Abstract
BACKGROUND AND PURPOSE
A class of drugs known as KATP-channel openers induce cardioprotection. This study examined the effects of the novel KATP-channel opener, the fluorine-containing pinacidil derivative, flocalin, on cardiac-specific KATP-channels, excitability of native cardiac myocytes and on the ischaemic heart.
EXPERIMENTAL APPROACH
The action of flocalin was investigated on: (i) membrane currents through cardiac-specific KATP-channels (IKATP) formed by KIR6.2/SUR2A heterologously expressed in HEK-293 cells (HEK-2936.2/2A); (ii) excitability and intracellular Ca2+ ([Ca2+]i) transients of cultured rat neonatal cardiac myocytes; and (iii) functional and ultrastructural characteristics of isolated guinea-pig hearts subjected to ischaemia-reperfusion.
KEY RESULTS
Flocalin concentration-dependently activated a glibenclamide-sensitive IKATP in HEK-2936.2/2A cells with an EC50 = 8.1 ± 0.4 µM. In cardiac myocytes, flocalin (5 µM) hyperpolarized resting potential by 3–5 mV, markedly shortened action potential duration, reduced the amplitude of [Ca2+]i transients by 2–3-fold and suppressed contraction. The magnitude and extent of reversibility of these effects depended on the type of cardiac myocytes. In isolated hearts, perfusion with 5 µmol·L−1 flocalin, before inducing ischaemia, facilitated restoration of contraction during reperfusion, decreased the number of extrasystoles, prevented the appearance of coronary vasoconstriction and reduced damage to the cardiac tissue at the ultrastructural level (state of myofibrils, membrane integrity, mitochondrial cristae structure).
CONCLUSION AND IMPLICATIONS
Flocalin induced potent cardioprotection by activating cardiac-type KATP-channels with all the benefits of the presence of fluorine group in the drug structure: higher lipophilicity, decreased toxicity, resistance to oxidation and thermal degradation, decreased metabolism in the organism and prolonged therapeutic action.
Keywords: KATP channel; KATP-channel openers; flocalin; cardiac excitability, cardioprotection
Introduction
Increased basal potassium conductance causes cell hyperpolarization of electrically excitable cells and inhibition of their physiological activity. One of the most important endogenous systems of cardioprotection during myocardial ischaemia and infarction relies on the activation of sarcolemmal ATP-dependent potassium channels (KATP) (Noma, 1983; Kane et al., 2005; channel nomenclature follows Alexander et al., 2009), whose functional state is regulated by the ratio of the key cytosolic metabolic ligands, ATP and ADP, with ATP promoting closure and Mg-ADP opening of the channels. KATP channels are closed under normal conditions, and concentrations of intracellular ATP but readily open when the metabolic state of the cardiac myocytes is compromised such as during myocardial ischaemia. Opening of KATP channels leads to hyperpolarization, action potential (AP) shortening, a decrease in voltage-dependent Ca2+ entry, reduction in contractility, decline in cellular metabolism and eventually to a decrease in oxygen consumption. A decrease in Ca2+ entry also slows down catabolic processes that help prevent degradation of the plasma membrane phospholipids and formation of the pathogenic pro-constrictory and arrhythmogenic eicosanoids (Charnock, 1999).
Consistent with this role, exogenous activation of KATP channels with the class of drugs known as KATP-channel openers facilitates cardioprotection and improves outcome after acute impairment of myocardial circulation (Jahandir and Terzic, 2005). At the same time, KATP-channel openers are known to be associated with considerable side effects, including disturbances in cardiac rhythm such as ventricular fibrillation, inhibition of insulin production and hypotension (Bhatnagar and Bolli, 1999). Thus, elaboration of new KATP-channel openers, in particular fluorine-containing ones, remains important. Introduction of fluorine into the chemical structure of a compound often leads to a decrease in toxicity and enhancement of stability (Begue and Bonnet-Delpon, 2008), without impairment of biological activity. Furthermore, the fluorocarbon (C-F) bond is stronger than a hydrocarbon (C-H) one, which increases the resistance of the molecule to oxidation and thermal degradation, decreases its metabolic turnover and consequently prolongs therapeutic action. In addition, lipophilicity of the fluorine-containing compounds is much higher than of their hydrogen-containing analogues (Yamazaki et al., 2009).
Among the fluorine-containing cardiovascular-specific KATP-channel openers, a promising member is flocalin [N-(4-difluoromethoxyphenyl)-N′-pinacolyl-N′′-cyanoguanidine], which differs from its better known precursor pinacidil by the presence of a benzene ring with a difluoromethoxy group instead of a pyridyl ring (Yagupolskii et al., 1997; 2001;) (Figure 1). One major advantage of flocalin is a significantly lower toxicity compared to pinacidil: its LD50 in Wistar rats was found to be 2150 mg·kg−1, which is about 3.6 times higher than that reported for pinacidil (600 mg·kg−1; Moibenko et al., 2009). Despite this, detailed analysis of flocalin's action on cardiac-specific KATP channels, cardiomyocyte excitability and ischaemic cardiac tissue is still lacking. In this study, we used HEK-293 cells stably expressing KIR6.2 and SUR2A subunits of cardiac-specific KATP channel (HEK-2936.2/2A), cultured rat neonatal cardiomyocytes and whole guinea pig hearts to address these issues. Our data show that flocalin is an effective KIR6.2 /SUR2A KATP channel opener, which decreases cardiomyocyte excitability and Ca2+ entry, thereby reducing the negative impact of ischaemia-reperfusion on cardiac muscle.
Figure 1.
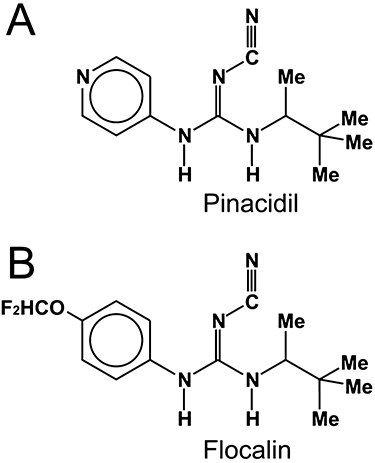
Chemical structures of pinacidil and flocalin. Flocalin (B) is characterized by the presence of benzene ring with difluoromethoxy group instead of a pyridyl ring for pinacidil (A). Me – methyl (CH3) group.
Methods
Cell culture
All animal care and experimental procedures complied with ‘Guidelines of the “European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes” ’ and were approved by the Bogomoletz Institute of Physiology NASU Ethical Committee. Isolation and culturing of neonatal rat cardiac myocytes was performed under aseptic conditions according to the general procedure described elsewhere (Rogers et al., 1986). Briefly, 3 day old pups were decapitated, their hearts quickly removed and placed into the ice-cold Ca2+-free physiological saline (PS, in mmol·L−1): 144 NaCl, 4 KCl, 1 MgSO4, 1.2 KH2PO4, 0.43 Na2HPO4, 5 sodium pyruvate, 10 HEPES, 10 glucose, pH 7.35 (adjusted with NaOH). The hearts were cut into small pieces (∼1 mm3), which were transferred to the room temperature Ca2+-free PS bubbled with carbogen (gas mixture of 95% O2 and 5% CO2). Enzymatic digestion was performed in the same solution supplemented with 0.46 mg·mL−1 collagenase (Type IA, Sigma-Aldrich, St. Louis, MO) at 37°C for 30 min with slight agitation and bubbling with carbogen. Then, the tissue was transferred into an enzyme-free solution, where it was dispersed by pipetting. The suspension was centrifuged at 500× g, the supernatant was removed and the pellet was resuspended in PS with Ca2+ concentration increased to 0.2 mmol·L−1. The Ca2+ concentration in this solution was then gradually increased to 1.5 mmol·L−1 over 1 h. After another centrifugation at 500× g, the cell pellet was resuspended in Dulbecco's Modified Eagle Medium culture medium supplemented with 10% of foetal calf serum (Gibco-Invitrogen, Carlsbad, CA). Cells were plated onto gelatin-coated glass cover slips at approximate density of 100 000 cells·cm−2 and placed in a Petri dish filled with culture medium. Cells were maintained in the incubator in 5% CO2+ 95% O2 atmosphere at 37°C for 1–3 days. The culture medium was changed every 24 h.
HEK-293 cells stably expressing KIR6.2 /SUR2A (HEK-2936.2/2A cells) cardiac-type KATP channel were created, as detailed elsewhere (Cui et al., 2001). Cells were cultured in MEM culture medium supplemented with 10% of foetal calf serum (Gibco-Invitrogen). The medium was changed every day and the cells were split every 4 days.
Electrophysiology, solutions and [Ca2+]i measurements
Whole-cell patch-clamp experiments on cultured cardiac myocytes and HEK-2936.2/2A cells were performed using PC-ONE (Dagan Corp, Minneapolis, MN, USA) amplifier and pClamp 8.0 software (Molecular Devices, Union City, CA, USA) for data acquisition and analysis. Patch pipettes for the whole-cell recordings were fabricated from borosilicate glass capillaries (World Precision Instr., Inc., Sarasota, FL, USA) on a horizontal puller (Sutter Instruments Co., Novato, CA, USA) and had resistances of 2–3 MΩ when filled with intracellular solutions.
Basic extracellular solutions used for electrophysiological recordings contained (in mmol·L−1): 144 NaCl, 5.4 KCl, 1.8 CaCl2, 1.2 MgCl2, 1 NaH2PO4, 10 HEPES, 10 glucose, pH 7.4 (adjusted with NaOH). Recording patch pipettes were filled with intracellular solution containing (in mmol·L−1): 10 KCl, 10 KOH, 105 K-aspartate, 15 NaCl, 1 MgCl2, 10 HEPES, 4 Mg-ATP, 5 sucrose, pH 7.2 (adjusted with HCl). Changes of external solutions and application of drugs were performed using a multi-barrel puffing micropipette with a common outflow positioned in close proximity to the cell under investigation. During the experiment, the cell was continuously superfused with the solution via a puffing pipette to reduce possible artefacts related to the switch from a static to a moving solution and vice versa. Complete external solution exchange was achieved in <1 s.
Electrophysiological data were analyzed using pClamp 8.0 (Molecular Devices, Union City, CA), Origin 7.0 (OriginLab Corp., Northampton, MA) and Matlab (MathWorks Corp., Natick, MA) software. Action potential duration (APD) was measured at different levels of repolarization, 20, 50 or 90%, from AP amplitude. When constructing current-voltage (I-V) and dose–response relationships, the amplitudes of the currents were normalized to the cell membrane capacitance to provide current densities (nA·pF−1). To obtain a dose–response relationship, current densities for each flocalin concentration were averaged and fitted to the Hill equation, after which the data were normalized to the maximal level of the Hill fit.
[Ca2+]i transients were measured in Fluo-4AM-loaded rat neonatal cardiac myocytes using inverted Olympus IX71 fluorescence microscope equipped with CFW-1312C digital camera (Scion Corp., Frederick, ME), operating at 7.5 frames·s−1. Signal intensity was averaged for the myocyte's area and normalized to the baseline intensity before any intervention was administered.
Whole heart and ultrastructural studies
Male guinea pigs weighing 250–350 g were killed by cervical dislocation, their hearts were quickly removed and attached to the Langendorf perfusion system. Hearts were perfused with the standard oxygenated Krebs-Henselate solution at 37°C. The perfusion rate was maintained at 12.5 ± 0.5 mL·min−1 using peristaltic pump while recording the following functional parameters using the Mingograf 34 (Elema, Solna, Sweden): perfusion pressure in coronary vessels under conditions of constant perfusion rate, contractile activity of the heart measured according to the pressure change within the latex balloon inserted into the left ventricle, and the frequency of the contractions. Ischaemia-reperfusion protocol consisted of a 20 min ischaemic period caused by cessation of the perfusion followed by a 40 min long reperfusion. To study the effects of flocalin, the heart was perfused for 5 min with 5 µmol·L−1 flocalin-supplemented Krebs-Henseleit solution prior to inducing the ischaemia.
After functional experiments, the hearts were used for ultrastructural electron microscopy studies. For this purpose the tissue was fixed and embedded in epoxy resin. Ultrathin tissue slices were contrasted by uranyl acetate and lead citrate. Sarcolemma integrity was determined using electron microscopy tracer, colloid lanthanum (Shaklai and Tavassoli, 1982). For this, the tissue was first fixed with lanthanum for 12 h under continuous agitation followed by final fixation in a mixture of 3% lanthanum and 2.5% glutaraldehyde in cacodylate buffer, post-fixation in 1% osmic acid, drying and epoxy embedment. The preparations were examined with a electron microscope Jem-100CX (JEOL Co., Tokyo, Japan).
Data analysis
All experiments were conducted several times, and the results were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed using Student's t-test and anova tests followed by Tukey-Kramer post-tests. Values of P < 0.05 were taken to show statistical significance between means.
Materials
All chemicals except for flocalin were obtained from Sigma-Aldrich, and flocalin was synthesized in the Institute of Organic Chemistry NASU (Kiev, Ukraine). Flocalin and glibenclamide were dissolved in dimethyl sulphoxide (DMSO) as 40 mmol·L−1 stocks and added to the basic extracellular solution in the appropriate amount to achieve desired final concentration. Concentration of DMSO in the working solutions did not exceed 0.05%.
Results
The action of flocalin on heterologously expressed KATP channels
Since flocalin is a fluorine-containing analogue of pinacidil (Figure 1), a known KATP-channel opener with the highest specificity to vascular and cardiac subtypes of the channel, we first sought to examine whether flocalin could activate these channels in HEK-2936.2/2A cells stably expressing a combination of KIR6.2 and SUR2A subunits that form cardiac-specific KATP channel.
As documented in Figure 2A,B, in the presence of 4 mmol·L−1 intracellular Mg-ATP HEK-2936.2/2A, cells showed only small, time-independent baseline current, over a wide range of membrane potentials, Vm = −110 to +90 mV. The density of this current at Vm = +60 mV did not exceed 10 pA·pF−1. However, application of flocalin (1–20 µmol·L−1) caused the development of big time-independent current characterized by weak inward rectification and reversal potential close to K+ equilibrium potential (EK, which for our ionic conditions constituted −83 mV), indicating that it is carried by K+ (Figure 2C). Activation of this current was concentration-dependent and its density in the presence of flocalin (20 µmol·L−1) and Vm = +60 mV reached 0.31 ± 0.3 nA·pF−1. The KATP-channel inhibitor glibenclamide (10 µmol·L−1) decreased flocalin-activated K+ current to the levels even lower than the baseline (Figure 2B) and this was identified as the opening of KATP-channels heterologously expressed in HEK-2936.2/2A cells (IK,ATP). The fact that glibenclamide blocked flocalin-activated IK,ATP in HEK-2936.2/2A cells to the levels lower than the baseline current suggested to us some basal activity of KATP-channels even in the absence of flocalin. Weak inward rectification of flocalin-activated IK,ATP, which becomes especially evident at Vm > −50 mV, as well as the position of its reversal potential are well illustrated by the I-V relationships acquired in response to both voltage ramps and square pulses to different Vm (Figure 2C).
Figure 2.

Flocalin activates heterologously expressed cardiac-type KATP channels. (A) Representative recordings of the baseline (middle panel) and 20 µmol·L−1 flocalin-activated (lower panel) membrane currents in HEK-2936.2/2A stably transfected with KIR6.2 /SUR2A in response to the voltage-step protocol shown on top. (B) Representative recordings of the baseline current and currents in the presence of 5, 10 and 20 µmol·L−1 flocalin, as well as of 20 µmol·L−1 flocalin plus 10 µmol·L−1 glibenclamide (Floc + Glib) in response to the voltage-ramp protocol shown on top; dashed lines in A and B denote zero current and zero voltage levels. (C) I-V relationships of 20 µmol·L−1 flocalin-activated IKATP measured from square pulses (symbols, mean ± SEM, n = 5) and ramp (smooth line) voltage-clamp protocols. (D) Dose–response relationship of IKATP activation by flocalin (symbols – experimental data points, mean ± SEM, n = 5–7) and the fit to the Hill equation; the values of IC50 and co-operativity (Hill) coefficient, p, are shown on the plot.
A concentration-response relationship of IK,ATP activation by flocalin was constructed by measuring current density at Vm = +90 mV in the presence of different concentrations of the drug. A fit of experimental data points with the Hill equation provided the EC50 values of 8.1 ± 0.4 µmol·L−1 and a co-operativity (Hill) coefficient p of 1.7 ± 0.1 (Figure 2D), suggesting positive co-operativity of drug interaction with the channel.
Thus, the data obtained in HEK-2936.2/2A cells indicated that flocalin is indeed an effective opener of cardio-specific KATP channels, and that this action of the drug may largely underlie its cardioprotective properties.
The effects of flocalin on electrical activity of neonatal rat cultured cardiomyocytes
Next, we examined how flocalin influenced electrical excitability of cardiomyocytes as measured by resting potential (Vr) and parameters of the AP. These experiments were carried out by exposing patch-clamped neonatal rat cardiomyocytes to flocalin while continuously monitoring their Vr and APs in the current-clamp mode. The generation of APs was evoked by 400 pA depolarizing current pulses of 2 ms duration applied at a frequency of 0.2 Hz. Since the intracellular pipette solution did not contain Ca2+-chelating agents, the generation of each AP was accompanied by cardiac myocyte contraction. A typical experimental protocol included: (i) establishment of the whole-cell configuration; (ii) 3–5 min dialysis of the myocyte with pipette solution to allow all changes in Vr and AP related to the replacement of intracellular milieu to occur; (iii) recording of the myocyte baseline electrical activity following stabilization of Vr and AP parameters; (iv) exposure of the myocyte to flocalin and observation of flocalin-evoked changes; and (v) washout of the flocalin after these changes reached a steady-state level.
Figure 3 presents the results of typical experiments of the effects of 5 µmol·L−1 flocalin on the AP of cardiac myocytes from various parts of neonatal rat heart, as judged from the initial shape of the AP. In the ventricular epicardial (Figure 3A), endocardial (Figure 3B) as well as atrial (Figure 3C) myocytes, flocalin hyperpolarized Vr by around 3–5 mV, notably shortened the AP duration (measured at 90% repolarization, APD90) and virtually abolished any contraction. All changes were largely reversible upon flocalin withdrawal, with AP duration in epicardial cells being the least reversible (Figure 3B). Flocalin showed the fastest onset and washout, in terms of changes in Vr and parameters of the AP in endocardial ventricular myocytes (Figure 3A) and the slowest in atrial myocytes (Figure 3C). In endocardial cells, flocalin also produced the most pronounced alteration of the shape of AP due to preferential shortening of the AP plateau phase (Figure 3A), whilst in other cell types, the AP shortening was more uniform.
Figure 3.

The effects of flocalin on excitability of the rat neonatal cultured cardiac myocytes. (A–C) Representative recordings of the control AP, AP in the presence of 5 µmol·L−1 flocalin and AP after flocalin washout in the ventricular epicardial- (A), endocardial- (B) as well as in atrial-type (C) cardiac myocytes; the insets on panels A–C show the dynamics of the action potential duration (APD90) and resting potential (Vr) changes in response to flocalin application (marked by horizontal bars). (D, E) Quantification of the changes of the AP amplitude (D) and of its maximal rate of rise (E) in the ventricular epicardial-, endocardial- as well as atrial-type cardiac myocytes in response to flocalin (5 µmol·L−1) and following its washout relative to the control pre-drug values; mean ± SEM, n = 5–7.
Flocalin did not influence the amplitude and the maximal rate of rise (onset rate) of the AP in the ventricular endocardial myocytes, but notably decreased these parameters in the ventricular epicardial and atrial myocytes (Figure 3D,E). However, in the epicardial ventricular myocytes, flocalin-evoked changes in the amplitude and onset rate of the AP were fully reversible, whilst in the atrial myocytes, they were virtually irreversible after 2 min of drug washout. The difference in the extent of reversibility of flocalin effects on the temporal and amplitude parameters of the AP in epicardial and atrial myocytes may be indicative of the drug's action on several types of ion channels that are differentially expressed in these cells.
All the effects of flocalin on the APs of various types of cardiac myocytes could be effectively antagonized by the KATP-channel inhibitor glibenclamide (10 µmol·L−1, Figure 4), consistent with the specific targeting by flocalin of cardiac KATP-channels. In the presence of flocalin and glibenclamide, the AP duration was even slightly prolonged compared to the control, suggesting some basal activity of KATP-channels.
Figure 4.

The effects of flocalin on excitability of the rat neonatal cultured cardiac myocytes are antagonized by glibenclamide. (A–C) Representative recordings of the control AP, AP in the presence of 5 µmol·L−1 flocalin and AP after addition of glibenclamide (5 µmol·L−1) to flocalin-containing solution in the ventricular epicardial- (A), endocardial- (B) as well as in atrial-type (C) cardiac myocytes; in the panel C flocalin and flocalin plus glibenclamide curves coincide.
In the cardiac myocytes that showed spontaneous beating, we could record non-stimulated generation of periodic APs with the frequency of 1–2 Hz. Figure 5A shows an example of such activity in the endocardial-type ventricular myocyte, as judged from the shape of its AP (see inset of Figure 5B). Application of 10 µmol·L−1 flocalin resulted in the poorly reversible hyperpolarization of the myocyte Vr by about 8–12 mV and in the substantial reduction of the frequency of APs and spontaneous contractions (Figure 5A). On average, the frequency decreased two- to threefold, although the myocytes were quite divergent with respect to this parameter. Flocalin also dramatically shortened the duration of spontaneous APs (measured at 50% repolarization, APD50, Figure 5B), which resulted in the pronounced decrease of the amplitude of intracellular Ca2+ ([Ca2+]i) transients that accompanied each AP (Figure 5C) and to the marked subsiding of spontaneous beating.
Figure 5.

The effects of flocalin on spontaneously beating rat neonatal cultured cardiac myocytes. (A) Representative recordings of the electrical activity of the spontaneously beating endocardial-type ventricular myocyte during application of 10 µmol·L−1 flocalin (marked by horizontal bar). (B) The dynamics of the action potential duration (APD50) shortening in the same myocyte in response to flocalin (open symbols – pre-drug, filled symbols – flocalin); the insets show the recordings of the APs at expanded time-scale in the absence and in the presence of flocalin taken at time periods marked by short horizontal bars at A and B. (C) Representative recordings of the [Ca2+]i transients from another Fluo-4-loaded myocyte during application of 10 µmol·L−1 flocalin (marked by horizontal bar).
The effects of flocalin on isolated hearts
To determine whether flocalin can exert cardioprotection following ischaemia-reperfusion injury we performed experiments on guinea-pig isolated, Langendorf-perfused, hearts. Termination of the heart perfusion with oxygenated Krebs-Henseleit solution led to the rapid cessation of the contractions, whilst restoration of the perfusion after 20 min of ischaemia required 63.7 ± 10 s (n = 11) for contractions to reappear (Figure 6A). Ischaemia-reperfusion resulted in the typical impairment of cardiac function: (i) increase of the perfusion pressure in coronary vessels; (ii) reduced force of contraction of the myocardium of the left ventricle; (iii) increased diastolic pressure; and (iv) irregular heart rhythm during reperfusion (Figure 7A). These changes are largely explained by the pro-oxidative effects of reperfusion due to increased levels of oxygen free radicals (Misra et al., 2009).
Figure 6.
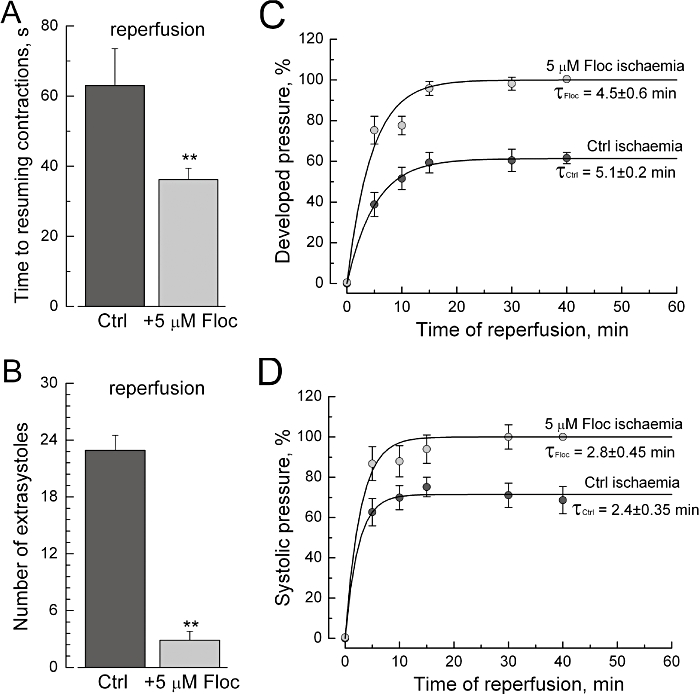
The effects of flocalin on the functional properties of isolated guinea-pig hearts. (A, B) Heart perfusion with flocalin (5 µmol·L−1) prior to ischaemia accelerates time to resuming contractions in response to reperfusion (A) and reduces the number of extrasystoles during reperfusion (B) compared to the control, non-flocalin conditions (Ctrl); mean ± SEM, n = 26. (C, D) Heart perfusion with flocalin prior to ischaemia improves recovery of the developed (C) and systolic (D) pressure during reperfusion compared to the control, non-flocalin conditions (Ctrl); mean ± SEM, n = 26; time ‘0’ corresponds to the beginning of reperfusion; smooth lines are the best fits of experimental data points with exponential functions with time constants shown near the curves.
Figure 7.
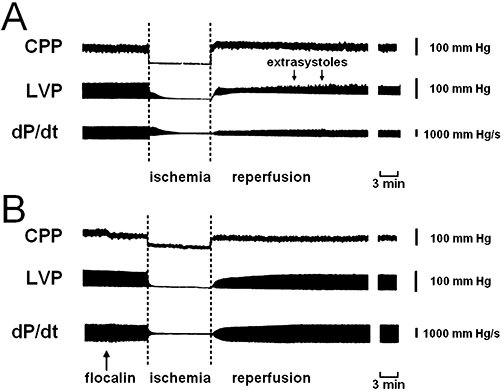
Flocalin improves the recovery of cardiac haemodynamics during reperfusion of the ischaemic myocardium. (A, B) Changes of the coronary artery perfusion pressure (CPP), left ventricle pressure (LVP) and of dP/dt contractility index during ischaemia-reperfusion of isolated guinea-pig hearts under control conditions (A) and with flocalin (5 µM) infusion prior to inducing ischaemia (B, shown by arrow). Flocalin facilitates the re-initiation of the contractions of ischaemic myocardium, prevents coronary vasoconstriction and appearance of extrasystoles (shown by arrows in A) during reperfusion.
The negative consequences of the ischaemia-reperfusion on cardiac function could be reduced by using flocalin (Figures 6 and 7). Perfusion of hearts (5 min) with oxygenated Krebs solution supplemented with flocalin (5 µM) before inducing ischaemia-reperfusion shortened almost twofold the time required for the re-initiation of contractions in response to reperfusion (Figure 6A), decreased by eightfold the maximal number of extrasystolic contractions during the 30 min reperfusion period (Figure 6B), essentially increased developed and systolic pressures and prevented raised end-diastolic pressure (Figure 6C,D) in the left ventricle of ischaemic heart (Figure 7B) as well as the reduced coronary artery perfusion pressure due to lowering of coronary vessels tone (Figure 7B).
The cardioprotective actions of flocalin were substantiated by ultrastructural data. As documented in the electron microscopy images of Figure 8A, 20 min long heart ischaemia followed by 40 min long reperfusion resulted in substantial contracture of the myofibrils, impairment of membrane integrity associated with high counts of intracellularly localized colloid lanthanum particles and by mitochondrial damage mostly evidenced by irregular cristae structure. However, when the 5 min flocalin (5 µmol·L−1) perfusion preceded the induction of ischaemia-reperfusion pathological ultrastructural changes appeared much less pronounced Figure 8B. In particular, cardiomyocyte swelling became minimal and the colloid lanthanum granules mostly localized extracellularly, suggesting plasma membrane-stabilizing effects.
Figure 8.

Flocalin reduces post-ischaemic damage to the guinea heart at the ultrastructural level. (A) Electron microphotograph (magnification 8000) showing profound contractures of the myofilaments (MF), mitochondria (M) swelling and irregular cristae structure, high counts of colloid lanthanum particles in the cytoplasm and outer membrane of mitochondria (arrows) due to impairment of plasma membrane integrity in the cardiac myocyte of the control post-ischaemic heart; Er – erythrocyte. (B) Electron micrograph (magnification 8000) of the subcellular structures of cardiac myocytes from post-ischaemic heart pre-treated with flocalin (5 µM): myofilaments (MF) and mitochondria (M) are well preserved, colloid lanthanum particles (arrows) are localized extracellularly; Eth – endothelium of the capillary.
Discussion and conclusions
Our results showed that the fluorine-containing pinacidil derivative, flocalin, was an effective opener of the cardiac-specific sarcolemmal KATP channel. The EC50 for the activation of IKATP mediated by heterologously expressed KIR6.2 /SUR2A subunits in HEK-293 cells by flocalin was found to be 8.1 ± 0.4 µmol·L−1, which is close to the EC50−10 µmol·L−1 reported for the activation of cardiac-type IKATP by pinacidil (Shindo et al., 1998). Thus, the introduction of a difluoromethoxy group into the structure did not influence its KATP-channel-activating potency, at least for the cardiac-type channel. It was claimed that flocalin is more effective in lowering the blood pressure and is less toxic in whole animal tests compared to its precursor pinacidil (Moibenko et al., 2009). It was also shown to be a better relaxant of isolated rat urinary bladder strips (Lymarenko, 2005). As with pinacidil, the targets of flocalin action also include the vascular and mitochondrial KATP channels (Strutyns'kyi et al., 2000; Strutyns'kyi et al., 2008). The increased efficacy of the drug in vivo and in non-cardiac tissues may be explained by its more potent action on these particular sites as well as by its higher stability.
Application of flocalin effectively reduces excitability of isolated cardiac myocytes by hyperpolarizing their Vr and profoundly shortening APD due to KATP-channel activation. These effects were found to vary in magnitude and extent of reversibility depending on the type of cardiac myocyte. The most marked APD shortening, reaching ∼85% with 5 µmol·L−1 flocalin, and the poorest reversibility upon drug withdrawal was observed for ventricular endocardial-type myocytes, whilst for the least affected epicardial cells, APD shortening did not exceed 50%. The divergence of the degree of APD shortening depending on the region of cardiac tissue has been previously reported for pinacidil (Steinberg et al., 1988). In addition to these effects, in ventricular epicardial and atrial myocytes, flocalin inhibited the amplitude of the AP by 15–20% and the maximal rate of its rise by 40–45% suggesting that the drug may also influence amplitude and/or kinetics of the inward Na+ and Ca2+ currents. The fact that in endocardial myocytes we did not notice such effects, whilst in epicardial and atrial myocytes they differed in their reversibility (reversible in the former and poorly reversible in the latter) suggests that molecular nature or regulation of sodium and calcium channels in these cell types are different.
The inhibition of cardiac myocyte excitability by flocalin due to activation sarcolemmal KATP channels translates into a marked decrease of [Ca2+]i transients during each AP and attenuation of contractions. Furthermore, our experiments in guinea pig isolated hearts show that the use of flocalin, prior to the induction of ischaemia-reperfusion, significantly reduced the damage to the cardiac muscle. On a functional level, this was shown by almost twofold faster post-ischaemic resumption of cardiac contractions and developed pressure as well as in much lower incidence of disturbances of cardiac rhythm. The improved recovery of the myocardium after ischaemia in the presence of flocalin can be explained by less structural damage including, reduced contractures of the myofibrils, better membrane integrity and more regular cristae structure in mitochondria. Much of these cardioprotective effects stem from opening of KATP channels by flocalin, which leads to diminished electrical and contractile activity, reduced cellular metabolism and a decrease in voltage-dependent Ca2+ entry. The latter helps to prevent Ca2+-dependent degradation of membrane phospholipids and elevation of the content of arachidonic acid (AA) and of its derivatives, leukotrienes and thromboxanes, which have arhythmogenic and coronary constrictory actions.
Indeed, our previous biochemical studies on cardiac tissue samples from canine hearts subjected to local ischaemia have shown threefold increase in the level of AA in the infarction zone compared to the remote zone (from 2.3 ± 0.2 to 6.7 ± 0.9 nM·mg−1 protein), whereas administration of flocalin prior to the induction of ischaemia was able to effectively reduce AA levels in this zone by 48% (to 3.5 ± 0.5 nM·mg−1) (Moibenko et al., 2009). Flocalin-evoked decrease in the AA content is indicative of the inhibition of phospholipid breakdown due to suppression of the activity of Ca2+-dependent cytosolic phospholipases A2 (cPLA2). Inhibition of Ca2+-dependent phospholipid breakdown serves as a good indication of flocalin-induced KATP activation, cardiomyocyte hyperpolarization, APD shortening and associated decrease of Ca2+ entry.
Another effect of flocalin administration seen in biochemical assays on tissue samples from ischaemic canine heart was fourfold reduction of the level of leukotriene C4 (from 36.3 ± 5.5 to 9.5 ± 1.7 pM·mg−1 protein) and 44% reduction of thromboxane B2 in the necrotic zone (Moibenko et al., 2009). Both of these eicosanoids are known to produce constriction of coronary vessels and to induce arrhythmogenesis therefore the decrease in their levels is in a good agreement with our functional data showing that flocalin lowers coronary perfusion pressure and eliminates disturbances of the heart rhythm. The use of flocalin was also found to have a potent antioxidative effect by essentially preventing the ischaemia-reperfusion-evoked increase of reactive species, H2O2 and oxidative stress marker, malondialdehyde, in the infarction zone and in arterial blood. Flocalin also prevented the decline of catalase and superoxide dismutase activity during ischaemia-reperfusion (Moibenko et al., 2009; Strutyns'kyi et al., 2009a). It is noteworthy that flocalin decreased by almost twofold the area of the infracted necrotic zone (Strutyns'kyi et al., 2009b).
An essential part of flocalin effects in the whole animal and at the tissue level may also result from the drug's action on vascular-type and mitochondrial KATP channels (Strutynskyi and Moibenko, 2000; Strutyns'kyi et al., 2008). Nevertheless, our data indicate that this fluorine-containing pinacidil derivative represents a promising cardioprotector with cardiac sarcolemmal KATP channels representing one of the major sites of action. In addition to its pharmacological effects, flocalin also provides all the benefits of a fluorine group in the drug structure: higher lipophilicity, decreased toxicity, resistance to oxidation and thermal degradation, decreased metabolism in the organism and prolonged therapeutic action.
Acknowledgments
This research was funded by the National Academy of Sciences of Ukraine.
Glossary
Abbreviations
- AA
arachidonic acid
- AP
action potential
- APD
action potential duration
- [Ca2+]i
intracellular calcium concentration
- cPLA2
Ca2+-dependent cytosolic phospholipase A2
- EK
K+ equilibrium potential
- HEK-2936.2/2A
HEK-293 cells stably transfected with KIR6.2 and SUR2A KATP-channel subunits
- IK,ATP
current through KATP channels
- I-V
current-voltage relationship
- KATP
ATP-dependent potassium channel, Vm, membrane potential
- Vr
resting potential
Conflict of interest
The authors declare no conflict of interests.
Supporting Information
Teaching Materials; Figs 1–8 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 4th edn. Br J Pharmacol. 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begue J-P, Bonnet-Delpon D. Effects of fluorine substitution on biological properties. In: Begue J-P, Bonnet-Delpon D, editors. Bioorganic and Medicinal Chemistry of Fluorine. Hoboken, New Jersey: John Wiley & Sons, Inc.; 2008. pp. 72–98. [Google Scholar]
- Bhatnagar A, Bolli R. Modulation of KATP channels to protect the ischemic myocardium: clinical implications. Exp Clin Cardiol. 1999;4:20–22. [Google Scholar]
- Charnock JS. Omega-3 polyunsaturated fatty acids and ventricular fibrillation: the possible involvement of eicosanoids. Prostaglandins Leukot Essent Fatty Acids. 1999;61:243–247. doi: 10.1054/plef.1999.0096. [DOI] [PubMed] [Google Scholar]
- Cui Y, Giblin JP, Clapp LH, Tinker A. A mechanism for ATP-sensitive potassium channel diversity: functional coassembly of two pore-forming subunits. Proc Natl Acad Sci USA. 2001;98:729–734. doi: 10.1073/pnas.011370498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahandir A, Terzic A. KATP channel therapeutics at the bedside. J Mol Cell Cardiol. 2005;39:99–112. doi: 10.1016/j.yjmcc.2005.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane GC, Liu XK, Yamada S, Olson TM, Terzic A. Cardiac KATP channels in health and disease. J Mol Cell Cardiol. 2005;38:937–943. doi: 10.1016/j.yjmcc.2005.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lymarenko IV. The comparative study of the effect of new fluorine-containing guanidine-derivate compounds and pinacidil on contractile function of the urinary bladder in vitro. Fiziol Zh [Ukrainian] 2005;51:88–91. [PubMed] [Google Scholar]
- Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- Moibenko AA, Strutynskyi RB, Yagupolskii LM, Mohort NA. Organization of industrial production of flocalin – new myotropic spasmolytic and cardioprotector. Sci Innov [Ukrainian] 2009;1:80–84. [Google Scholar]
- Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147–148. doi: 10.1038/305147a0. [DOI] [PubMed] [Google Scholar]
- Rogers TB, Gaa ST, Allen IS. Identification and characterization of functional angiotensin II receptors on cultured heart myocytes. J Pharmacol Exp Ther. 1986;236:438–444. [PubMed] [Google Scholar]
- Shaklai M, Tavassoli M. Lanthanum as an electron microscopic stain. J Histochem Cytochem. 1982;30:1325–1330. doi: 10.1177/30.12.6185564. [DOI] [PubMed] [Google Scholar]
- Shindo T, Yamada M, Isomoto S, Horio Y, Kurachi Y. SUR2 subtype (A and B)-dependent differential activation of the cloned ATP-sensitive K+ channels by pinacidil and nicorandil. Br J Pharmacol. 1998;124:985–991. doi: 10.1038/sj.bjp.0701927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg MI, Ertel P, Smallwood JK, Wyss V, Zimmerman K. The relation between vascular relaxant and cardiac electrophysiological effects of pinacidil. J Cardiovasc Pharmacol. 1988;12:S30–S40. doi: 10.1097/00005344-198812002-00007. [DOI] [PubMed] [Google Scholar]
- Strutyns'kyi RB, Moibenko OO. Modeling of KATP channel activity in normotensive and hypertensive animals. Fiziol Zh [Ukrainian] 2000;46:54–60. [PubMed] [Google Scholar]
- Strutyns'kyi RB, Moibenko OO, Iahupol's'kyi LM. The vasomotor effects of new fluorine-containing synthetic activators of ATP-dependent potassium channels. Fiziol Zh [Ukrainian] 2000;46:17–23. [PubMed] [Google Scholar]
- Strutyns'kyi RB, Pyvovar SM, Tumanovska LV, Moibenko OO. Cardioprotective effects of flokalin: relative role of activation of sarcolemmal and mitochondrial adenosine triphosphate-dependent potassium channels. Fiziol Zh [Ukrainian] 2008;54:15–23. [PubMed] [Google Scholar]
- Strutyns'kyi RB, Kotsiuruba AV, Neshcheret AP, Shysh AN, Rovenets RA, Moibenko AA. Cardioprotective effects of flokalin in experiments in vivo: influence on biochemical parameters of blood at ischemia-reperfusion of a myocardium. Fiziol Zh [Ukrainian] 2009a;55:12–19. [PubMed] [Google Scholar]
- Strutyns'kyi RB, Neshcheret AP, Tumanovs'ka LV, Rovenets RA, Moibenko AA. Cardioprotective effects of flokalin in experiments in vivo: influence on hemodynamic and damage of a myocardium at its ischemia-reperfusion. Fiziol Zh [Ukrainian] 2009b;55:9–16. [PubMed] [Google Scholar]
- Yagupolskii LM, Maletina II, Shavaran SS, Petko KI, Klebanov BM. N-1,2,2-trimethylpropyl-N′-cyano-N″-arylguanidines with fluorine containing substitutes in aromatic ring that have the hypotensive and cardiotonic action. 1997. Patent of Ukraine 17071.
- Yagupolskii LM, Maletina II, Petko KI, Fedyuk DV, Handrock R, Shavaran SS, et al. New fluorine-containing hypotensive preparations. J Fluor Chem. 2001;109:87–94. [Google Scholar]
- Yamazaki T, Taguchi T, Iwao Ojima I. Unique properties of fluorine and their relevance to medicinal chemistry and chemical biology. In: Ojima I, editor. Fluorine in Medicinal Chemistry and Chemical Biology. West Sussex, UK: John Wiley & Sons, Inc.; 2009. pp. 3–46. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


