whether our analytic decisions affected the results. In one study (20), there appeared to be a discrepancy between the reported number of people with depressive symptoms (n = 10) and the percentage of participants with depressive symptoms (2.2%). Therefore, we compared the results with the raw numbers and the recalculated data based on the reported percentages. Another study (21) reported the total number of participants and ORs based on imputed data; therefore, we compared the results with the total number of participants and with ORs based on imputed data. Results of these sensitivity analyses showed that in both cases, using alternative data did not change the overall results.
Figure 1.
Forest plots showing the OR and 95% CI of depression in IGM compared with NGM and PDD. I-V, fixed-effects estimate (inverse variance method); D+L, random-effects estimate (Der Simonian and Laird method). (A high-quality color representation of this figure is available in the online issue.)
Depression in undiagnosed type 2 diabetes
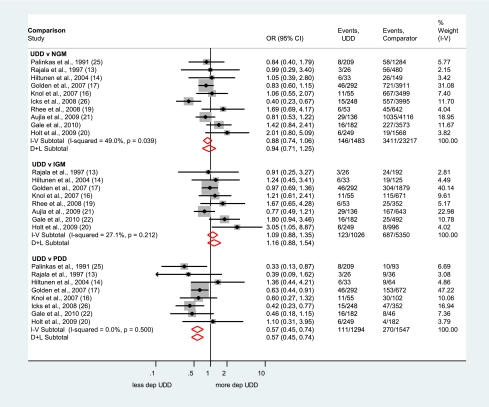
Three comparisons were carried out for the prevalence of depression in UDD: 10 studies (18,19,21,22,24–27,29,30) comparing with NGM, eight studies (18,19,21,22,24–27) comparing with IGM, and eight studies (18,19,21,22,25,27,29,30) comparing with PDD. The I-squared statistic was moderately large for the comparison of UDD versus NGM (I-squared = 49.0%; P = 0.039 for the Cochran's Q test for heterogeneity), suggesting that the random-effects model was the most appropriate for this comparison. However, a single study (30) accounted for all heterogeneity beyond that due to chance, and when this study was omitted from the analysis, the I-squared statistic diminished to 0% and the fixed- and random-effects pooled estimates became 0.98 (95% CI 0.81–1.19). Harbord's test for reporting bias just reached statistical significance (P = 0.03). For consistency, only the random-effects models for each comparison were considered.
The Forest plots of the ORs and 95% CI of each study and the pooled OR for comparisons of UDD versus NGM are shown in Fig. 2: the pooled OR of 0.94 (95% CI 0.71–1.25) indicated that the risk for being depressed was not significantly different. Furthermore, the odds of depression did not differ significantly between UDD versus IGM (OR 1.16, 95% CI 0.88–1.54). Finally, the risk of depression was significantly lower in individuals with UDD versus PDD (OR 0.57, 95% CI 0.45–0.74).
Figure 2.
Forest plots showing the OR and 95% CI of depression in UDD compared with NGM, IGM, and PDD. I-V, fixed-effects estimate (inverse variance method); D+L, random-effects estimate (Der Simonian and Laird method). (A high-quality color representation of this figure is available in the online issue.)
Sensitivity analyses
We conducted sensitivity analyses to determine whether using OR controlled for demographic factors affected the results. In all the studies that provided adjusted OR, NGM was always the reference; thus, we were not able to conduct sensitivity analyses for comparisons of IGM or UDD with PDD or IGM with UDD. Results did not change in the sensitivity analyses. A pooled OR of 0.93 (95% CI 0.85–1.04) was obtained for the comparison of IGM versus NGM (21–23,26–28) and an OR of 1.04 (95% CI 0.85–1.28) for the comparison of UDD versus NGM (21,22,26,27,30).
CONCLUSIONS
The results of the present meta-analysis clearly show that people who have impaired glucose metabolism or undiagnosed type 2 diabetes are not at increased risk for depression compared with people in the general population or people with normal glucose metabolism. When compared with people with known type 2 diabetes, individuals with impaired glucose metabolism or unknown diabetes have significantly lower risk of having depressive symptoms. This result could be regarded as support for the “psychological burden hypothesis” (31), which states that the burden of knowing that you have diabetes and having a chronic illness to manage, or complications to cope with contributes to higher levels of depression. By definition, people with IGM and undiagnosed diabetes have both higher levels of blood glucose than people with normal glucose metabolism or people in the general population. Results of the present meta-analysis indicate that higher blood glucose levels per se in the prediabetic or early diabetes stages are not associated with an increased level of depressive symptoms.
One explanation for the lower risk of depression in UDD compared with PDD might relate to differences in the number of complications between people with UDD and people with PDD. Although diabetes complications can occur in people with undiagnosed diabetes, these are more likely to be found in people with longstanding diabetes (32,33). The results of the current meta-analysis would then concur with a large cross-sectional population-based study that showed that, compared with healthy control subjects, diabetes alone did not increase the chances of depressive symptoms but having diabetes and diabetes complications did (34). However, a recent study showed that the risk of depressive disorder is increased in the 2 years after diagnosis of type 2 diabetes in the absence of diabetes complications (35). In another study, it was found that diabetes distress did not become associated with depressive symptoms until after 1 year of living with diabetes (36). In yet another study, people who were prescribed a more intensive treatment developed more depressive symptoms in the first 3 years after detection of type 2 diabetes than individuals on less intensive treatment (37). These studies suggest that factors other than diabetes complications (e.g., fear of complications, burden of treatment) may increase the risk of depressive symptoms. However, because none of the studies included in the meta-analysis assessed diabetes complications, it was not possible to refute or support this argument. Future studies into depression and undiagnosed diabetes should assess diabetes complications in these groups.
There are several limitations to this study. First, the number of people with undiagnosed diabetes in the included investigations was quite small despite the fact that many were large-scale population-based studies. Second, the meta-analysis draws on observational cohort studies, and it is appropriate to analyze adjusted rather than unadjusted effect estimates. However, because only half of the studies provided adjusted effect estimates and controlled for important demographic confounders, we used the unadjusted ORs in our analyses. However, when calculating pooled ORs based on the studies that did provide ORs controlled for demographics, the outcome did not change. Given these results and the low heterogeneity, we are confident that the results in the current study are reliable. Third, although in all studies oral glucose tolerance tests were used to establish participants’ glucose metabolism classification, it is possible that unmeasured differences in blood glucose level between the previously diagnosed and undiagnosed diabetes groups may explain their differences in depression. Because these data were not routinely available in the published reports, we contacted authors of the more recent articles to obtain these data and used them to calculate weighted pooled mean blood glucose levels for each group. Whereas blood glucose levels did not differ between NGM (mean 5.1, 95% CI 3.9–6.3) and IGM (5.8, 4.6–7.0) and between UDD (8.8, 7.6–10.0) and PDD (8.3, 7.1–9.5), differences between the diabetes groups (UDD and PDD) and individuals without diabetes (NGM and IGM) were significant. These results suggest that despite differences in depression between those with versus those without diabetes, blood glucose levels did not differ within these broader categories. Fourth, the possibility of reporting bias cannot be ruled out. There was weak evidence of reporting bias for the comparison of undiagnosed diabetes versus normal glucose metabolism, but the number of studies here, as for other comparisons, was too low for strong inference.
The relatively low level of heterogeneity observed in most comparisons (I-squared ranging from 0 to 27%) was not amenable to productive exploration using meta-regression; this was because it is recommended that at least 10 studies per study level variable explored are required if spurious associations are to be avoided, and a complete set of data for this number of studies was unfortunately not available for study level variables of interest (e.g., age, sex, fasting plasma glucose).
Fifth, the studies in this meta-analysis used cross-sectional data and therefore do not provide evidence regarding the time frame in which depression develops after the diagnosis of type 2 diabetes. A recent study reported that antidepressant medication use showed a temporary peak during the year of diagnosis of type 2 diabetes, suggesting that the risk of depressive symptoms is increased soon after diagnosis and recedes thereafter in the absence of another incident risk factor (38).
Finally, only one of the included studies (27) used diagnostic criteria to determine depression status. In this study, the prevalence of depression was particularly increased in people with previously diagnosed diabetes (compared with NGM) and in people with undiagnosed diabetes, although for the latter, this failed to reach significance. IGM was not significantly associated with the increased prevalence of major depressive disorder. These findings suggest that diabetes, but not IGM, is associated with increased prevalence of major depressive disorder. However, the numbers in this study were small, and further research is needed.
Overall, the results of this meta-analysis show that the risk of depression is not directly related to elevated blood glucose levels. One conclusion, in line with the results of the current meta-analysis, is that the burden of diabetes and its complications are the main determinants of depressive symptoms in individuals with diabetes (16). Future research should examine the constituents of this burden.
Acknowledgments
No potential conflicts of interest relevant to this article were reported.
A.N. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. G.N. researched data and reviewed the manuscript. I.C. researched data. M.C. researched data, contributed to discussion, and reviewed the manuscript. K.W. researched data and reviewed and edited the manuscript. C.E.L. and M.P. researched data, contributed to discussion, and reviewed and edited the manuscript. F.P. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript.
The authors thank Sue Bayliss of the Unit of Public Health, Epidemiology and Biostatistics, University of Birmingham, U.K., for help with the literature searches.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc10-1414/-/DC1.
References
- 1.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with type 2 diabetes: a systematic review and meta-analysis. Diabet Med 2006;23:1165–1173 [DOI] [PubMed] [Google Scholar]
- 2.Nouwen A, Winkley K, Twisk J, et al. Type 2 diabetes mellitus as a risk factor for the onset of depression: a systematic review and meta-analysis. Diabetologia 2010;53:2480–2486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schram MT, Baan CA, Pouwer F. Depression and quality of life in patients with diabetes: a systematic review from the European Depression in Diabetes (EDID) research consortium. Curr Diabetes Rev 2009;5:112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katon WJ, Rutter C, Simon G, et al. The association of comorbid depression with mortality in patients with type 2 diabetes. Diabetes Care 2005;28:2668–2672 [DOI] [PubMed] [Google Scholar]
- 5.Bruce DG, Davis WA, Starkstein SE, Davis TM. A prospective study of depression and mortality in patients with type 2 diabetes: the Fremantle Diabetes Study. Diabetologia 2005;48:2532–2539 [DOI] [PubMed] [Google Scholar]
- 6.Egede LE, Zheng D, Simpson K. Comorbid depression is associated with increased health care use and expenditures in individuals with diabetes. Diabetes Care 2002;25:464–470 [DOI] [PubMed] [Google Scholar]
- 7.de Groot M, Anderson R, Freedland KE, Clouse RE, Lustman PJ. Association of depression and diabetes complications: a meta-analysis. Psychosom Med 2001;63:619–630 [DOI] [PubMed] [Google Scholar]
- 8.Pouwer F, Skinner TC, Pibernik-Okanovic M, et al. Serious diabetes-specific emotional problems and depression in a Croatian-Dutch-English Survey from the European Depression in Diabetes [EDID] Research Consortium. Diabetes Res Clin Pract 2005;70:166–173 [DOI] [PubMed] [Google Scholar]
- 9.Lustman PJ, Anderson RJ, Freedland KE, de Groot M, Carney RM, Clouse RE. Depression and poor glycemic control: a meta-analytic review of the literature. Diabetes Care 2000;23:934–942 [DOI] [PubMed] [Google Scholar]
- 10.Adriaanse MC, Dekker JM, Spijkerman AM, et al. Diabetes-related symptoms and negative mood in participants of a targeted population-screening program for type 2 diabetes: The Hoorn Screening Study. Qual Life Res 2005;14:1501–1509 [DOI] [PubMed] [Google Scholar]
- 11.Egede LE. Effect of comorbid chronic diseases on prevalence and odds of depression in adults with diabetes. Psychosom Med 2005;67:46–51 [DOI] [PubMed] [Google Scholar]
- 12.Fujinami A, Ohta K, Obayashi H, et al. Serum brain-derived neurotrophic factor in patients with type 2 diabetes mellitus: relationship to glucose metabolism and biomarkers of insulin resistance. Clin Biochem 2008;41:812–817 [DOI] [PubMed] [Google Scholar]
- 13.Krabbe KS, Nielsen AR, Krogh-Madsen R, et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007;50:431–438 [DOI] [PubMed] [Google Scholar]
- 14.Pickup JC. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care 2004;27:813–823 [DOI] [PubMed] [Google Scholar]
- 15.Castrén E, Rantamäki T. Role of brain-derived neurotrophic factor in the aetiology of depression: implications for pharmacological treatment. CNS Drugs 2010;24:1–7 [DOI] [PubMed] [Google Scholar]
- 16.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: results from the World Health Surveys. Lancet 2007;370:851–858 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S, Eds. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.0). The Cochrane Collaboration [Internet], 2008. Available from www.cochrane-handbook.org Accessed 30 June 2010
- 18.Rajala U, Keinänen-Kiukaanniemi S, Kivelä SL. Non-insulin-dependent diabetes mellitus and depression in a middle-aged Finnish population. Soc Psychiatry Psychiatr Epidemiol 1997;32:363–367 [DOI] [PubMed] [Google Scholar]
- 19.Hiltunen L, Keinänen-Kiukaanniemi S, Läärä E, Kivelä S-L. Self-perceived health and symptoms of elderly persons with diabetes and impaired glucose tolerance. Age Ageing 1996;25:59–66 [DOI] [PubMed] [Google Scholar]
- 20.Yaffe K, Blackwell T, Kanaya AM, Davidowitz N, Barrett-Connor E, Krueger K. Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology 2004;63:658–663 [DOI] [PubMed] [Google Scholar]
- 21.Knol MJ, Heerdink ER, Egberts AC, et al. Depressive symptoms in subjects with diagnosed and undiagnosed type 2 diabetes. Psychosom Med 2007;69:300–305 [DOI] [PubMed] [Google Scholar]
- 22.Golden SH, Lee HB, Schreiner PJ, et al. Depression and type 2 diabetes mellitus: the multiethnic study of atherosclerosis. Psychosom Med 2007;69:529–536 [DOI] [PubMed] [Google Scholar]
- 23.Adriaanse MC, Dekker JM, Heine RJ, et al. Symptoms of depression in people with impaired glucose metabolism or type 2 diabetes mellitus: The Hoorn Study. Diabet Med 2008;25:843–849 [DOI] [PubMed] [Google Scholar]
- 24.Rhee MK, Musselman D, Ziemer DC, et al. Unrecognized glucose intolerance is not associated with depression: Screening for Impaired Glucose Tolerance study 3 (SIGT 3). Diabet Med 2008;25:1361–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holt RI, Phillips DI, Jameson KA, Cooper C, Dennison EM, Peveler RC, Hertfordshire Cohort Study Group The relationship between depression and diabetes mellitus: findings from the Hertfordshire Cohort Study. Diabet Med 2009;26:641–648 [DOI] [PubMed] [Google Scholar]
- 26.Aujla N, Abrams KR, Davies MJ, Taub N, Skinner TC, Khunti K. The prevalence of depression in white-European and South-Asian people with impaired glucose regulation and screen-detected type 2 diabetes mellitus. PLoS ONE 2009;4:e7755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gale CR, Kivimaki M, Lawlor DA, Carroll D, Phillips AC, Batty GD. Fasting glucose, diagnosis of type 2 diabetes, and depression: the Vietnam experience study. Biol Psychiatry 2010;67:189–192 [DOI] [PubMed] [Google Scholar]
- 28.Bouwman V, Adriaanse MC, van ’t Riet E, Snoek FJ, Dekker JM, Nijpels G. Depression, anxiety and glucose metabolism in the general Dutch population: the new Hoorn study. PLoS ONE 2010;5:e9971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palinkas LA, Barrett-Connor E, Wingard DL. Type 2 diabetes and depressive symptoms in older adults: a population-based study. Diabet Med 1991;8:532–539 [DOI] [PubMed] [Google Scholar]
- 30.Icks A, Kruse J, Dragano N, et al. Are symptoms of depression more common in diabetes? Results from the Heinz Nixdorf Recall study. Diabet Med 2008;25:1330–1336 [DOI] [PubMed] [Google Scholar]
- 31.Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: is there a link? Diabetes Care 2000;23:1556–1562 [DOI] [PubMed] [Google Scholar]
- 32.Dankner R, Geulayov G, Olmer L, Kaplan G. Undetected type 2 diabetes in older adults. Age Ageing 2009;38:56–62 [DOI] [PubMed] [Google Scholar]
- 33.Bourdel-Marchasson I, Helmer C, Barberger-Gateau P, et al. Characteristics of undiagnosed diabetes in community-dwelling French elderly: the 3C study. Diabetes Res Clin Pract 2007;76:257–264 [DOI] [PubMed] [Google Scholar]
- 34.Pouwer F, Beekman AT, Nijpels G, et al. Rates and risks for co-morbid depression in patients with type 2 diabetes mellitus: results from a community-based study. Diabetologia 2003;46:892–898 [DOI] [PubMed] [Google Scholar]
- 35.O’Connor PJ, Crain AL, Rush WA, Hanson AM, Fischer LR, Kluznik JC. Does diabetes double the risk of depression? Ann Fam Med 2009;7:328–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner TC, Carey ME, Cradock S, et al. Depressive symptoms in the first year from diagnosis of type 2 diabetes: results from the DESMOND trial. Diabet Med 2010;27:965–967 [DOI] [PubMed] [Google Scholar]
- 37.Thoolen BJ, de Ridder DT, Bensing JM, Gorter KJ, Rutten GE. Psychological outcomes of patients with screen-detected type 2 diabetes: the influence of time since diagnosis and treatment intensity. Diabetes Care 2006;29:2257–2262 [DOI] [PubMed] [Google Scholar]
- 38.Kivimäki M, Tabák AG, Lawlor DA, et al. Antidepressant use before and after the diagnosis of type 2 diabetes: a longitudinal modeling study. Diabetes Care 2010;33:1471–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]