Abstract
BACKGROUND AND PURPOSE
The treatment of scleroderma-related digital ulcers is still a therapeutic challenge. The most effective drugs are prostacyclin analogues. However, their usage is limited to an intravenous route of administration and by their frequent side effects. The objective of this study was to test whether treprostinil, iloprost and epoprostenol can induce sustained vasodilatation in rats when delivered locally using cutaneous iontophoresis.
EXPERIMENTAL APPROACH
Treprostinil, iloprost and epoprostenol were delivered by cathodal and anodal iontophoresis onto the hindquarters of anaesthesized rats (n = 8 for each group). Skin blood flow was quantified using laser Doppler imaging and cutaneous tolerance was assessed from day 0 to day 3.
KEY RESULTS
Cathodal but not anodal iontophoresis of treprostinil (6.4 mM), iloprost (0.2 mM) and epoprostenol (1.4 mM) induced a significant and sustained increase in cutaneous blood flow. The effects of treprostinil and iloprost were significantly different from those of treprostinil vehicle. Only weak effects were observed when both drugs were applied locally without current. Skin resistance was unchanged in areas treated with prostacyclin analogues. Finally, skin tolerance was good, with no evidence of epidermal damage.
CONCLUSIONS AND IMPLICATIONS
Cathodal iontophoresis of treprostinil and iloprost increases cutaneous blood flow with a good local tolerance. The effects of cathodal iontophoresis of these drugs should be investigated in humans, as they could have potential as new local therapies for digital ulcers in patients with scleroderma.
Keywords: iontophoresis, prostaglandin I2, prostaglandin analogue, treprostinil, iloprost, microcirculation, rat
Introduction
Systemic sclerosis (SSc) is a rare disease affecting the microcirculation and its treatment poses a difficult therapeutic challenge. Most SSc patients suffer from severe Raynaud's phenomenon, and 30–50% of them exhibit cutaneous ulcers on their fingers, which can lead to amputation (Herrick, 2000). Indeed, despite long-term treatment with calcium channel blockers they are liable to serious handicap. Nitrates have been used for decades for the treatment of Raynaud's phenomenon, but not digital ulcers (Franks, 1982). More recently, bosentan, a non-specific endothelin receptor antagonist, has been indicated to prevent digital ulcers in patients at risk, but has no efficacy on existing ulcers (Korn et al., 2004). Iloprost, a prostacyclin (PGI2) analogue used intravenously, is the only drug approved for the treatment of existing digital ulcers (Wigley et al., 1992). In addition, treprostinil, a PGI2 analogue used subcutaneously, and epoprostenol (intravenous PGI2) are used as second- or third-line treatment in scleroderma patients with pulmonary arterial hypertension. However, the therapeutic effect of prostaglandin analogues is counterbalanced by potentially serious systemic side effects related to their potent vasodilator properties, such as severe headaches, facial flushing, tachycardia and systemic hypotension.
In order to get around the toxicity of systemic treatments, topical drugs have been proposed for SSc-related digital ulcers. For example, new topical nitrate formulations (Chung et al., 2009) have been proposed for Raynaud's phenomenon, but they have no efficacy in the treatment of existing ulcers. In addition to classical topical administration methods (i.e. cream or gel), iontophoresis is a simple, non-invasive transdermal drug delivery method using a low-intensity electric current. Some authors have highlighted the potential of iontophoresis of vasodilator drugs as a treatment for digital ulcers (Murray et al., 2005; 2008;). Indeed, the iontophoretic route has the advantage of optimizing drug diffusion at the site of injury while limiting systemic drug exposure. We recently showed that iontophoresis of sodium nitroprusside (SNP) leads to a non axon reflex-dependent, increase in cutaneous vascular conductance on the forearm of patients with secondary Raynaud's phenomenon (Roustit et al., 2009). Local administration of PGI2 analogues could have potential for the treatment of digital ulcers. However, to our knowledge, iontophoresis of PGI2 analogues has never been tested, either in animals or in humans.
The main objective of the present study was to test whether iontophoretically administered vasodilatator drugs such as PGI2 (epoprostenol) and PGI2 analogues (iloprost and treprostinil) induce an increase in cutaneous blood flow in rats. As a secondary objective, we also assessed the cutaneous tolerance of PGI2 analogues when administered iontophoretically.
Methods
Animals
Fifty male Wistar rats (8 weeks old, 300–400 g), obtained from CERJ (Le Genest-St-Isle, France), were housed in controlled conditions conforming to the current French legislation and fed standard rat chow. The protocol was approved by the Rhone Alps Region Animal Ethics Committee (number 309). The rats were subjected to a day/night cycle of 12 h/12 h and were provided with food and water ad libitum. Three days before the iontophoresis, the fur on the back, the hind legs and the back of the neck was removed by shaving with electric clippers followed by the application of hair removal cream (Veet®, Massy, France). The skin was then wiped with a wet compress and dried. After the experiments, rats were kept, as before, for 3 days for close examination of the skin and then killed by lethal dose of pentobarbital.
Drugs
The PGI2 analogues and intravenous PGI2 we used were: treprostinil (Bioprojet Pharma, Paris, France) 2.5 mg mL−1 solution (MW 390.5 g mol−1; pKa 4.5); iloprost (Bayer Sante, Puteaux, France) 0.1 mg mL−1 solution (MW 478.6 g mol−1); and epoprostenol (GlaxoSmithKline, Marly-le-Roi, France) 0.5 mg 50 mL−1 solvent (glycine, sodium chloride, sodium hydroxide, water for injection) (PGI2 MW 352.5 g mol−1; pKa 7.5) (Alexander et al., 2008). Sodium nitroprusside (Serb, Paris, France) (MW 215.9 g mol−1), a nitrate that induces the activation of soluble guanylyl cyclase through NO release, was used as a positive control. Isotonic sodium chloride (NaCl 0.9%) (Aguettant, Lyon, France) was used as a control solution.
Drugs were administered as commercially available solutions for human use: treprostinil solution at 2.5 mg mL−1 (6.4 mM), iloprost solution at 0.1 mg mL−1 (0.2 mM), epoprostenol solution at 0.01 mg mL−1 (0.028 mM) and sodium nitroprusside at 12.5 mg mL−1 (57.8 mM). For each solution, the pH was determined before iontophoresis using a microprocessor-based pH meter (pH 210, Hanna Instruments, Woonsocket, RI, USA). Iloprost and treprostinil solutions pH were 6 and 6.5, respectively, which are suitable for epidermal application. However, the epoprostenol solution purchased was spontaneously at pH 12 at 0.01 mg mL−1. We therefore adjusted the pH to 6.5 by adding 1 N HCl, and tested both solutions (pH 12 and pH 6.5). After iontophoresis, the pH of all solutions was retested using the semi-quantitative Universal and Special Indicator papers (Macherey-Nagel, Düren, Germany).
The two most potent drugs (treprostinil and iloprost) were tested at three different concentrations. Solutions of treprostinil (6.4 mM) and iloprost (0.2 mM) were diluted with NaCl 0.9% to 0.64 mM and 0.064 mM, and 0.02 mM and 0.002 mM, respectively; as NaCl has been shown to induce less axon reflex non-specific vasodilatation in human skin than pure water (Durand et al., 2002). Pilot experiments in rats with our protocol confirmed these findings.
We asked Bayer Sante and Bioprojet to provide us with the exact compositions of the vehicles for iloprost and treprostinil (placebo form), respectively, or of each drug solution in order to reconstitute it. We had a positive answer only for treprostinil, for which the exact composition of the drug, provided by the manufacturer, was: sodium citrate 6.3 mg mL−1, hydrochloric acid 0.2 mg mL−1, metacresol 3 mg mL−1, sodium hydroxide 0.32 mg mL−1, sodium chloride 5.3 mg mL−1 and water for injection. Treprostinil vehicle was then reconstituted, adjusted for pH and tested as a control.
All solutions were prepared on the same day as iontophoresis.
Experimental procedures
The rats were anaesthetized with sodium pentobarbital (50 mg kg−1 i.p.) and were maintained in the prone position for the duration of the whole experiment, by placing them on the table with the back uppermost (Figure 1); two rats were studied simultaneously. Experiments were performed in a temperature-controlled room, and the rats were placed on a thermal pad, temperature being maintained at 37.5°C, adjusted using a rectal probe in one rat, connected to the thermal pad (Harvard apparatus). Mean arterial blood pressure (three readings) was measured by plethysmography using the tail cuff method, before and immediately after iontophoresis. Before iontophoresis each rat was closely inspected to ensure that the hairless skin in the back and the hind legs was intact. Photographs were taken before the start of iontophoresis, immediately after the iontophoresis and 3 days later. A cutaneous score was used to assess skin tolerance, based on the International Contact Dermatitis Research Group scoring (Fregert, 1981). Negative reactions were coded grade 0; weak reactions (grade 1) are characterized as non-vesicular erythema. Strong positive reactions (grade 2) are characterized as erythema associated with vesicles. Extreme positive reactions (grade 3) are bullous lesions. Irritant reactions (that we coded grade 4) are characterized by necrosis.
Figure 1.
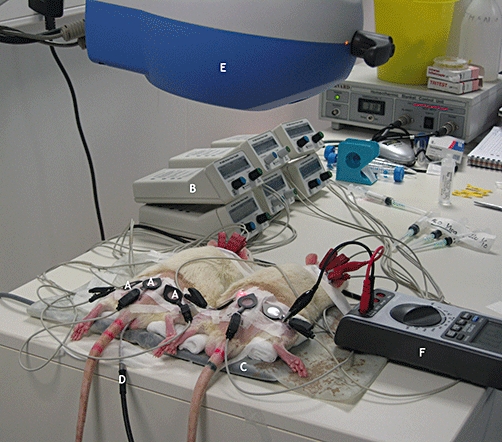
Experimental setup: three iontophoresis chambers (A) connected to power supplies (B) were placed on the back and the hind legs of two shaved rats placed in the prone position on a thermal pad (C), temperature being maintained at 37.5°C and adjusted using a rectal probe (D) in one rat. Laser Doppler imaging (E) was performed over the area including all iontophoresis chambers. Cutaneous resistance was determined during iontophoresis with an amperometric biosensor unit (F).
Histopathological examination of full-thickness skin biopsies from treated and untreated rats skin areas was realized in experiments 2 and 3 detailed thereafter. Eighty biopsies were fixed in AFA fluid (5% acetic acid, 75% absolute ethylic alcohol, and 18% water; Carlo Erba), embedded in paraffin and stained with haematoxylin, eosin, safran. In order to evaluate the effect of the treatment on the skin, various features were searched for, such as hyperkeratosis and epidermolytic aspects. In the stratum corneum, the degree of hyperkeratosis was evaluated, and the presence of parakeratosis was noted. The granular layer was evaluated for perinuclear vacuolar changes, cytolysis and the appearance of the keratohyaline granules. The spinous layer was investigated for the development of these features. Vasculitis, which is a histological diagnosis defined as inflammation targeting blood vessel walls and compromising their function, leading to haemorrhagic and/or ischaemic events, was also assessed. Furthermore, inflammation accompanied by infiltration of neutrophils, lymphocytes or mast cells at the dermo-epiderma interface was evaluated.
Iontophoresis protocol and laser Doppler imaging (LDI)
Iontophoresis probes were used together with LDI (PeriIont System and PeriScan PIM 3 System, Perimed, Järfälla, Sweden). Two or three probes, depending on the area of intact skin available, were placed on the hairless skin of the lower back/hind legs, each probe having a 1.2-cm2 circular contact surface area. A cotton swab was placed under the two hind legs to help maintain the skin surface close to horizontal during the experiment. Passive probes were placed on the back of the neck. Before iontophoresis, baseline LDI data were recorded for 5 min with the laser head placed 20 cm above the skin. The resolution was set at ‘medium’ (2 mm step length) and LDI scans were taken every minute. Given the fact that only two or three sites were simultaneously available on each individual rat, we randomly assigned the order in which the different drugs and controls were used in each experiment. Following data recording, the regions of interest were chosen beneath the probes and from adjacent untreated skin areas, and monitored to ensure the stability of the blood flux (flow rate per area) in each animal. Under our experimental conditions flux values in these control areas were stable in all animals (data not shown).
Cutaneous resistance was determined during iontophoresis with an amperometric biosensor unit (Multimeter system DVM1200, Velleman, Gavere, Belgium). For these measurements, one electrode was connected to the passive probe on the neck of the rat and the other to a probe positioned on a hind leg. Measurements were commenced 5 min after the start of iontophoresis. This delay was chosen in pilot experiments and was aimed at monitoring voltage after the start of iontophoresis. Voltage (expressed in volts) was recorded and skin resistance calculated and expressed in Ω (Ferrell et al., 2002).
The following experiments were performed:
Experiment 1
Iontophoresis was performed for 20 min with the current intensity set at 100 µA using both anodal and cathodal currents (n = 8 for each series). Iontophoresis chambers contained 180 µL drug solution and NaCl 0.9% was used as a control. LDI acquisition was performed throughout the whole procedure.
Experiment 2
We tested the effects of treprostinil and iloprost in the cathodal direction in comparison with treprostinil vehicle in the same animals. LDI acquisition was again performed throughout the whole procedure.
Experiment 3
We tested the effects of treprostinil and iloprost in the cathodal direction at three different concentrations in the same animal. LDI acquisition was again performed throughout the whole procedure and during an additional 60 min after the end of iontophoresis.
Experiment 4
We determined whether iloprost, treprostinil and NaCl 0.9% induced vasodilatation through passive diffusion. Iontophoresis probes were filled with the same drug solutions, but were not connected to the current generator. The length of acquisition was 20 min from application of the drug, the initial scan being used as a reference for baseline flux.
Data analysis
Data are expressed as area under the curve (AUC20min or AUC80min for the third experiment), and expressed as a percentage of baseline flux (%BL.s). Baseline flux was averaged over 5 min.
Qualitative data are expressed as numerical values and percentage in parenthesis. Quantitative data are expressed as mean and SD and were analysed by anova, and with 2 × 2 t-tests for unpaired or paired analyses as required, corrected by Bonferroni's correction. P-values less than 0.05 were considered statistically significant.
Results
Effect of cathodal and anodal iontophoresis of iloprost, treprostinil and epoprostenol on cutaneous flux: initial screening
At the cathode, treprostinil 6.4 mM (115 669 ± 49 812%BL.s, P < 0.001), iloprost 0.2 mM (60 180 ± 43 069%BL.s, P = 0.005) and epoprostenol 0.028 mM pH 12 (42 865 ± 24 922%BL.s, P = 0.0018) induced an increase in AUC20min significantly different from 0 (Figure 2A). As expected, SNP induced a significant increase in AUC20min (41 937 ± 28 785%BL.s, P = 0.008). However, epoprostenol 0.028 mM pH 6.5 (14 557 ± 18 012%BL.s) and NaCl (18 071 ± 16 677%BL.s) did not induce a significant increase in skin blood flux, expressed as AUC20min.
Figure 2.
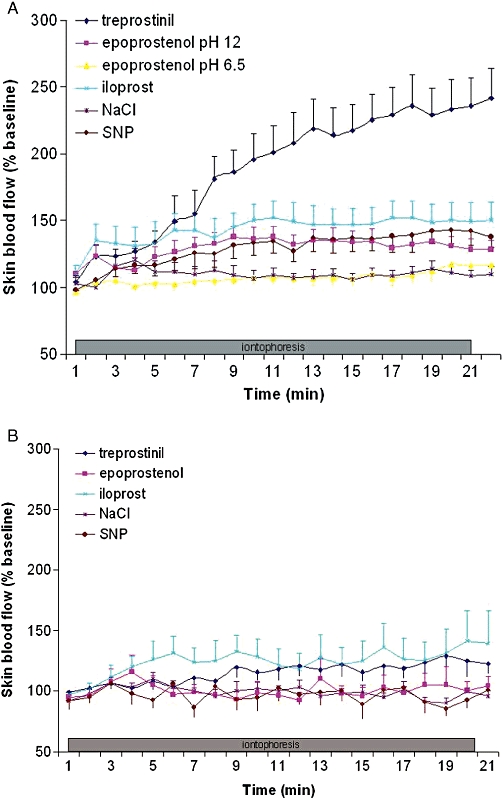
Effects of iontophoresis (A) in the cathodal direction and (B) in the anodal direction, of iloprost 0.2 mM, treprostinil 6.4 mM, epoprostenol 0.028 mM at pH 12 and pH 6.5, NaCl 0.9 % and sodium nitroprussiate (SNP) 57.8 mM, on cutaneous blood flow expressed as % of baseline.
At the anode, although we observed a non-significant trend towards a small increase in AUC20min (36 792 ± 49 268%BL.s, P = 0.07) for iloprost 0.2 mM, this was only significantly different from 0 for treprostinil 6.4 mM (AUC20min 24 695 ± 26 339%BL.s, P = 0.048) (Figure 2B). No increase in AUC20min or iontophoresis end flux was observed for SNP, for epoprostenol 0.028 mM at either pH and for NaCl.
Paired analysis of the effect of treprostinil 6.4 mM and iloprost 0.2 mM in comparison with treprostinil buffer at the cathode
Treprostinil 6.4 mM (107 340 ± 42 269%BL.s, P < 0.0001) and iloprost 0.2 mM (49 484 ± 21 842%BL.s, P < 0.001) induced an increase in AUC20min in comparison with the vehicle for treprostinil (Figure 3).
Figure 3.
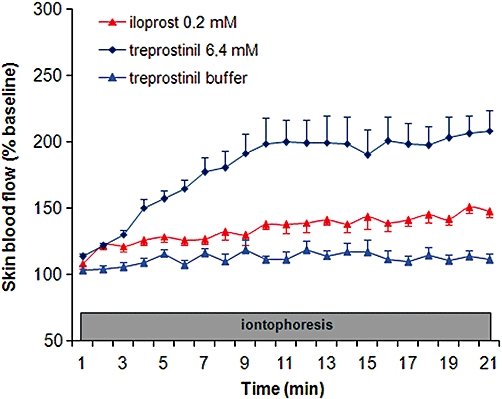
Paired analysis of the effect of treprostinil 6.4 mM and iloprost 0.2 mM in comparison with treprostinil buffer at the cathode, on cutaneous blood flow expressed as % of baseline.
Concentration-dependent effect of cathodal iontophoresis of iloprost and treprostinil on cutaneous flux
Three different concentrations of iloprost and treprostinil were studied. Treprostinil at 6.4 mM, 0.64 mM and 0.064 mM induced an increase in AUC80min that did not differ between concentrations (270 963 ± 117 637%BL.s, 342 568 ± 379 205%BL.s, 190 418 ± 108 746%BL.s, respectively, NS) (Figure 4A).
Figure 4.
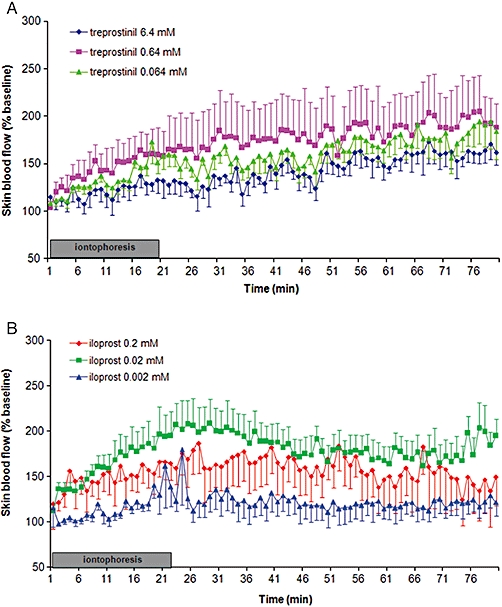
Paired dose-dependent effect of cathodal iontophoresis of (A) treprostinil at 6.4 mM, 0.64 mM and 0.064 mM and (B) iloprost at 0.2 mM, 0.02 mM and 0.002 mM, on cutaneous blood flow expressed as % of baseline.
Iloprost at 0.02 mM induced a significant increase in AUC80min in comparison with iloprost 0.002 mM (379 597 ± 196 125%BL.s vs. 96 730%BL.s ± 145 138, respectively, P = 0.02) (Figure 4B). Iloprost at 0.2 mM tended to increase the AUC (270 126%BL.s ± 303 908) compared with 0.002 mM (P = 0.08). Unlike with treprostinil, the maximal effect of iloprost was observed at the end of the iontophoresis (20 min), and decreased thereafter. However, it did not return to baseline at 80 min.
Immediately after iontophoresis, for all solutions the pH was still compatible with its use as a skin application (pH ranging from 6.5 to 7.9).
Cutaneous resistance
The values for cutaneous resistance during iontophoresis of treprostinil 6.4 mM (45 ± 7 KΩ) and iloprost 0.2 mM (44 ± 5 KΩ) were not significantly different from that obtained with NaCl (45 ± 7 KΩ, NS). When these solutions were diluted, skin resistances were still not different from that with NaCl: 46 ± 14 KΩ for treprostinil 0.64 mM, 49 ± 14 KΩ for treprostinil 0.064 mM, 51 ± 14 KΩ for iloprost 0.02 mM and 48 ±8 KΩ for iloprost 0.002 mM.
Passive transdermal diffusion of iloprost and treprostinil
Treprostinil did not increase cutaneous flux when applied without iontophoresis (Figure 5). Iloprost at 0.02 mM but not 0.2 mM induced a small increase in cutaneous flux, as reflected by a non-significant trend towards an increased AUC20min (iloprost 26 048 ± 30 792%BL.s at 0.02 mM vs. 15 178 ± 22 251%BL.s at 0.2 mM, NS).
Figure 5.
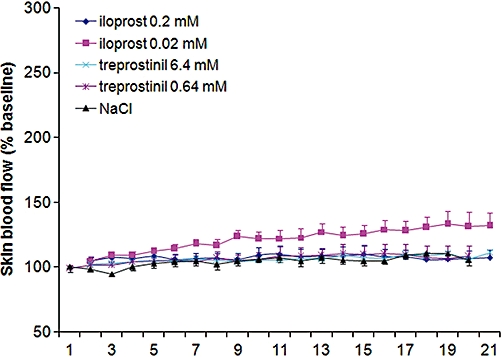
Effect of the passive transdermal diffusion of iloprost 0.2 mM and treprostinil 6.4 mM on cutaneous flux expressed as % baseline blood flow.
Skin and systemic tolerance of the iontophoresis
No side effects of the treatments were observed on the skin. All the cutaneous scores were 0 both immediately after the procedure and at day 3. None of the various histopathological features listed in Methods were found in any of the skin biopsies (Figure 6). No significant drop in mean arterial pressure after iontophoresis was observed with any of the drugs used. During the screening phase, systolic blood pressure was 105 ± 32 mmHg before and 113 ± 30 mmHg after iontophoresis of treprostinil 6.4 mM, 105 ± 19 mmHg before and 112 ± 23 mmHg after iloprost 0.2 mM, 120 ± 30 mmHg before and 112 ± 30 mmHg after epoprostenol 0.028 mM pH 12, 100 ± 14 mmHg before and 108 ± 17 mmHg after epoprostenol 0.028 mM pH 6.5, and 109 ± 32 mmHg before and 114 ± 25 mmHg after NaCl. During experiment 2, systolic blood pressure was 136 ± 27 mmHg before iontophoresis and 129 ± 26 mmHg after. During experiment 3, systolic blood pressure was 106 ± 19 mmHg before iontophoresis and 112 ± 26 mmHg after concentration-dependent iontophoresis of treprostinil and 114 ± 28 mmHg before and 111 ± 33 mmHg after concentration-dependent iontophoresis of iloprost.
Figure 6.
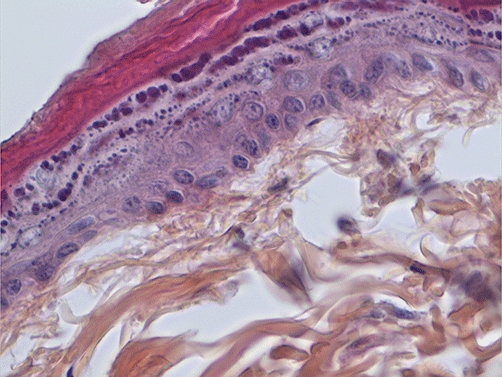
Histopathological examination of a full-thickness skin biopsy taken after iontophoresis of treprostinil 6.4 mM. The sample was embedded in paraffin and stained with haematoxylin, eosin and safran (x40).
Discussion and conclusion
This is the first demonstration that cutaneous cathodal iontophoresis of treprostinil and iloprost, two PGI2 analogues, induces a large and sustained increase in cutaneous blood flow in rats. This pharmacodynamic effect was produced without any local toxic effects on the skin.
Iontophoresis refers to the facilitated movement of ionized molecules through application of an electrical field (Kalia et al., 2004). It has the advantage over passive diffusion of enabling the delivery of polarized and/or larger drugs across the dermal barrier. Iontophoresis enhances the transport of drugs by two major mechanisms in addition to passive diffusion, electrorepulsion and electro-osmosis. Electrorepulsion refers to the ion-electric field interaction that provides a force, which drives ionized drugs through the skin. Electro-osmosis refers to the bulk motion of the solvent that carries ionic or neutral solutes with the solvent stream and is mostly observed when iontophoresis is applied using an anodal current (Dixit et al., 2007). Many factors are critical for transdermal drug delivery using iontophoresis, among these the most important are the drug concentration and molecular weight, the drug's charge, the solution pH (which directly influences ionization), the current strength, and skin hydration and resistance (Dixit et al., 2007). In our study, we tried to optimize these factors: all PGI2 analogues are weak acids that may be theoretically repulsed by a cathodal current when ionized. Our observations are consistent with this hypothesis as we mostly observed increased cutaneous flux with cathodal currents for all drugs. We also observed a weak trend towards increased skin flow for iloprost and treprostinil with anodal currents, and this is probably due to non-specific electro-osmosis. The pH of treprostinil and iloprost solutions, whether diluted or not, was between 5 and 6.5, which is close to skin pH and higher than treprostinil pKa, ensuring a large fraction of ionized molecules. In contrast, skin blood flow increased when the epoprostenol solution was at pH 12, but not when at 6.5, which may be because most epoprostenol is not ionized at neutral pH given its higher pKa value of 7.5. The second possibility is that the stability of PGI2 is highly dependent on pH being quite stable only at alkaline pH. The molecular weight of the drugs was not a limitation in this study, as they are far below the limit for iontophoresis. We applied a relatively high current (100 µA over 20 min, i.e. 120 mC), and it is unclear whether a lower current strength would provide a similar increase in skin blood flow. However, we have observed similar increases in skin blood flux during iontophoresis of 57.8 mM SNP for 20 min at 100 µA through rat skin to that found when we used a 20-µA current in humans (Blaise et al., 2010). This suggests that hairless rat skin is a suitable model for topical and transdermal drug delivery studies, probably due to the similar lipid content and water uptake properties of rat and human skin (Morimoto et al., 1992). In our study, the choice of drug concentrations was based on the currently available concentrations of these drugs for human use and hence the initial concentrations of the drugs differed, and this was the reason why a comparison of the potency of those drugs was not performed. We did not observe a clear-cut concentration-dependent effect for either treprostinil or iloprost (i.e. all treprostinil concentrations induced a large vasodilator response, while for iloprost there was no effect at 2 µM and a maximal effect at 20 µM). This probably reflects the lack of linearity between drug concentration and drug iontophoretic flux across the skin, which has been observed with most drugs used in solutions containing background electrolytes (Kalia et al., 2004). Indeed, our observation is consistent with the general tendency for the flux concentration profile to plateau as concentration increases. This observation is important, as it will help in the choice of drug concentrations to be used in future experiments with healthy human volunteers. We ruled out the potential confounding effect of the excipients for treprostinil, as we were unable to detect any variation in skin blood flow with them. Unfortunately, we were unable to obtain either an iloprost placebo or the exact composition of the drug's excipient, despite several requests to the manufacturer, and are thus unable to provide such a clear conclusion for iloprost. This is a potential limitation of our study. Iloprost and treprostinil are both PGI2 analogues acting through stimulation of IP receptors. The difference observed following cessation of iontophoresis is therefore probably due to a different pharmacokinetic profile in the skin.
Skin resistance is an important parameter that influences the degree of permeability and drug diffusion, and can be measured easily during iontophoresis (Ferrell et al., 2002). In order to rule out variations in skin resistance to the different drugs as a confounding factor, we recorded the potential difference at each site 5 min after the start of iontophoresis. We found no difference in skin resistance between treprostinil, iloprost, whether diluted or not, and NaCl.
A good candidate for therapeutic iontophoresis in patients with skin ulcers would be an ionized drug without cutaneous toxicity and with a large and sustained pharmacodynamic effect at the site of transdermal delivery enabling a once a day delivering protocol, without major systemic side effects. Transdermal iontophoresis is considered to be a safe procedure, associated with only moderate erythema and tingling sensations (Kumar and Lin, 2008). In terms of local toxicity, we observed no local side effects in the 3 days following iontophoresis of the different drugs and the pH remained compatible with topical application. We attempted to ascertain whether any skin irritation or burning occurred. No clinical or histopathological effects were observed. In terms of systemic effects, no variation in arterial pressure was observed, as assessed by the tail cuff method. However, we did not perform continuous blood pressure monitoring for these animals, and this might be considered as a limitation in our study. However, continuous blood pressure monitoring is included in our protocol for future experiments using healthy human volunteers. A fairly recent report showed that SNP and acetylcholine leads to an increased digital flux (assessed using LDI) in eight patients with SSc, five of which exhibited limited cutaneous SSc and three of which diffuse cutaneous SSc (Murray et al., 2008). This suggests that dermal thickening is not a major obstacle to the iontophoresis flux. However, such a question will need to be addressed in our future experiments.
Microvascular function can be routinely studied in human skin using non-invasive LDI (Turner et al., 2008) and thermal hyperaemia is commonly used as a reference for maximal vasodilatation (Cracowski et al., 2006). We did not normalize flux data over maximal vasodilatation in this study as the duration of the experiment would have to have been much longer and more complex, as local heating for 40 min is required to reach a stable thermal plateau. The present study was designed as a drug screening protocol, and given the large effects observed compared with baseline flux, we consider our data as sufficient to initiate human studies in which we will normalize flux over baseline and thermal plateau.
Iontophoresis has been associated with confounding non-specific axon reflex vasodilatation (Cracowski et al., 2006). However, we did not observe this non-specific effect when using NaCl, despite using a current of 100 µA during 20 min. Indeed, in pilot experiments, we tested the effects of both NaCl 0.9% and pure water as vehicles and as a large axon reflex vasodilatation was seen only with pure water, we chose NaCl as the diluent for the drugs (Durand et al., 2002). Also, as a consequence, we did not use lidocaine/prilocaine cream as previously used in human iontophoresis experiments (Blaise et al., 2010).
In conclusion, we showed that cathodal iontophoresis of treprostinil and iloprost induces a large and sustained increase in cutaneous blood flow in rats. This effect was observed using treprostinil concentrations as low as 64 µM and iloprost as low as 20 µM. Weak or no effects of these drugs were observed when they were applied without current. No local side effects were observed. The next step will be to confirm this proof-of-concept in healthy human volunteers. Treprostinil and iloprost cathodal iontophoresis could then be investigated in the future as a new local therapy for digital ulcers in patients with scleroderma.
Acknowledgments
We thank the patients' association ‘Association des Sclérodermiques de France’, and the ‘Groupe Français de Recherche sur la Sclérodermie’ for their financial support, Sandrine Cachot for her technical support, Bioproject Pharma for the composition of the treprostinil excipient and Alison Foote for editing the manuscript.
Glossary
Abbreviations
- PGI2
prostacyclin
- SNP
sodium nitroprusside
- SSc
systemic sclerosis
Conflicts of interest
J.-L. C. and M. R. received unrestricted research grants from Pfizer, Actelion, Ampli and Boiron for their studies.
Financial support
Association des Sclérodermiques de France; Groupe Français de Recherche sur la Sclérodermie.
Supporting Information
Teaching Materials; Figs 1–6 as PowerPoint slide.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edition. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaise S, Hellmann M, Roustit M, Isnard S, Cracowski JL. Oral sildenafil increases skin hyperaemia induced by iontophoresis of sodium nitroprusside in healthy volunteers. Br J Pharmacol. 2010;160:1128–1134. doi: 10.1111/j.1476-5381.2010.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung L, Shapiro L, Fiorentino D, Baron M, Shanahan J, Sule S, et al. MQX-503, a novel formulation of nitroglycerin, improves the severity of Raynaud's phenomenon: a randomized, controlled trial. Arthritis Rheum. 2009;60:870–877. doi: 10.1002/art.24351. [DOI] [PubMed] [Google Scholar]
- Cracowski JL, Minson CT, Salvat-Melis M, Halliwill JR. Methodological issues in the assessment of skin microvascular endothelial function in humans. Trends Pharmacol Sci. 2006;27:503–508. doi: 10.1016/j.tips.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Dixit N, Bali V, Baboota S, Ahuja A, Ali J. Iontophoresis – an approach for controlled drug delivery: a review. Curr Drug Deliv. 2007;4:1–10. doi: 10.2174/1567201810704010001. [DOI] [PubMed] [Google Scholar]
- Durand S, Fromy B, Bouye P, Saumet JL, Abraham P. Current-induced vasodilation during water iontophoresis (5 min, 0.10 mA) is delayed from current onset and involves aspirin sensitive mechanisms. J Vasc Res. 2002;39:59–71. doi: 10.1159/000048994. [DOI] [PubMed] [Google Scholar]
- Ferrell WR, Ramsay JE, Brooks N, Lockhart JC, Dickson S, McNeece GM, et al. Elimination of electrically induced iontophoretic artefacts: implications for non-invasive assessment of peripheral microvascular function. J Vasc Res. 2002;39:447–455. doi: 10.1159/000064515. [DOI] [PubMed] [Google Scholar]
- Franks AG., Jr Topical glyceryl trinitrate as adjunctive treatment in Raynaud's disease. Lancet. 1982;1:76–77. doi: 10.1016/s0140-6736(82)90215-x. [DOI] [PubMed] [Google Scholar]
- Fregert S. Manual of Contact Dermatitis, 2nd edn. Copenhagen: Munksgaard; 1981. pp. 71–76. [Google Scholar]
- Herrick AL. Vascular function in systemic sclerosis. Curr Opin Rheumatol. 2000;12:527–533. doi: 10.1097/00002281-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Kalia YN, Naik A, Garrison J, Guy RH. Iontophoretic drug delivery. Adv Drug Deliv Rev. 2004;56:619–658. doi: 10.1016/j.addr.2003.10.026. [DOI] [PubMed] [Google Scholar]
- Korn JH, Mayes M, Matucci Cerinic M, Rainisio M, Pope J, Hachulla E, et al. Digital ulcers in systemic sclerosis: prevention by treatment with bosentan, an oral endothelin receptor antagonist. Arthritis Rheum. 2004;50:3985–3993. doi: 10.1002/art.20676. [DOI] [PubMed] [Google Scholar]
- Kumar MG, Lin S. Transdermal iontophoresis: impact on skin integrity as evaluated by various methods. Crit Rev Ther Drug Carrier Syst. 2008;25:381–401. doi: 10.1615/critrevtherdrugcarriersyst.v25.i4.30. [DOI] [PubMed] [Google Scholar]
- Morimoto Y, Hatanaka T, Sugibayashi K, Omiya H. Prediction of skin permeability of drugs: comparison of human and hairless rat skin. J Pharm Pharmacol. 1992;44:634–639. doi: 10.1111/j.2042-7158.1992.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Murray AK, Herrick AL, Gorodkin RE, Moore TL, King TA. Possible therapeutic use of vasodilator iontophoresis. Microvasc Res. 2005;69:89–94. doi: 10.1016/j.mvr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Murray AK, Moore TL, King TA, Herrick AL. Vasodilator iontophoresis a possible new therapy for digital ischaemia in systemic sclerosis? Rheumatology (Oxford) 2008;47:76–79. doi: 10.1093/rheumatology/kem314. [DOI] [PubMed] [Google Scholar]
- Roustit M, Blaise S, Cracowski JL. Sodium nitroprusside iontophoresis on the finger pad does not consistently increase skin blood flow in healthy controls and patients with systemic sclerosis. Microvasc Res. 2009;77:260–264. doi: 10.1016/j.mvr.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Turner J, Belch JJ, Khan F. Current concepts in assessment of microvascular endothelial function using laser Doppler imaging and iontophoresis. Trends Cardiovasc Med. 2008;18:109–116. doi: 10.1016/j.tcm.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Wigley FM, Seibold JR, Wise RA, McCloskey DA, Dole WP. Intravenous iloprost treatment of Raynaud's phenomenon and ischemic ulcers secondary to systemic sclerosis. J Rheumatol. 1992;19:1407–1414. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


