Abstract
BACKGROUND AND PURPOSE
Two non-psychoactive cannabinoids, cannabidiol (CBD) and cannabichromene (CBC), are known to modulate in vitro the activity of proteins involved in nociceptive mechanisms, including transient receptor potential (TRP) channels of vanilloid type-1 (TRPV1) and of ankyrin type-1 (TRPA1), the equilibrative nucleoside transporter and proteins facilitating endocannabinoid inactivation. Here we have tested these two cannabinoids on the activity of the descending pathway of antinociception.
EXPERIMENTAL APPROACH
Electrical activity of ON and OFF neurons of the rostral ventromedial medulla in anaesthetized rats was recorded extracellularly and tail flick latencies to thermal stimuli were measured. CBD or CBC along with various antagonists were injected into the ventrolateral periaqueductal grey.
KEY RESULTS
Cannabidiol and CBC dose-dependently reduced the ongoing activity of ON and OFF neurons in anaesthetized rats, whilst inducing antinociceptive responses in the tail flick-test. These effects were maximal with 3 nmol CBD and 6 nmol CBC, and were antagonized by selective antagonists of cannabinoid CB1 adenosine A1 and TRPA1, but not of TRPV1, receptors. Both CBC and CBD also significantly elevated endocannabinoid levels in the ventrolateral periaqueductal grey. A specific agonist at TRPA1 channels and a synthetic inhibitor of endocannabinoid cellular reuptake exerted effects similar to those of CBC and CBD.
CONCLUSIONS AND IMPLICATIONS
CBD and CBC stimulated descending pathways of antinociception and caused analgesia by interacting with several target proteins involved in nociceptive control. These compounds might represent useful therapeutic agents with multiple mechanisms of action.
Keywords: pain, cannabinoids, endocannabinoids, TRP channels, adenosine, serotonin, receptors, transporters, brainstem
Introduction
The periaqueductal grey (PAG) has a key role in the descending modulation of nociception (Behbehani, 1995; Fields, 2000). Although this region does have direct projections to the spinal cord (Sandkühler and Gebhart, 1984), it uses the rostral ventromedial medulla (RVM), a site that projects directly to the spinal cord dorsal horn, as an important intermediate in pain modulation (Basbaum and Fields, 1984; Fields et al., 1995). Glutamate and γ-aminobutyric acid play a critical role in processing pain at the PAG-RVM level (Behbehani and Fields, 1979; Moreau and Fields, 1986; Harris and Hendrickson, 1987; Millan et al., 1987). Three different neuronal classes are found in the RVM (Fields et al., 1991): ‘neutral cells’, which show no modification in spontaneous activity associated with nociceptive stimulation; ON cells, which show a burst of activity just before withdrawal reflexes; and OFF cells, which are inhibited just before withdrawal reflexes. These neurons usually respond in opposite ways to pharmacological stimulation with antinociceptive substances: systemic or local injections of µ-opioid or cannabinoid CB1 receptor agonists sufficient to inhibit nociceptive reflexes usually inhibit the ongoing and tail-flick-related activities of ON cells whilst increasing the activities and reducing the pauses of OFF cells (Fields et al., 1983; Heinricher and Tortorici, 1994; Meng et al., 1998; Fields, 2004). We have recently reported that also local stimulation of transient receptor potential vanilloid-type 1 (TRPV1) channels (Palazzo et al., 2002; Maione et al., 2006; Starowicz et al., 2007) or adenosine A1 receptors (Maione et al., 2007) in the ventrolateral (vl) PAG exerts antinociceptive effects in rats by stimulating a similar descending pathway, and resulting in the stimulation and inhibition of OFF and ON cell ongoing activities respectively. Interestingly, TRPV1 receptor activation in the vl-PAG interacts with both CB1 (Maione et al., 2006) and µ-opioid (Maione et al., 2009) receptor-mediated stimulation of such pathway.
Plant-derived cannabinoids, and in particular the two most abundant ones in Cannabis, Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD), are known to possess strong analgesic and anti-inflammatory properties and their clinical use is now supported by several preclinical studies and clinical trials (see Mechoulam et al., 2007; Karst and Wippermann, 2009; Rahn and Hohmann, 2009; for reviews). While most of the pharmacological effects of THC appear to be mediated selectively by cannabinoid CB1 and CB2 receptors, CBD is capable of interacting with several molecular targets involved in the control of pain. These include: (i) one of the enzymes involved in the inactivation of endocannabinoids, the fatty acid amide hydrolase (FAAH), as well as the as yet unclarified mechanism of endocannabinoid cellular reuptake (Watanabe et al., 1996; Bisogno et al., 2001) – indeed, inhibitors of endocannabinoid inactivation have been found to exert antinociceptive and antihyperalgesic activity in several studies (Petrosino and Di Marzo, 2010, for review); (ii) the TRPV1 channel (Bisogno et al., 2001), the desensitization of which causes analgesia and can be mediated by CBD; (iii) the equilibrative nucleoside transporters, which CBD inhibits at sub-micromolar concentrations (Carrier et al., 2006), thereby causing elevation of adenosine signalling and associated pharmacological actions, including analgesia and inhibition of inflammation; and (iv) the 5-HT1A receptor (Russo et al., 2005; Campos and Guimarães, 2008; Resstel et al., 2009; Magen et al., 2010; Zanelati et al., 2010), which has been implicated in the descending modulation of nociception (Kishimoto et al., 2001; Huo et al., 2008). Furthermore, CBD also stimulates and desensitizes the ankyrin-type 1 transient receptor potential channel (TRPA1), and this property is shared with other non-psychotropic plant cannabinoids, of which cannabichromene (CBC) is the one with highest potency and selectivity (De Petrocellis et al., 2008). TRPA1 is emerging as an important player in nociception (Cai, 2008), and its direct activation and desensitization by the synthetic CB1/CB2 receptor agonist WIN55,212-2 and the CB2 receptor agonist AM1241 causes TRPA1-dependent antinociceptive effects in vivo (Akopian et al., 2008). Although inactive at TRPV1 channels and FAAH, CBC can inhibit endocannabinoid degradation by interfering with endocannabinoid cellular uptake more potently than CBD (Ligresti et al., 2006).
In view of the well-documented presence in the vl-PAG of several of the potential molecular targets of CBD and/or CBC (FAAH, putative endocannabinoid transporter, ENTs, TRPV1) (Maione et al., 2006; 2008;), and of the possible presence in this area also of TRPA1, which is very often co-localized with TRPV1 in sensory neurons, and was suggested to be expressed in the brainstem (Sun et al., 2009), we decided to investigate the effects of the injection of these phytocannabinoids (see Figure 1 for their chemical structures) into the vl-PAG on RVM ON and OFF cell activity and tail-flick-related nociception in anaesthetized rats.
Figure 1.
Chemical structures of cannabidiol (CBD) and cannabichromene (CBC).
Methods
Animals and treatments
All animal care and experimental procedures complied with Italian (D.L. 116/92) and EECabO.J. of E.C. L358/1 (18/12/86) regulations on the protection of laboratory animals and were approved by the Animal Ethics Committee of the Second University of Naples. All efforts were made to minimize animal suffering and to reduce the number of animals used. A total of 260 Wistar male rats (250–300 g) were used (Harlan, Milan, Italy). Rats were housed three per cage under controlled illumination (12:12 h light : dark cycle; light on 06:00 h) and environmental conditions (ambient temperature 20–22°C, humidity 55–60%) for at least 1 week before the commencement of experiments. Rat chow and tap water were available ad libitum.
Groups of 12–16 animals per treatment were used in order to have at least 6–8 ON and OFF cell recordings with each animal being used for a single cell recording.
Rats receiving intra-vl-PAG microinjections of vehicle or different doses of CBD and CBC, alone or in combination with antagonists were grouped as follows:
A group of rats received an intra-vl-PAG microinjection of 200 nL of vehicle (0.2% dimethyl sulfoxide, DMSO, in rtificial CSF).
Groups of rats received intra-vl-PAG microinjections of same volume of CBD (1.5, 3 and 6 nmol) alone or CBD (3 nmol) in combination with the selective TRPV1 antagonist, 5′-iodo-resiniferatoxin (I-RTX, 1 nmol), or the selective CB1 receptor antagonist, 1-(2,4-dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-(1-piperidyl)pyrazole-3-carboxamide (AM 251, 0.5 nmol), or the selective adenosine A1 receptor antagonist, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX, 0.05 nmol), or the selective TRPA1 antagonist (Z)-4-(4-chlorophenyl)-3-methylbut-3-en-2-oxime (AP18, 6 nmol), or the selective 5-HT1A receptor antagonist, N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide (WAY100635, 0.34 nmol).
Groups of rats received intra-vl-PAG microinjections of CBC (3 and 6 nmol) alone or CBC (6 nmol) in combination with I-RTX (1 nmol), AM251 (0.5 nmol), DPCPX (0.05 nmol) or AP18 (6 nmol).
A group of rats received intra-vl-PAG microinjections of OMDM-2 (1.5 and 3 nmol), a selective inhibitor of endocannabinoid cellular reuptake (Ortar et al., 2003).
A group of rats received intra-vl-PAG microinjections of mustard oil (3 and 6 nmol), a classical agonist at TRPA1 channels.
As, to our knowledge, no other study has been published describing the effects of the drugs using a similar administration route in the rat, we performed preliminary experiments (not shown) with several doses of all drugs in order to find the lowest doses able to change RVM cell activities and/or tail-flick latencies or, in the case of the antagonists, the highest doses inactive per se.
Electrophysiological analyses and tail flick test
In order to perform direct intra-vl-PAG administrations of drugs or respective vehicle, rats were anaesthetized with pentobarbital (50 mg kg−1, i.p.) and a 26-gauge, 12 mm-long stainless steel guide cannula was stereotaxically lowered until its tip was 1.5 mm above the vl-PAG by applying coordinates (A: −7.8 mm and L: 0.5 mm from bregma, V: 4.3 mm below the dura) from the atlas of Paxinos and Watson (1986). The cannula was anchored with dental cement to a stainless steel screw in the skull. We used a David Kopf stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA) with the animal positioned on a homeothermic temperature control blanket (Harvard Apparatus Limited, Edenbridge, Kent, UK). Only those rats whose microinjected site was located within the vl-PAG, recognized by histological methods, were used for data computation (Figure 2).
Figure 2.
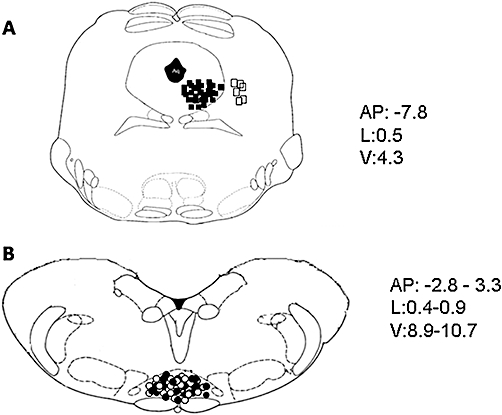
Schematic illustration of the location of periaqueductal gray (PAG) microinjection sites (A) and rostral ventromedial medulla (RVM) ON or OFF cell recording sites (B). Vehicle or drug microinjections were performed in the ventrolateral (vl)-PAG (filled squares) (A). Open squares indicate the microinjection sites performed outside the vl-PAG, which were neither associated with change in RVM cell activity nor with tail-flick latency. Moreover, cell recordings performed by lowering a tungsten electrode into the RVM and ON cells (filled circles) or OFF cell (open circles) sites (B) are shown. Many sites are not shown to avoid symbol overlapping. Distances (mm) from the interaural line are indicated.
Anaesthesia was maintained with a continuous infusion of propofol (5–10 mg kg−1 h−1, i.v.), through a non-sterile catheter (polyethylene tubing, ID 0.58 mm, OD 0.96 mm, Becton Dickinson & Co., Franklin Lakes, NJ, USA) inserted into the left jugular vein, and adjusted so that tail flicks were elicited with a constant latency of 4–5 s. A thermal stimulus was elicited by a radiant heat source of a tail flick unit (Ugo Basile, Varese, Italy), focused on the rat tail approximately 3–5 cm from the tip. The intensity of the radiant heat source was adjusted to 50 mW (corresponding to 50 mJ per second) at the beginning of each experiment in order to elicit a constant tail-flick latency. A glass-insulated tungsten filament electrode (3–5 MW) (FHC Frederick Haer & Co., Bowdoin, ME, USA) was lowered into the RVM using the following stereotaxic coordinates: 2.8–3.3 mm caudal to lambda, 0.4–0.9 mm lateral and 8.9–10.7 mm depth from the surface of the brain (Paxinos and Watson, 1986) (Figure 2). RVM noxious stimuli-responding neurons were identified by the characteristic OFF cell pause and ON cell burst immediately prior to tail flick responses (Fields et al., 1991). The recorded signals were amplified and displayed on both analogue and a digital storage oscilloscope to ensure that the unit under study was unambiguously discriminated throughout the experiment. Signals were also fed into a window discriminator, whose output was processed by an interface (CED 1401) (Cambridge Electronic Design Ltd, Cambridge, UK) connected to a Pentium III PC. Spike2 software (CED, version 4) was then used to create peristimulus rate histograms online and to store and analyse digital records of single-unit activity offline. The configuration, shape and height of the recorded action potentials were monitored and recorded continuously using a window discriminator and Spike2 software for online and offline analyses. Once an ON or OFF cell was identified from its background activity, we optimized spike size before all treatments. This study only included neurons whose spike configuration remained constant and could clearly be discriminated from the background activity throughout the entire experiment. For each neuron the ongoing activity was obtained by averaging the firing rate (spikes s−1) for 50 s before the tail flick trials (carried out every 5 min). Moreover, the peak height of the tail-flick-related burst (spikes s−1) of the ON cells and the duration of the tail-flick-related pause (the time elapsing between the pause onset and the first action potential following tail flick) of OFF cells were also quantified. Recording sites were recognized with an electrolytic lesion at the conclusion of the experiment. The locations of all the studied neurons were reconstructed and plotted on standardized sections. Cells located outside the RVM were excluded from the study (Figure 2).
Tail flick latencies and extracellular recordings were considered before and after microinjecting drugs, or respective vehicle, into the vl-PAG. When CBD or CBC was administered in combination with I-RTX, AM251, DPCPX, AP18 or WAY100635, the two drugs were always co-injected. Tail flick latencies were monitored in the same rats undergoing RVM ON and OFF cell recordings.
Analysis of endocannabinoid levels in the PAG
In order to perform the endocannabinoid analysis, a different cohort of rats was used. Rats were decapitated 20 min after intra-PAG drug/vehicle microinjections, brains were rapidly removed and immersed in oxygenated ice-cold artificial cerebrospinal fluid. A brainstem slice of 1.30–1.35 mm was cut throughout the rostral-caudal PAG using a vibrotome (Vibratome 1500, Warner Instruments, CT, USA) (interaural from + 1.9 mm to + 0.7 mm, Paxinos and Watson, 1986). The slice of tissue containing the PAG/dorsal raphe was then further dissected under a optical microscope for microsurgery to isolate the vl-PAG (M650, Wild Heerbrugg, Glattbrugg, Switzerland) to be homogenized accordingly to the following protocol. In brief, tissues were homogenized in five volumes of chloroform/methanol/Tris HCl 50 mM (2:1:1) containing 20 pmol of d8-arachidonoyl ethanolamide (AEA) or d5-2-arachidonoyl glycerol (2-AG). Deuterated standards were synthesized from commercially available deuterated arachidonic acid and ethanolamine or glycerol, as described, respectively, in Devane et al. (1992) and Bisogno et al. (1997). Homogenates were centrifuged at 13 000×g for 16 min (4°C), the aqueous phase plus debris was collected and extracted again twice with one volume of chloroform. The organic phases from the three extractions were pooled and the organic solvents evaporated in a rotating evaporator. Lyophilized extracts were resuspended in chloroform/methanol (99:1, v v−1). The solutions were then purified by open bed chromatography on silica as described in Bisogno et al. (1997). Fractions eluted with chloroform/methanol 9:1 by volume (containing AEA and 2-AG) were collected, the excess solvent was evaporated with a rotating evaporator, and aliquots were analysed by isotope dilution-liquid chromatography/atmospheric pressure chemical ionization/mass spectrometry (LC-APCI-MS) carried out under conditions described previously (Marsicano et al., 2002) and allowing the separation of the four compounds. Mass spectrometric (MS) detection was carried out in the selected ion monitoring mode using m/z-values of 356 and 348 (molecular ions + 1 for deuterated and undeuterated AEA) and 384.35 and 379.35 (molecular ions + 1 for deuterated and undeuterated 2-AG).The area ratios between the signals of the deuterated and undeuterated compounds varied linearly with varying amounts of undeuterated compounds (30 fmol–100 pmol). AEA and 2-AG levels in unknown samples were therefore calculated on the basis of their area ratios with the internal deuterated standard signal areas. For 2-AG, the areas of the peaks corresponding to 1(3)-and 2-isomers were added together. The amounts of endocannabinoids were expressed as pmol g−1 of wet tissue weight.
Data analysis
Results were expressed as means ± SEM of latency time to the tail withdrawal reflex or spikes s−1 obtained by averaging the ongoing cell firing recorded in 50 s before tail flick trials (which were carried out every 5 min). Tail-flick-related ON cell burst was calculated as means ± SEM of the number of spikes in the 10 s interval starting from the beginning of the increase in the cell frequency. The duration of the cell pause was expressed as means ± SEM of the time elapsing between the pause onset and the 1st spike after the tail flick. Comparisons between pretreatment and post-treatment ongoing and tail-flick-related cell activity changes were performed by anova for repeated measures. Comparisons between different treated groups of rats were performed by using Wilcoxon signed-ranks test. P < 0.05 was considered statistically significant.
Materials
5′-iodo-resiniferatoxin, DPCPX, AM 251, OMDM-2, and propofol were supplied by Tocris (Bristol, UK) and AP-18, WAY100635, pentobarbital and mustard oil were from Sigma-Aldrich (Milano, Italy). CBD and CBC were obtained from GW Pharmaceuticals, Salisbury, UK. Receptor and channel nomenclature follows Alexander et al. (2009)
Results
Effect of intra-vl-PAG CBD and CBC on ongoing ON and OFF cell activity in anaesthetized rats
The results were based on RVM neurons (one cell recorded from each animal per treatment) at a depth of 9900–10 955 µm from the surface of the brain. All recorded neurons, identified as OFF cells by the characteristic pause induced by the tail flick trail, were spontaneously active and discharged with a mean frequency of 7.58 ± 0.6 spikes s−1 (Figure 3B).
Figure 3.
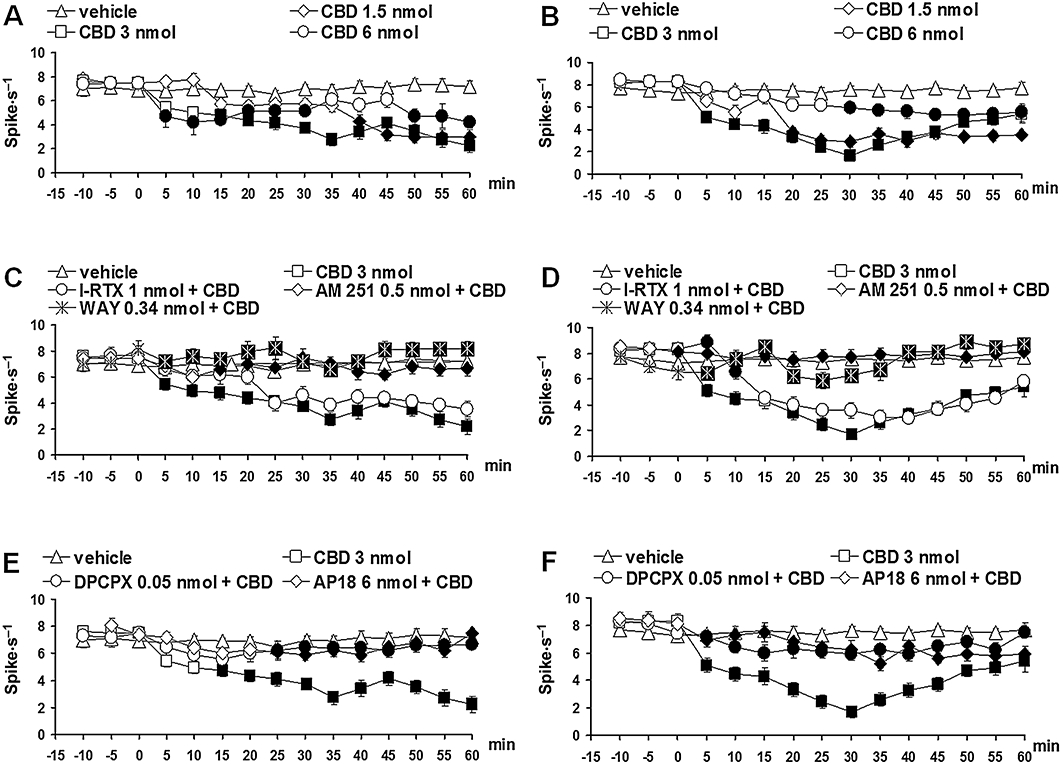
Effect of vehicle, cannabidiol (CBD) (1.5, 3 and 6 nmol) alone or CBD (3 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol), DPCPX (0.05 nmol), AP18 (6 nmol) or WAY100635 (0.34 nmol) on the spontaneous firing of rostral ventromedial medulla ON (A, C and E) or OFF (B, D and F) cells. A and B show the effect of intra-ventrolateral periaqueductal gray (vl-PAG) microinjections of vehicle and CBD (1.5, 3 and 6 nmol). C and D show the effect of intra-vl PAG administration of CBD (3 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol) or WAY100635 (0.34 nmol). E and F show the effect of intra-ventrolateral periaqueductal gray administration of CBD (3 nmol) in combination with DPCPX (0.05 nmol) or AP18 (6 nmol). Each point represents the mean ± standard error of the mean (SEM) of 6–8 neurons. Filled symbols indicate values significantly different (P < 0.05) from vehicle or CBD (3 nmol). DPCPX, 1,3-dipropyl-8-cyclopentylxanthine; I-RTX, 5′-iodo-resiniferatoxin.
Neurons identified as ON cells by a burst of activity just before tail flick responses were spontaneously active in 33.2% of the cases and inactive in the remaining cases. ON cells with spontaneous activity were chosen in order to characterize drug-related changes on both ongoing and tail-flick-related activity. The population of ON cells with spontaneous activity had a mean frequency of 7.12 ± 0.4 spikes s−1 (Figure 3A).
Microinjection of vehicle did not change the spontaneous activity of either ON (F(1,14) = 0; P > 0.05) or OFF cells (F(1,14) = 0.02; P > 0.05). Microinjections of CBD (1.5, 3 and 6 nmol) into the vl-PAG caused a decrease on the firing activity of the ON cells. CBD (3 nmol) induced a greater effect with a maximal decrease observed 35 min after microinjection (F(1,14) = 28.64; P < 0.01) (Figure 3A). The same treatment produced a decrease in the firing activity of the OFF cells, which was maximal 30 min after the microinjection of CBD (3 nmol) (F(1,14) = 88.65; P < 0.01) (Figure 3B). CBD (3 nmol) also reduced tail-flick-related ON cell burst (F(1,14) = 8.71; P < 0.05) (Figure 4A) but was devoid of activity on the OFF cell pause. The effects induced by CBD (3 nmol) on the ON and OFF cell were prevented when AM251 (0.5 nmol) (Figures 3C and D, 4B), DPCPX (0.05 nmol), AP18 (6 nmol) (Figures 3E and F, 4C) or WAY-100635 (0.34 nmol) (Figures 3C and D, 4B) were co-injected. I-RTX (1 nmol) only antagonized the effect of CBD (3 nmol) on the OFF cells at 5 and 10 min after drug microinjection (Figure 3D).
Figure 4.
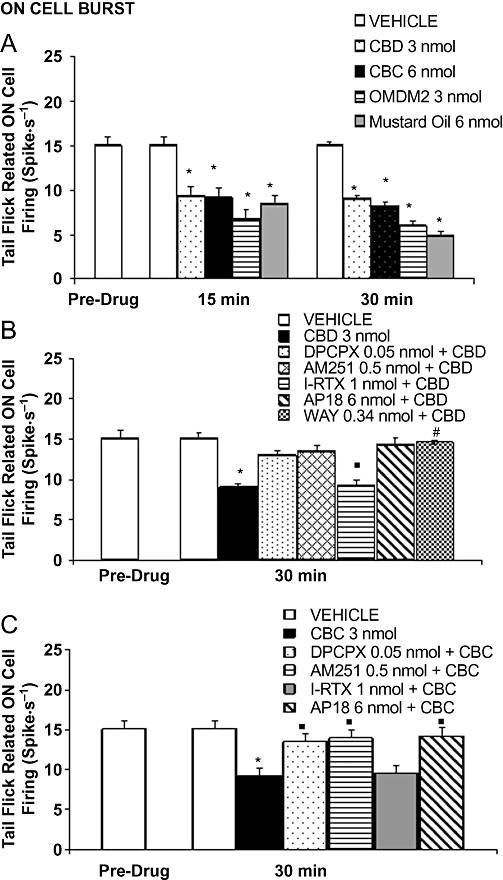
Effects of intra-ventrolateral periaqueductal gray microinjections of vehicle, cannabidiol (CBD) (3 nmol), cannabichromene (CBC) (6 nmol), OMDM-2 (3 nmol) and mustard oil (6 mol) on the tail flick induced RVM ON burst of firing (A). B shows the effect of vehicle, CBD (3 nmol) alone or CBD (3 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol), DPCPX (0.05 nmol), AP18 (6 nmol) or WAY100635 (0.34 nmol). C shows the effect of vehicle, CBC (6 nmol) alone or CBC (6 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol), DPCPX (0.05 nmol) and AP18 (6 nmol). Each histogram represents the mean ± SEM of 6–8 neurons. * indicates values significantly different (P < 0.05) from vehicle and ° from CBD (3 nmol) or CBC (6 nmol). DPCPX, 1,3-dipropyl-8-cyclopentylxanthine; I-RTX, 5′-iodo-resiniferatoxin.
Cannabichromene (3 and 6 nmol) induced effects similar to those elicited by CBD, that is, a decrease on the ongoing activity of the ON and OFF cells. In particular, the decrease in the ON cell ongoing activity was maximal 30 min after the injection of CBC (6 nmol) (F(1,14) = 22.65; P < 0.01) (Figure 5A). CBC (6 nmol) also reduced tail-flick-related ON cell burst (F(1,14) = 9.15; P < 0.01) (Figure 4A) without changing the OFF cell pause. The decrease in the OFF cell ongoing activity started 5 min after CBC (6 nmol) microinjection and was maximal 35 min after its administration (F(1,14) = 49.97; P < 0.01) (Figure 5D). Similarly to CBD, the effect of CBC (6 nmol) was antagonized by AM251 (0.5 nmol) (Figures 4C, 5C and D), DPCPX (0.05 nmol) and AP18 (6 nmol) (Figures 4C, 5E and F), although not by I-RTX (1 nmol) (Figures 4C, 5C and D).
Figure 5.
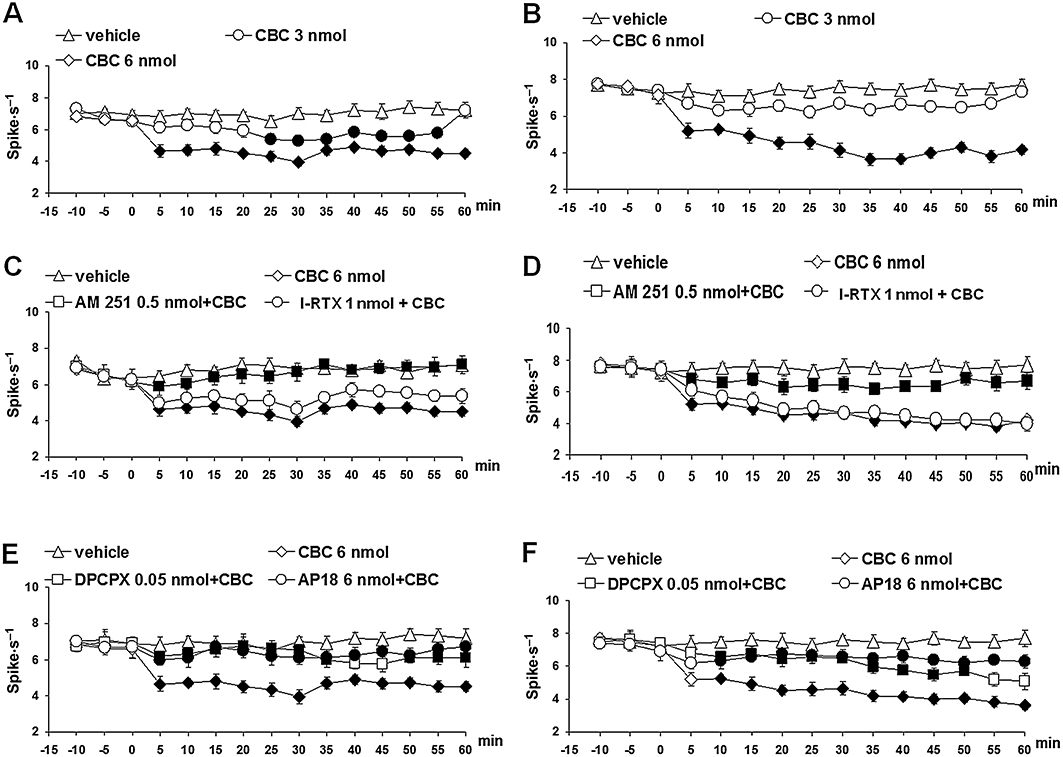
Effect of vehicle, cannabichromene (CBC) (3 and 6 nmol) alone or CBC (6 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol), DPCPX (0.05 nmol) and AP18 (6 nmol) on the spontaneous firing of RVM ON (A, C and E) or OFF (B, D and F) cells. A and B show the effect of intra-vl PAG microinjections of vehicle and CBC (3 and 6 nmol). C and D show the effect of intra-ventrolateral periaqueductal gray administration of CBC (6 nmol) in combination with AM251 (0.5 nmol) or I-RTX (1 nmol). E and F show the effect of intra-vl PAG administration of CBC (6 nmol) in combination with DPCPX (0.05 nmol) or AP18 (6 nmol). Each point represents the mean ± standard error of the mean (SEM) of 6–8 neurons. Filled symbols indicate values significantly different (P < 0.05) from vehicle and from CBC (6 nmol). DPCPX, 1,3-dipropyl-8-cyclopentylxanthine; I-RTX, 5′-iodo-resiniferatoxin; vl-PAG, ventrolateral periaqueductal grey.
Effect of intra-vl-PAG CBD and CBC on tail-flick-related nociception in anaesthetized rats
Tail flicks were elicited every 5 min for at least 10 min prior to microinjecting drugs or respective vehicle into the vl-PAG. Data related to pretreatment intervals were considered as basal tail flick latencies (4.5 ± 0.3 s). Intra-vl PAG microinjection of vehicle did not change the tail-flick latency as compared with basal values. Tail-flick latency proved to be significantly increased by CBD (1.5 and 3 nmol). The dose of 3 nmol CBD produced the highest antinociceptive effect (F(1,30) = 38.12; P < 0.01) while the dose of 6 nmol was devoid of antinociceptive effect (Figure 6A). The antinociceptive effect of CBD (3 nmol) was blocked by AM251 (0.5 nmol) or WAY100635 (0.34 nmol) (Figure 6C), DPCPX (0.05 nmol) or AP18 (6 nmol). I-RTX (1 nmol) was instead able to block CBD (3 nmol) antinociceptive effect only at 15 min after drug microinjection (Figure 6C).
Figure 6.
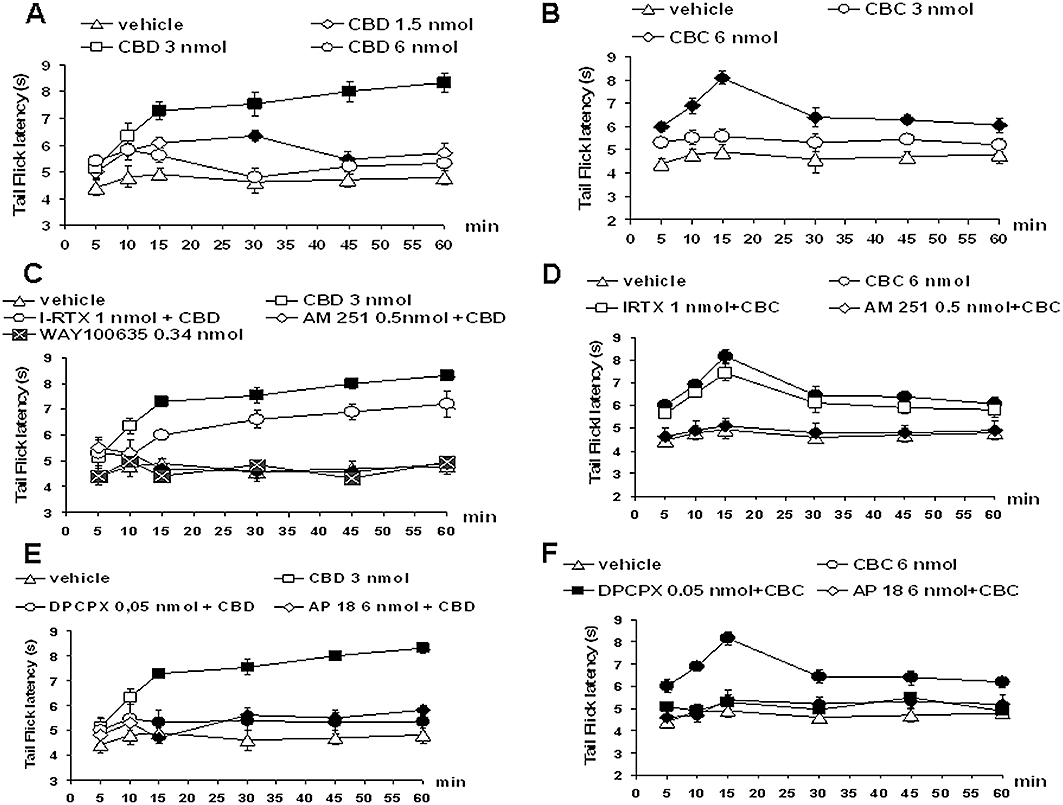
Effect of vehicle, cannabidiol (CBD) (1.5, 3 and 6 nmol) and cannabichromene (CBC) (3 and 6 nmol) alone or CBD (3 nmol) or CBC (6 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol), DPCPX (0.05 nmol) and AP18 (6 nmol) on the tail-flick latency. A shows the effect of intra-vl PAG microinjections of vehicle and CBD (1.5, 3 and 6 nmol). B shows the effect of intra-vl PAG administration of CBC (3 and 6 nmol). C shows the effect of intra-vl PAG administration of CBD (3 nmol) in combination with AM251 (0.5 nmol), I-RTX (1 nmol) or WAY100635 (0.34 nmol). D shows the effect of intra-vl PAG administration of CBC (6 nmol) in combination with AM251 (0.5 nmol) or I-RTX (1 nmol). E shows the effect of intra-vl PAG administration of CBD (3 nmol) in combination with DPCPX (0.05 nmol) or AP18 (6 nmol). F shows the effect of intra-vl PAG administration of CBC (6 nmol) in combination with DPCPX (0.05 nmol) or AP18 (6 nmol). Each point represents the mean ± standard error of the mean (S.E.M) of 12–16 rats. Filled symbols indicate values significantly different (P < 0.05) from vehicle or from CBD (3 nmol) and CBC (6 nmol). DPCPX, 1,3-dipropyl-8-cyclopentylxanthine; I-RTX, 5′-iodo-resiniferatoxin; vl-PAG, ventrolateral periaqueductal grey.
Tail-flick latency was also increased by CBC (3 and 6 nmol) with a maximal effect at the higher dose used (F(1,30) = 40.70; P < 0.01) (Figure 6B) and at 15 min after its administration. Such an antinociceptive effect was prevented by AM251 (0.5 nmol) (Figure 6D), DPCPX (0.05 nmol) or AP18 (6 nmol) (Figure 6F) but not by I-RTX (1 nmol) (Figure 6D).
Effect of intra-vl-PAG injection of mustard oil and OMDM-2 on ongoing ON and OFF cell activity and tail-flick-related nociception in anaesthetized rats
In order to substantiate the involvement of TRPA1 channels and endocannabinoid cellular uptake in the effects of the phytocannabinoids, we next tested pharmacological tools specific for these two targets. Both OMDM-2 (1.5 and 3 nmol), a selective and potent inhibitor of endocannabinoid cellular uptake (Ortar et al., 2003), and mustard oil isothiocyanate (3 and 6 nmol), the prototypical activator of TRPA1 (Jordt et al., 2004) injected into the vl-PAG, reduced ON and OFF cell ongoing activity (Figure 7A–D). In particular, OMDM-2 (1.5 nmol) decreased the ON cell ongoing activity with a noticeable delay (35 min). The highest dose of OMDM-2 (3 nmol) decreased the ON cell ongoing activity as early as 15 min after its administration and showed a maximal effect 35 min after its administration (F(1,14) = 116.46; P < 0.01) (Figure 7A). OMDM-2 also decreased the OFF cell ongoing activity but only at the higher dose used (3 nmol). This effect was evident after 15 min and maximal 35 min after its administration (F(1,14) = 135.98; P < 0.01) (Figure 7B). The lowest dose of mustard oil (3 nmol) did not change the ON cell ongoing activity. The highest dose (6 nmol) decreased the ON cell ongoing activity after 20 min showing a maximal effect after 35 min from its administration (F(1,14) = 23.82; P < 0.01) (Figure 7C). The highest dose of mustard oil (6 nmol) also decreased the OFF cell ongoing activity already after 5 min from its administration showing a maximal effect 35–40 min after its administration (F(1,14) = 28.55; P < 0.01) (Figure 7D). OMDM-2 (3 nmol) and mustard oil (6 nmol) decreased also the tail-flick-induced ON cell burst (Figure 4A) but were devoid of activity on the OFF cell pause. OMDM-2 (3 nmol) and mustard oil (6 nmol) increased the tail-flick latency (F(1,30) = 22.49 and F(1,30) = 8.98 respectively; both P < 0.01) (Figure 7E and F).
Figure 7.
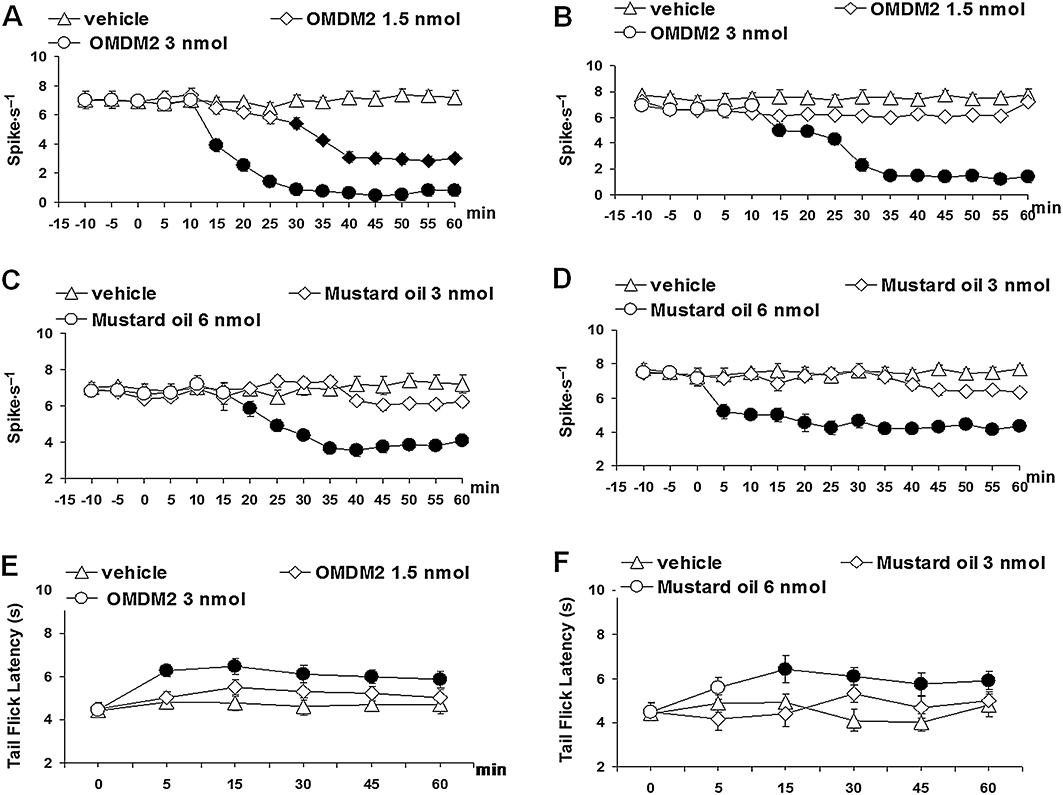
Effect of vehicle, OMDM-2 (1.5 and 3 nmol) or mustard oil (3 and 6 nmol) on the spontaneous firing of rostral ventromedial medulla ON (A and C) and OFF (B and D) cells and on nocifensive behaviour through tail flick test (E and F). Each point represents the mean ± standard error of the mean (SEM) of 6–8 neurons (A and D) or 12–16 rats (E and F) per groups. Filled symbols indicate values significantly different (P < 0.05) from vehicle.
Effect of intra-vl-PAG CBD and CBC on endocannabinoid levels in the PAG
The effect of the intra vl-PAG injection of maximally active doses of CBD (3 nmol) and CBC (6 nmol) on PAG endocannabinoid levels of lipid extracts of the PAG were determined by LC-MS (Table 1). CBD significantly elevated 2-AG by 2.6-fold, but not anandamide levels. CBC, instead, significantly elevated both 2-AG levels, by almost 3.9-fold and anandamide levels, by almost 1.7-fold.
Table 1.
Amounts of anandamide and 2-arachidonoylglycerol (2-AG) in the rat periaqueductal grey (PAG) after injection of cannabidiol (CBD) or cannabichromene (CBC)
| Anandamide (pmol g−1 tissue) | 2-AG (nmol g−1 tissue) | |
|---|---|---|
| Vehicle | 90.1 ± 15.8 | 7.8 ± 2.1 |
| CBD (3 nmol) | 107.7 ± 14.4 | 20.5 ± 4.4* |
| CBC (6 nmol) | 148.7 ± 21.0* | 30.1 ± 6.1** |
Injection of vehicle (0.2% DMSO in aCSF), or cannabidiol (CBD, 3 nmol in 0.2 µL of vehicle) or cannabichromene (CBC, 3 nmol in 0.2 µL of vehicle) were made into the ventrolateral PAG. Data are means ± SEM of n = 4 determinations.
P < 0.05
P < 0.01 as assessed by anova followed by Bonferroni's test.
Discussion
We have described here for the first time the dose-related effects of intra-vl-PAG injections of two major non-psychotropic phytocannabinoids, CBD and CBC, on the activity of the descending pathway of antinociception in anaesthetized rats. We found that the two compounds behave in a similar way by producing tail-flick-related antinociceptive responses accompanied by the expected decrease in ON cell ongoing activity and by a paradoxical decrease of OFF cell ongoing activity, in the RVM. Indeed, most of the analgesic compounds that have been studied on RVM ON and OFF cell activity, for example, µ-opioid, CB1 cannabinoid and adenosine A1 receptor agonists, have been found to reduce ON cell and stimulate OFF cell activity after intra-PAG injections (Fields et al., 1983; Heinricher and Tortorici, 1994; Meng et al., 1998; Fields, 2004; de Novellis et al., 2005; Maione et al., 2007). Therefore, in view of the fact that we are proposing here that the action of CBD and CBC in the vl-PAG mechanisms is mediated by CB1 and adenosine A1 receptors, it is difficult to explain why these compounds inhibited both ON and OFF cell ongoing activity. It is possible that the one of the mechanisms of action that we have suggested here for these two compounds, that is, the activation of TRPA1 channels, by stimulating glutamatergic signalling in the vl-PAG, as recently shown for another brainstem region, the nucleus tractus solitarius (Sun et al., 2009), activates descending pathways that do not only turn off ON cells but also interfere with the activity of OFF cells in the RVM (see Rea et al., 2007). Against this possibility is the previous finding that stimulation, in the vl-PAG, of TRPV1 receptors, which also enhance glutamatergic signalling in this area as well as in the RVM (Starowicz et al., 2007), reduces ON cell but stimulates OFF cell activity. On the other hand, we have recently reported that N-arachidonoyl-5-HT, a mixed inhibitor of endocannabinoid inactivation and a blocker of TRPV1 receptors, also causes antinociception accompanied by inhibition of both ON and OFF cell activity (de Novellis et al., 2008). Also CBD and CBC inhibit endocannabinoid inactivation (Ligresti et al., 2006), and hence, elevate endocannabinoid levels, as shown in the present study, and this might explain also why their effects were found here to be sensitive to the CB1 antagonist, AM251 (the two phytocannabinoids have almost negligible intrinsic affinity for CB1 receptors). Furthermore, again like N-arachidonoyl-5-HT, by activating TRPA1, CBC and CBD might also desensitize and hence block TRPV1 [see (Jeske et al., 2006; Akopian et al., 2008) for examples of such mechanism]. Therefore, it is possible that these compounds act on both RVM ON and OFF cell activity in a way similar to N-arachidonoyl-5-HT.
Indeed, we found here that, when reproducing the putative effect of CBC and CBD on endocannabinoid cellular uptake by using an intra-vl-PAG injection of a synthetic inhibitor of such mechanism, inhibition of both ON and OFF cell activity in the RVM, as well as inhibition of tail-flick-related nociception, were again observed, although in this case the former effect was somewhat delayed as compared with those of the phytocannabinoids. Thus, inhibition of ON-cell ongoing activity in the RVM might be sufficient to cause antinociceptive activity even in the presence of inhibition of OFF cell activity, a finding that is supported by more than one recent study (de Novellis et al., 2008), and which suggests that ON cells might be more important than OFF cells in determining the output of the descending PAG-RVM pathway of antinociception (Heinricher and Neubert, 2004; Kincaid et al., 2006; Neubert et al., 2004; Bee and Dickenson, 2007; Xu et al., 2007; Heinricher and Ingram, 2008; Heinricher et al., 2009). However, considering that the highest dose of CBD reduced ON-cell activity without exerting any antinociceptive effect in the tail-flick test, some discrepancies seem to emerge between the behavioural and electrophysiological experiments in the current study, thus raising important questions about ON-cell activity as a functional correlate of descending pain pathway activity. Indeed, this finding seems to contradict the idea that the ongoing activity of both ON and OFF cells modulates nociceptive responsiveness (Heinricher et al., 1989; Jinks et al., 2004; Foo and Mason, 2005). Furthermore, it is noteworthy that previous studies have shown that ON-cell bursts can be completely inhibited, without any consequent change of tail-flick latency (Heinricher and McGaraughty, 1998). It is possible that ON-cell firing provides a complex and critical regulatory pronociceptive output that might be more important in specific pathological conditions (Bederson et al., 1990; Wiertelak et al., 1997), whereas in healthy animals, as in the case of our experiments, ON-cell discharge may not always be a potent regulator of moment-to-moment variations in nociceptive responsiveness.
We also observed here that, similar to CBD and CBC, mustard oil isothiocyanate, a specific activator of TRPA1 with no direct action on TRPV1 or on endocannabinoid inactivation, and OMDM-2, a specific inhibitor of endocannabinoid cellular re-uptake, when injected into the vl-PAG, inhibited both ON and OFF cell ongoing activity in the RVM and nociception. However, both compounds produced a somewhat delayed response as compared to CBC and CBD, and their analgesic effect in the tail-flick response was lower. These observations, together with the data obtained previously with selective adenosine A1 and CB1 receptor agonists, whilst supporting the hypothesis that the two phytocannabinoids act at multiple targets in the vl-PAG, also suggest that it is the combination of several effects that confers on CBC and CBD their unique ‘pharmacological fingerprint’ in terms of modulation of ON and OFF cell activity.
As mentioned above, the stimulatory effect of CBD and CBC on PAG endocannabinoid levels and the antagonism of CBD and CBC actions by AM251, observed here, support the involvement of mechanisms of endocannabinoid inhibition in the mode of action of the two phytocannabinoids. Interestingly, while CBD is more efficacious than CBC at inhibiting FAAH, CBC is significantly more potent at inhibiting endocannabinoid cellular uptake (Ligresti et al., 2006). This might explain why CBC elevated both anandamide and 2-AG levels, because both endocannabinoids are good substrates for the putative membrane endocannabinoid transporter (Bisogno et al., 2002), whereas FAAH inhibition was previously shown to elevate anandamide but not 2-AG levels in the whole brain (Kathuria et al., 2003). Nevertheless, it has also been previously shown that the selective FAAH inhibitor, URB597, when injected into the vl-PAG, can produce elevation of both anandamide and 2-AG levels (Maione et al., 2006). Clearly, CBD is a much less potent FAAH inhibitor than URB597 (Bisogno et al., 2001; Kathuria et al., 2003), thus possibly explaining why this compound, in terms of its differential effect on the levels of the two endocannabinoids, seemed to be acting in this study more as a weak endocannabinoid uptake inhibitor than as a blocker of FAAH.
Our findings indicate that CBD and CBC exert their effects by inhibiting not only endocannabinoid, but also adenosine inactivation. In fact, their actions were antagonized here not only by AM251, but also by the selective adenosine A1 receptor antagonist DPCPX. This finding is in agreement with previous evidence indicating that CBD can produce several pharmacological effects via inhibition of the equilibrative nucleoside transporters (Carrier et al., 2006), and subsequent enhancement of adenosine tone at the various adenosine receptors (Liou et al., 2008; Magen et al., 2009; Castillo et al., 2010). However, it must be emphasized that no direct evidence exists for the interaction of CBC with the equilibrative adenosine transporter (ENT), although Carrier et al. (2006) did show that several ‘classical’ (including THC) and non-classical cannabinoids can exert this property, thus suggesting that the structural prerequisite for cannabinoid interaction with this transporter might be such to allow its interaction also with CBC.
Cannabidiol has been reported to exert some of its pharmacological actions by enhancing the activity of the 5-HT1A receptor (Russo et al., 2005; Campos and Guimarães, 2008; Resstel et al., 2009; Magen et al., 2010; Zanelati et al., 2010). This receptor is involved in supra-spinal antinociception and is expressed in the PAG, and its activation in this brain area was associated with inhibition of γ-aminobutyric acid release in the PAG, but not yet with descending analgesia (Behbehani et al., 1993; Kishimoto et al., 2001; Huo et al., 2008). We report here that the effect of CBD on ON and OFF neurons of the RVM and on tail-flick latency is also antagonized by a selective 5-HT1a antagonist, thus adding further complexity to the action of this phytocannabinoid. Further studies with selective 5-HT1A receptor agonists are now required to assess whether this mechanism can account for the somewhat surprising inhibition of both ON and OFF neuron activity caused by intra-vl-PAG injection of CBD and CBC.
Indeed, one might wonder whether the effects found here with CBC and CBD on ON and OFF cell activity are the results of sequential or simultaneous activation of several targets. When looking at the delayed actions of OMDM-2 and mustard oil, it is possible to hypothesize that the effect of the two phytocannabinoids on TRPA1 and endocannabinoid uptake occurs after those on the ENT. It is possible that adenosine elevation by the two compounds, and the subsequent activation of adenosine A1 receptors causes, on the one hand, elevation of endocannabinoid levels, which would then be enhanced by the inhibitory effect on endocannabinoid cellular uptake, and on the other hand activation of TRPA1. Although there is no evidence of adenosine A1 receptor activation of TRPA1, previous data have shown that this receptor can instead sensitize TRPV1 channels (Vaughan et al., 2006), the gating of which might lead to TRPA1 activation (Akopian et al., 2007; Salas et al., 2009). However, the only evidence so far pointing to an interaction between adenosine A1 receptors and endocannabinoid release showed inhibition, rather than stimulation, by the former over the latter (Hoffman et al., 2010). Moreover, one should not neglect the possibility that the differences observed in the time-course of the effects of CBC and CBD on behaviour and cell firing may be merely due to differences in the pharmacokinetics of these drugs, such as differential rates of diffusion through the PAG, differential susceptibility to metabolism/degradation or vascular uptake.
In conclusion, the present findings indicate, for the first time, that two non-psychotropic phytocannabinoids, CBD and CBC, produce antinociceptive effects also at the supraspinal level by interacting with several targets involved in the control of pain. They also provide unprecedented in vivo evidence for the targeting by these compounds of TRPA1 channels and endocannabinoid inactivating mechanisms, which might open new avenues in their therapeutic exploitation (Cai, 2008; Petrosino and Di Marzo, 2010). Further studies will be now necessary to identify other pharmacological effects of CBD and CBC that are due, at least in part, to these mechanisms.
Acknowledgments
This study was partly supported by a research grant from GW Pharmaceuticals.
Glossary
Abbreviations
- 2-AG
2-arachidonoyl glycerol
- AEA
arachidonoyl ethanolamide (anandamide)
- CB1
cannabinoid receptor type 1
- CBC
cannabichromene
- CBD
cannabidiol
- FAAH
fatty acid amide hydrolase
- RVM
rostral ventromedial medulla
- THC
Δ9-tetrahydrocannabinol
- TRPA1
transient receptor potential channels of ankyrin type-1
- TRPV1
transient receptor potential channels of vanilloid type-1
- vl-PAG
ventrolateral periaqueductal grey
Conflict of interest
The authors declare no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–7 as PowerPoint slide.
References
- Akopian AN, Ruparel NB, Jeske NA, Hargreaves KM. Transient receptor potential TRPA1 channel desensitization in sensory neurons is agonist dependent and regulated by TRPV1-directed internalization. J Physiol. 2007;583:175–193. doi: 10.1113/jphysiol.2007.133231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28:1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Fields HL, Barbaro NM. Hyperalgesia during naloxone-precipitated withdrawal from morphine is correlated with increased ON cell activity in the rostral ventromedial medulla. Somatosens Mot Res. 1990;7:185–203. doi: 10.3109/08990229009144706. [DOI] [PubMed] [Google Scholar]
- Bee LA, Dickenson AH. Rostral ventromedial medulla control of spinal sensory processing in normal and pathophysiological states. Neuroscience. 2007;147:786–793. doi: 10.1016/j.neuroscience.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Prog Neurobiol. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Fields HL. Evidence that an excitatory connection between the periaqueductal gray and nucleus raphe magnus mediates stimulation produced analgesia. Brain Res. 1979;170:85–93. doi: 10.1016/0006-8993(79)90942-9. [DOI] [PubMed] [Google Scholar]
- Behbehani MM, Liu H, Jiang M, Pun RY, Shipley MT. Activation of serotonin1A receptors inhibits midbrain periaqueductal gray neurons of the rat. Brain Res. 1993;612:56–60. doi: 10.1016/0006-8993(93)91643-7. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Maurelli S, Melck D, De Petrocellis L, Di Marzo V. Biosynthesis, uptake, and degradation of anandamide and palmitoylethanolamide in leukocytes. J Biol Chem. 1997;272:3315–3323. doi: 10.1074/jbc.272.6.3315. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Hanus L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, et al. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol. 2001;134:845–852. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, De Petrocellis L, Di Marzo V. Fatty acid amide hydrolase, an enzyme with many bioactive substrates. Possible therapeutic implications. Curr Pharm Des. 2002;8:533–547. doi: 10.2174/1381612023395655. [DOI] [PubMed] [Google Scholar]
- Cai X. A new tr(i)p to sense pain: TRPA1 channel as a target for novel analgesics. Expert Rev Neurother. 2008;8:1675–1681. doi: 10.1586/14737175.8.11.1675. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 2008;199:223–230. doi: 10.1007/s00213-008-1168-x. [DOI] [PubMed] [Google Scholar]
- Carrier EJ, Auchampach JA, Hillard CJ. Inhibition of an equilibrative nucleoside transporter by cannabidiol: a mechanism of cannabinoid immunosuppression. Proc Natl Acad Sci U S A. 2006;103:7895–7900. doi: 10.1073/pnas.0511232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A, Tolón MR, Fernández-Ruiz J, Romero J, Martinez-Orgado J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol Dis. 2010;37:434–440. doi: 10.1016/j.nbd.2009.10.023. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Vellani V, Schiano-Moriello A, Marini P, Magherini PC, Orlando P, et al. Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J Pharmacol Exp Ther. 2008;325:1007–1015. doi: 10.1124/jpet.107.134809. [DOI] [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- Fields HL. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565–575. doi: 10.1038/nrn1431. [DOI] [PubMed] [Google Scholar]
- Fields HL, Vanegas H, Hentall ID, Zorman G. Evidence that disinhibition of brain stem neurones contributes to morphine analgesia. Nature. 1983;306:684–686. doi: 10.1038/306684a0. [DOI] [PubMed] [Google Scholar]
- Fields HL, Heinricher MM, Mason P. Neurotransmitters in nociceptive modulatory circuits. Annu Rev Neurosci. 1991;14:219–245. doi: 10.1146/annurev.ne.14.030191.001251. [DOI] [PubMed] [Google Scholar]
- Fields HL, Malick A, Burstein R. Dorsal horn projection targets of ON and OFF cells in the rostral ventromedial medulla. J Neurophysiol. 1995;74:1742–1759. doi: 10.1152/jn.1995.74.4.1742. [DOI] [PubMed] [Google Scholar]
- Foo H, Mason P. Sensory suppression during feeding. Proc Natl Acad Sci U S A. 2005;102:16865–16869. doi: 10.1073/pnas.0506226102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RM, Hendrickson AE. Local circuit neurons in the rat ventrobasal thalamus – a GABA immunocytochemical study. Neuroscience. 1987;21:229–236. doi: 10.1016/0306-4522(87)90335-6. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, A Comprehensive Reference. Pain, Vol. 5. San Diego, CA: Academic Press; 2008. pp. 593–626. [Google Scholar]
- Heinricher MM, McGaraughty S. Analysis of excitatory amino acid transmission within the rostral ventromedial medulla: implications for circuitry. Pain. 1998;75:247–255. doi: 10.1016/s0304-3959(97)00226-1. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Neubert MJ. Neural basis for the hyperalgesic action of cholecystokinin in the rostral ventromedial medulla. J Neurophysiol. 2004;92:1982–1989. doi: 10.1152/jn.00411.2004. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tortorici V. Interference with GABA transmission in the rostral ventromedial medulla: disinhibition of off-cells as a central mechanism in nociceptive modulation. Neuroscience. 1994;63:533–546. doi: 10.1016/0306-4522(94)90548-7. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Barbaro NM, Fields HL. Putative nociceptive modulating neurons in the rostral ventromedial medulla of the rat: firing of ON and OFF cells is related to nociceptive responsiveness. Somatosens Mot Res. 1989;6:427–439. doi: 10.3109/08990228909144685. [DOI] [PubMed] [Google Scholar]
- Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AF, Laaris N, Kawamura M, Masino SA, Lupica CR. Control of cannabinoid CB1 receptor function on glutamate axon terminals by endogenous adenosine acting at A1 receptors. J Neurosci. 2010;30:545–555. doi: 10.1523/JNEUROSCI.4920-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo FQ, Qu CL, Li YQ, Tang JS, Jia H. GABAergic modulation is involved in the ventrolateral orbital cortex 5-HT 1A receptor activation-induced antinociception in the rat. Pain. 2008;139:398–405. doi: 10.1016/j.pain.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Jeske NA, Patwardhan AM, Gamper N, Price TJ, Akopian AN, Hargreaves KM. Cannabinoid WIN 55,212-2 regulates TRPV1 phosphorylation in sensory neurons. J Biol Chem. 2006;281:32879–32890. doi: 10.1074/jbc.M603220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks SL, Carstens E, Antognini JF. Isoflurane differentially modulates medullary on and off neurons while suppressing hind-limb motor withdrawals. Anesthesiology. 2004;100:1224–1234. doi: 10.1097/00000542-200405000-00026. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karst M, Wippermann S. Cannabinoids against pain. Efficacy and strategies to reduce psychoactivity: a clinical perspective. Expert Opin Investig Drugs. 2009;18:125–133. doi: 10.1517/13543780802691951. [DOI] [PubMed] [Google Scholar]
- Kathuria S, Gaetani S, Fegley D, Valiño F, Duranti A, Tontini A, et al. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Kincaid W, Neubert MJ, Xu M, Kim CJ, Heinricher MM. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33–41. doi: 10.1152/jn.00449.2005. [DOI] [PubMed] [Google Scholar]
- Kishimoto K, Koyama S, Akaike N. Synergistic mu-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacology. 2001;41:529–538. doi: 10.1016/s0028-3908(01)00100-9. [DOI] [PubMed] [Google Scholar]
- Ligresti A, Schiano Moriello A, Starowicz K, Matias I, Pisanti S, De Petrocellis L, et al. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J Pharmacol Exp Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- Liou GI, Auchampach JA, Hillard CJ, Zhu G, Yousufzai B, Mian S, et al. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Invest Ophthalmol Vis Sci. 2008;49:5526–5531. doi: 10.1167/iovs.08-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J Hepatol. 2009;51:528–534. doi: 10.1016/j.jhep.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Magen I, Avraham Y, Ackerman Z, Vorobiev L, Mechoulam R, Berry EM. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br J Pharmacol. 2010;159:950–957. doi: 10.1111/j.1476-5381.2009.00589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Bisogno T, de Novellis V, Palazzo E, Cristino L, Valenti M, et al. Elevation of endocannabinoid levels in the ventrolateral periaqueductal grey through inhibition of fatty acid amide hydrolase affects descending nociceptive pathways via both cannabinoid receptor type 1 and transient receptor potential vanilloid type-1 receptors. J Pharmacol Exp Ther. 2006;316:969–982. doi: 10.1124/jpet.105.093286. [DOI] [PubMed] [Google Scholar]
- Maione S, de Novellis V, Cappellacci L, Palazzo E, Vita D, Luongo L, et al. The antinociceptive effect of 2-chloro-2′-C-methyl-N6-cyclopentyladenosine (2′-Me-CCPA), a highly selective adenosine A1 receptor agonist, in the rat. Pain. 2007;131:281–292. doi: 10.1016/j.pain.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Maione S, Morera E, Marabese I, Ligresti A, Luongo L, Ortar G, et al. Antinociceptive effects of tetrazole inhibitors of endocannabinoid inactivation: cannabinoid and non-cannabinoid receptor-mediated mechanisms. Br J Pharmacol. 2008;155:775–782. doi: 10.1038/bjp.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Starowicz K, Cristino L, Guida F, Palazzo E, Luongo L, et al. Functional interaction between TRPV1 and micro-opioid receptors in the descending antinociceptive pathway activates glutamate transmission and induces analgesia. J Neurophysiol. 2009;101:2411–2422. doi: 10.1152/jn.91225.2008. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanus LO. Cannabidiol – recent advances. Chem Biodivers. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Meng ID, Manning BH, Martin WJ, Fields HL. An analgesia circuit activated by cannabinoids. Nature. 1998;395:381–383. doi: 10.1038/26481. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Członkowski A, Millan MH, Herz A. Activation of periaqueductal grey pools of beta-endorphin by analgetic electrical stimulation in freely moving rats. Brain Res. 1987;407:199–203. doi: 10.1016/0006-8993(87)91239-x. [DOI] [PubMed] [Google Scholar]
- Moreau JL, Fields HL. Evidence for GABA involvement in midbrain control of medullary neurons that modulate nociceptive transmission. Brain Res. 1986;397:37–46. doi: 10.1016/0006-8993(86)91367-3. [DOI] [PubMed] [Google Scholar]
- Neubert MJ, Kincaid W, Heinricher MM. Nociceptive facilitating neurons in the rostral ventromedial medulla. Pain. 2004;110:158–165. doi: 10.1016/j.pain.2004.03.017. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Mariani L, Palazzo E, Vita D, Marabese I, Scafuro M, et al. Periaqueductal grey CB1 cannabinoid and metabotropic glutamate subtype 5 receptors modulate changes in rostral ventromedial medulla neuronal activities induced by subcutaneous formalin in the rat. Neuroscience. 2005;134:269–281. doi: 10.1016/j.neuroscience.2005.03.014. [DOI] [PubMed] [Google Scholar]
- de Novellis V, Palazzo E, Rossi F, De Petrocellis L, Petrosino S, Guida F, et al. The analgesic effect of N-arachidonoyl-serotonin, a FAAH inhibitor and TRPV1 receptor antagonist, associated with changes in rostral ventromedial medulla and locus coeruleus cell activity in rats. Neuropharmacology. 2008;55:1105–1113. doi: 10.1016/j.neuropharm.2008.06.023. [DOI] [PubMed] [Google Scholar]
- Ortar G, Ligresti A, De Petrocellis L, Morera E, Di Marzo V. Novel selective and metabolically stable inhibitors of anandamide cellular uptake. Biochem Pharmacol. 2003;65:1473–1481. doi: 10.1016/s0006-2952(03)00109-6. [DOI] [PubMed] [Google Scholar]
- Palazzo E, de Novellis V, Marabese I, Cuomo D, Rossi F, Berrino L, et al. Interaction between vanilloid and glutamate receptors in the central modulation of nociception. Eur J Pharmacol. 2002;439:69–75. doi: 10.1016/s0014-2999(02)01367-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1986. [Google Scholar]
- Petrosino S, Di Marzo V. FAAH and MAGL inhibitors: therapeutic opportunities from regulating endocannabinoid levels. Curr Opin Investig Drugs. 2010;11:51–62. [PubMed] [Google Scholar]
- Rahn EJ, Hohmann AG. Cannabinoids as pharmacotherapies for neuropathic pain: from the bench to the bedside. Neurotherapeutics. 2009;6:713–737. doi: 10.1016/j.nurt.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea K, Roche M, Finn DP. Supraspinal modulation of pain by cannabinoids: the role of GABA and glutamate. Br J Pharmacol. 2007;152:633–648. doi: 10.1038/sj.bjp.0707440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS. 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats. Br J Pharmacol. 2009;156:181–188. doi: 10.1111/j.1476-5381.2008.00046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res. 2005;30:1037–1043. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- Salas MM, Hargreaves KM, Akopian AN. TRPA1-mediated responses in trigeminal sensory neurons: interaction between TRPA1 and TRPV1. Eur J Neurosci. 2009;29:1568–1578. doi: 10.1111/j.1460-9568.2009.06702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandkühler J, Gebhart GF. Relative contributions of the nucleus raphe magnus and adjacent medullary reticular formation to the inhibition by stimulation in the periaqueductal gray of a spinal nociceptive reflex in the pentobarbital-anesthetized rat. Brain Res. 1984;305:77–87. doi: 10.1016/0006-8993(84)91121-1. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Maione S, Cristino L, Palazzo E, Marabese I, Rossi F, et al. Tonic endovanilloid facilitation of glutamate release in brainstem descending antinociceptive pathways. J Neurosci. 2007;27:13739–13749. doi: 10.1523/JNEUROSCI.3258-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Bang SI, Jin YH. Transient receptor potential A1 increase glutamate release on brain stem neurons. Neuroreport. 2009;20:1002–1006. doi: 10.1097/WNR.0b013e32832d2219. [DOI] [PubMed] [Google Scholar]
- Vaughan RP, Szewczyk MT, Jr, Lanosa MJ, Desesa CR, Gianutsos G, Morris JB. Adenosine sensory transduction pathways contribute to activation of the sensory irritation response to inspired irritant vapors. Toxicol Sci. 2006;93:411–421. doi: 10.1093/toxsci/kfl061. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kayano Y, Matsunaga T, Yamamoto I, Yoshimura H. Inhibition of anandamide amidase activity in mouse brain microsomes by cannabinoids. Biol Pharm Bull. 1996;19:1109–1111. doi: 10.1248/bpb.19.1109. [DOI] [PubMed] [Google Scholar]
- Wiertelak EP, Roemer B, Maier SF, Watkins LR. Comparison of the effects of nucleus tractus solitarius and ventral medial medulla lesions on illness-induced and subcutaneous formalin-induced hyperalgesias. Brain Res. 1997;748:143–150. doi: 10.1016/s0006-8993(96)01289-9. [DOI] [PubMed] [Google Scholar]
- Xu M, Kim CJ, Neubert MJ, Heinricher MM. NMDA receptor-mediated activation of medullary pro-nociceptive neurons is required for secondary thermal hyperalgesia. Pain. 2007;127:253–262. doi: 10.1016/j.pain.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanelati TV, Biojone C, Moreira FA, Guimarães FS, Joca SR. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br J Pharmacol. 2010;159:122–128. doi: 10.1111/j.1476-5381.2009.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


