Abstract
BACKGROUND AND PURPOSE
The calcimimetic, (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet), which activates Ca2+-sensing receptors (CaR) in parathyroid glands, is used to treat hyperparathyroidism. Interestingly, CaR in perivascular nerves or endothelial cells is also thought to modulate vascular tone. This study aims to characterize the vascular actions of calcimimetics.
EXPERIMENTAL APPROACH
In rat isolated small mesenteric arteries, the relaxant responses to the calcimimetics, cinacalcet and (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) were characterized, with particular emphasis on the role of CaR, endothelium, perivascular nerves, K+ channels and Ca2+ channels. Effects of L-ornithine, which activates a Ca2+-sensitive receptor related to CaR (GPRC6A), were also tested.
KEY RESULTS
Cinacalcet induced endothelium-independent relaxation (pEC50 5.58 ± 0.07, Emax 97 ± 6%) that was insensitive to sensory nerve desensitization by capsaicin or blockade of large-conductance Ca2+-activated K+ channels by iberiotoxin. Calindol, another calcimimetic, caused more potent relaxation (pEC50 6.10 ± 0.10, Emax 101 ± 6%), which was attenuated by endothelial removal or capsaicin, but not iberiotoxin. The negative modulator of CaR, calhex 231 or changes in [Ca2+]o had negligible effect on relaxation to both calcimimetics. The calcimimetics relaxed vessels precontracted with high [K+]o and inhibited Ca2+ influx in endothelium-denuded vessels stimulated by methoxamine, but not ionomycin. They also inhibited contractions to the L-type Ca2+ channel activator, BayK8644. L-ornithine induced small relaxation alone and had no effect on the responses to calcimimetics.
CONCLUSION AND IMPLICATIONS
Cinacalcet and calindol are potent arterial relaxants. Under the experimental conditions used, they predominantly act by inhibiting Ca2+ influx through L-type Ca2+ channels into vascular smooth muscle, whereas Ca2+-sensitive receptors (CaR or GPRC6A) play a minor role.
Keywords: calcimimetics, cinacalcet, calindol, L-ornithine, mesenteric arteries, calhex 231, Ca2+-sensing receptors, GPRC6A, Ca2+ influx, endothelium
Introduction
The cell surface, extracellular Ca2+-sensing receptor (CaR), which is activated by Ca2+ ions, is crucial for maintaining a stable systemic [Ca2+]o, primarily through regulation of parathyroid hormone secretion and renal Ca2+ excretion (see reviews Brown and MacLeod, 2001). CaR is mainly coupled to Gq/11 proteins and subsequent activation of phospholipase C, generating intracellular Ca2+ signals, but it can also activate Gi proteins and mitogen-activated protein kinases (Brown and MacLeod, 2001). Of note, a number of endogenous (L-α-amino acids) and synthetic substances act as allosteric modulators of CaR (Jensen & Brauner-Osborne, 2007). Calcimimetics are positive modulators of CaR by potentiating the action of extracellular Ca2+. One particular example is (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet), which has been approved for the clinical treatment of uraemic secondary hypercalcaemia parathyroidism and hypercalcaemia in parathyroid cancer (Steddon and Cunningham, 2005). On the other hand, calcilytics, which negatively modulate CaR, might be useful in osteoporosis as parathyroid hormone also stimulates bone growth (Steddon and Cunningham, 2005).
Interestingly, CaR is also present in tissues not involved in systemic Ca2+ balance, including cardiovascular tissues (Brown and MacLeod, 2001). Indeed, several studies have shown that cinacalcet or other calcimimetics can lead to acute hypertension as well as chronic hypotension, possibly due to normalization of parathyroid hormone level and direct cardiovascular actions (Ogata et al., 2003; Odenwald et al., 2006; Fryer et al., 2007). Expression of CaR has been demonstrated in the vasculature; in perivascular sensory nerves (Bukoski et al., 1997), the endothelium (Weston et al., 2005; Molostvov et al., 2007) and the vascular smooth muscle (Smajilovic et al., 2006; Molostvov et al., 2007). Although the physiological [Ca2+] in serum is tightly regulated, changes in interstitial [Ca2+] might be sufficient to activate vascular CaR and thus CaR are involved in the regulation of vascular tone and blood pressure. Bukoski and co-workers demonstrated that increases in [Ca2+]o (from 1 to 5 mM), presumably via CaR, cause mesenteric relaxation that is dependent on perivascular sensory nerves and large conductance Ca2+-activated K+ channels (BKCa; Bukoski et al., 1997; Ishioka and Bukoski, 1999). More recently, activation of endothelial CaR, which is closely coupled to intermediate conductance Ca2+-activated K+ channels (IKCa), has also been found to elicit vasorelaxation (Weston et al., 2005).
The vasorelaxant actions of Ca2+ or CaR would be consistent with the beneficial effect of increased dietary Ca2+ intake in some forms of hypertension (McCarty, 2004). However, CaR in vascular smooth muscle cells has also been associated with vasocontraction (Wonneberger et al., 2000) and signalling via mitogen-activated protein kinases (Smajilovic et al., 2006; Molostvov et al., 2007). Therefore, the precise vascular effects of CaR and calcimimetics remain to be defined. In this study, using rat isolated small mesenteric arteries, we have characterized the relaxant responses to cinacalcet and the more recently developed calcimimetic, (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) (Kessler et al., 2004). In particular, the involvement of CaR, the endothelium, perivascular sensory nerves, K+ channels and Ca2+ channels was investigated. Recently, GRPC6A, a novel receptor that is sensitive to Ca2+ and closely related to CaR (Wellendorph et al., 2007), has also been identified in the endothelium of mesenteric and coronary arteries (Harno et al., 2008). Thus, the potential contribution of GPRC6A to the calcimimetic responses was also explored. Our results suggest that the two calcimimetics act as potent vasorelaxants mainly through inhibition of Ca2+ influx in smooth muscle cells independent of CaR or GPRC6A. Activation of K+ channels and, in the case of calindol, the endothelium and capsaicin-sensitive nerves also significantly contribute to their mesenteric relaxations.
Methods
Myographic studies
Male Wistar rats (200–350 g; Charles River UK Ltd, Kent, UK) were stunned by a blow to the back of their neck and killed by cervical dislocation. All animal care and use was in accordance with the UK Animal (Scientific Procedures) Act 1986. The third-order branches of the superior mesenteric artery, which provides blood supply to the intestine, were removed and cleaned of adherent tissue. Segments (2 mm in length) were mounted in a Mulvany–Halpern type wire myograph (Model 610 M; Danish Myo Technology, Aarhus, Denmark) and maintained at 37°C in gassed (95% O2/5% CO2) Krebs–Henseleit solution of the following composition (mM): NaCl 118, KCl 4.7, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, CaCl2 2, d-glucose 10 as previously described, unless otherwise stated (Ho and Randall, 2007). Arteries were equilibrated and set to a basal tension of 2 to 2.5 mN. The integrity of the endothelium was assessed by precontracting the vessel with 10 µM methoxamine (a α1-adrenoceptor agonist), followed by relaxation with 10 µM carbachol (a muscarinic acetylcholine receptor agonist); vessels showing relaxations of greater than 90% were designated as endothelium-intact. When endothelium was not required, it was removed by rubbing the intima with a human hair; carbachol-induced relaxation of less than 10% indicated successful removal.
In some experiments, the third-order branches of the superior mesenteric vein were similarly dissected and mounted on a wire myograph. Veins were equilibrated and set to a basal tension of 0.5 mN (Zhang et al., 2007) and precontracted with 60 mM KCl. Nomenclature of molecular targets, including receptors and ion channels, is used in accordance with the Guide to Receptor and Channels, British Journal of Pharmacology (Alexander et al., 2008).
Experiments in the presence of extracellular Ca2+
After the test for endothelial integrity, arteries were left for 30 min and then precontracted with 10 µM methoxamine. This was followed by construction of a cumulative concentration–relaxation curve to cinacalcet, calindol or L-ornithine. Preliminary experiments showed that washing could not fully reverse the effects of cinacalcet or calindol; therefore, only a single concentration–response curve to the calcimimetic was constructed in each preparation. The vehicle of calcimimetics had no significant relaxation (up to 0.6% ethanol vv−1; data not shown) in methoxamine-precontracted arteries. Most experiments were performed in matched vessels; effects of putative modulators or endothelial removal were compared with the control responses obtained in separate vessels of the same rat.
To investigate the relaxation mechanisms of calcimimetics, 4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide (calhex) 231 (a negative modulator of CaR; 3 µM) and iberiotoxin (a selective BKCa blocker; 50 nM) were added to the myograph bath 30 min before, kept present during, construction of the concentration–response curve. To examine the potential interaction between GRPC6A and calcimimetics, L-ornithine was added to myograph for 5 min before determination of relaxant responses to either cinacalcet or calindol. For functional desensitization of capsaicin-sensitive perivascular nerves, vessels were incubated with 10 µM capsaicin for 1 h, followed by washing out (Zygmunt et al., 1999). In some experiments, vessels were precontracted with high K+ (60 mM) Krebs–Henseleit solution, which was prepared by equimolar substitution of NaCl for KCl in the standard Krebs–Henseleit buffer described above. Further addition of a small amount of methoxamine (1 µM) was often required to achieve a stable precontracted tone. The tension generated by 60 mM KCl (plus methoxamine top-up: 10.6 ± 1.1 mN) was similar to that induced by 10 µM methoxamine in the test for endothelial integrity (9.0 ± 1.1 mN; 16 vessels).
To further investigate the interaction between the calcimimetics and CaR, some experiments were conducted using Krebs–Henseleit solution containing 0.5 mM, instead of 2 mM, CaCl2 after the endothelial integrity test. Mesenteric arteries were precontracted with 10 µM methoxamine, followed by construction of a cumulative concentration–relaxation curve to cinacalcet, calindol or CaCl2 (1–5 mM). The effects of calhex 231, iberiotoxin or capsaicin on relaxation to the calcimimetics or CaCl2 were determined as described above. Some arteries were pretreated with cinacalcet (1 µM) or calindol (0.3 µM) for 30 min, before construction of the relaxation curve to CaCl2. Preliminary experiments found that the lower [Ca2+]o significantly reduced methoxamine contractions (0.1–30 µM; data not shown); however, a similar precontracted tone was achieved, where necessary, by using a higher concentration of methoxamine (20 µM) (8.7 ± 0.7 mN as compared with 8.7 ± 0.7 mN under 2 mM [Ca2+]o; 25 vessels). For relaxation studies at 0.5 or 2 mM [Ca2+]o, the mean tension generated in the test for endothelial integrity was similar to that generated after incubation with a potential modulator (control, 8.6 ± 0.9 mN; vs. + calhex 231, 6.9 ± 0.5 mN, 20 vessels; control, 8.6 ± 0.8 mN; vs. + iberiotoxin, 9.6 ± 0.9 mN, 17 vessels; control, 9.3 ± 0.9 mN vs. + capsaicin, 10.1 ± 0.8 mN, 15 vessels; control, 8.4 ± 0.9 mN; vs. + L-ornithine, 9.7 ± 0.8 mN, 8 vessels; control, 10.8 ± 1.9 mN vs. + cinacalcet, 8.8 ± 1.4 mN, 7 vessels; control, 10.0 ± 1.2 mN vs. + calindol, 9.2 ± 1.2 mN, 7 vessels).
In endothelium-denuded mesenteric arteries, effects of calcimimetics on contractions induced by (4R)- and (4S)-1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-trifluoromet hyl)phenyl]-3-pyridinecarboxylic acid methyl ester (BayK8644) (an activator of the voltage-gated, L-type Ca2+ channels; 3 µM, Sanguinetti and Kass, 1984) were also determined. Preliminary experiments found that 3 µM BayK8644 produced consistent, although often transient, contractions in the presence of 15 mM KCl. This is consistent with previous reports that BayK8644 exerts greater effects on L-type Ca2+ channels at more depolarized conditions (Sanguinetti and Kass, 1984). Addition of KCl (15 mM) alone caused minimal contractions (0.2 ± 0.1 mN; eight vessels). In separate vessels, cinacalcet (10 µM), calindol (3 µM), verapamil (a L-type Ca2+ channel blocker; 10 µM) or their vehicle (0.1% vv−1 ethanol) was added to the bath, then 30 min later 15 mM KCl followed by 3 µM BayK8644.
In an additional set of experiments, cumulative concentration–relaxation curves to cinacalcet or calindol were also obtained from small mesenteric veins precontracted with 60 mM KCl.
Ca2+-free experiments
Influx of extracellular Ca2+ through plasma membrane Ca2+ channels was examined in endothelium-denuded mesenteric arteries depleted of intracellular Ca2+ stores according to the methods described previously (Ho and Hiley, 2003). Briefly, extracellular Ca2+ was removed by washing vessels with Ca2+-free Krebs–Henseleit solution (composition the same as normal Krebs–Henseleit buffer but with CaCl2 omitted). EGTA (1 mM) was added to the myograph bath, followed by a series of additions of 10 µM methoxamine in order to deplete intracellular Ca2+ stores as shown by loss of the contractile response. Vessels were then washed with Ca2+-free Krebs–Henseleit solution, 10 µM methoxamine was added, and a cumulative concentration–response curve to CaCl2 (10 µM–10 mM) was then obtained. Then, the washing process was repeated and a second CaCl2 curve constructed in the presence of vehicle (0.1% vv−1 ethanol), cinacalcet or calindol (with 30 min pre-incubation). Contractions were expressed as a percentage of the maximum contraction induced by CaCl2 in the vessel in the presence of 10 µM methoxamine alone. It was noted that the ethanol vehicle significantly potentiated contractions to CaCl2 (data not shown), thus effects of the calcimimetics were determined by comparing the second concentration–response curves with CaCl2 (in the presence of cinacalcet or calindol vs. vehicle).
To further examine the roles of voltage-gated Ca2+ channels, ionomycin (a Ca2+ ionophore) was used to facilitate Ca2+ entry. However, as the effects of ionomycin are irreversible, a modified protocol was used. Endothelium-denuded mesenteric arteries were depleted of intracellular Ca2+ stores as described above, followed by incubation with 10 µM ionomycin for 30 min. Contraction was then induced by readdition of 2 mM CaCl2, followed by vehicle (0.1% vv−1 ethanol), cinacalcet (10 µM), calindol (3 and 10 µM) or verapamil (10 µM). Preliminary experiments showed that an initial addition of ethanol vehicle (0.1% vv−1) caused an exaggerated contraction on top of the ionomycin-evoked response, but subsequent additions of ethanol vehicle had little effect (data not shown). Thus, in all experiments, ethanol vehicle (0.1% vv−1) was applied, before determination of responses to a further addition of vehicle, a calcimimetic or verapamil.
Data and statistical analysis
All relaxant responses are expressed as percentage relaxation of the tone induced by methoxamine or KCl. Values are given as mean ± SEM and n represents the number of animals used. Concentration–relaxation curves, with variable slopes, were obtained by fitting data using a sigmoidal logistic equation (Prism 4, GraphPad Software, Inc, San Diego, CA, USA). [Y = Bottom + (Top − Bottom)/(1 + 10((LogEC50−X) × Hillslope)), where X is a logarithm of drug concentration and Y is the response that starts from the Bottom and goes to the Top in a sigmoid shape]. The sigmoidal curves were also used to calculate pEC50, negative logarithm of the concentration of relaxant giving 50% of maximum response and Emax, maximal response. Statistical comparisons of pEC50 and Emax were made by Student's t-tests or one-way analysis of variance followed by Dunnett's post hoc tests, where appropriate (Prism 4, GraphPad Software, Inc, San Diego, CA, USA).
Relaxations and contractions to CaCl2 in the presence of methoxamine were examined by two-way analysis of variance followed by Bonferroni post hoc tests, whereas contractions to CaCl2 in the presence of ionomycin, or contractions to BayK8644, were analysed by one-way analysis of variance followed by Dunnett's post hoc tests (Prism 4, GraphPad Software, Inc, San Diego, CA, USA). P < 0.05 was taken as statistically significant.
Drugs
Methoxamine, carbachol, L-ornithine (Sigma Chemical Co., Poole, UK), iberiotoxin (Tocris Biosciences, Bristol, UK) were dissolved in deionized water. Cinacalcet [(R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride], calindol [(R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride], S-cinacalcet [(S)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride], S-calindol [(S)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride], calhex 231 (4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide) (Toronto Research Chemicals Inc., Ontario, Canada), capsaicin and verapamil (Sigma) were dissolved in 100% ethanol. Ionomycin (Sigma) and (+/−)-BayK8644 ((4R)- and (4S)-1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-trifluoromet hyl)phenyl]-3-pyridinecarboxylic acid methyl ester; Merck Chemicals Ltd, Nottingham, UK) were dissolved in 100% dimethyl sulphoxide.
Results
Relaxation to cinacalcet, calindol and L-ornithine in mesenteric arteries
Figure 1 demonstrates that cinacalcet and calindol, a more recently developed calcimimetic, induced concentration-dependent relaxation of rat small mesenteric arteries. The chemical structures of the two compounds are also shown in Figure 1. Relaxation to cinacalcet was endothelium-independent (Table 1; Figure 2A), and slightly less potent (pEC50, P < 0.01) than that of calindol (pEC50 = 6.10 ± 0.10, Emax = 101 ± 6, n = 6 vs. cinacalcet: pEC50 = 5.58 ± 0.07, Emax = 97 ± 6, n = 5; Figure 2B). Removal of the endothelium caused an apparent rightward displacement (P < 0.01) of the concentration–response curve to calindol (Table 2; Figure 2B).
Figure 1.
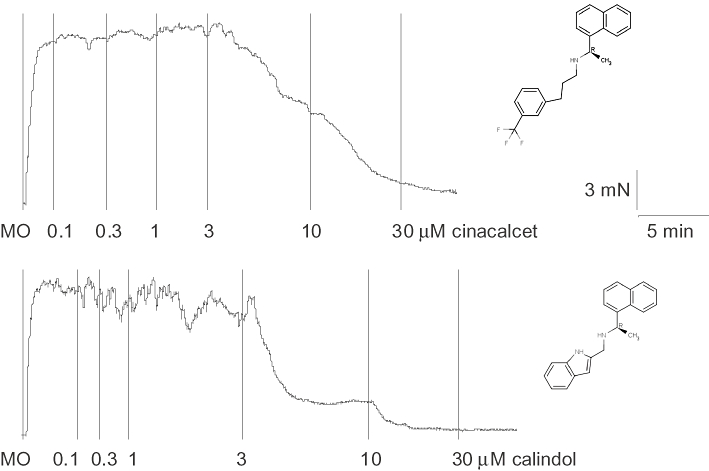
Original tracings showing concentration-dependent relaxation to (A) (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) and (B) (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) in separate mesenteric arteries of the rat. Vertical lines denote addition of drugs. MO, methoxamine (at 10 µM). Chemical structures of the calcimimetics are shown next to their corresponding tracings.
Table 1.
Concentration-dependent relaxation to cinacalcet in rat small mesenteric arteries
| pEC50 | Emax (%) | n | |
|---|---|---|---|
| +endothelium | 5.58 ± 0.07 | 97 ± 6 | 5 |
| −endothelium | 5.59 ± 0.06 | 91 ± 5 | 5 |
| −endothelium | |||
| Methoxamine-precontracted | 5.56 ± 0.07 | 96 ± 6 | 4 |
| KCl-precontracted | 5.24 ± 0.09** | 103 ± 11 | 5 |
| −endothelium | 5.60 ± 0.06 | 97 ± 5 | 4 |
| +50 nM iberiotoxin | 5.49 ± 0.10 | 95 ± 10 | 4 |
| +10 µM capsaicin | 5.51 ± 0.04 | 94 ± 5 | 5 |
| −endothelium | 5.50 ± 0.04 | 95 ± 7 | 4 |
| +3 µM calhex 231 | 5.50 ± 0.04 | 96 ± 4 | 4 |
| −endothelium (at 0.5 mM [Ca2+]o) | 5.55 ± 0.04 | 97 ± 4 | 4 |
| +endothelium | 5.64 ± 0.08 | 98 ± 6 | 4 |
| +1 mM L-ornithine | 5.63 ± 0.07 | 100 ± 5 | 4 |
Data are given as mean ± SEM. pEC50 and Emax were obtained as stated in Methods. n indicates number of animals used. All responses were obtained at 2 mM [Ca2+]o, unless otherwise stated.
P < 0.01 significantly different from the corresponding controls.
calhex 231, 4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide; cinacalcet, (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride.
Figure 2.
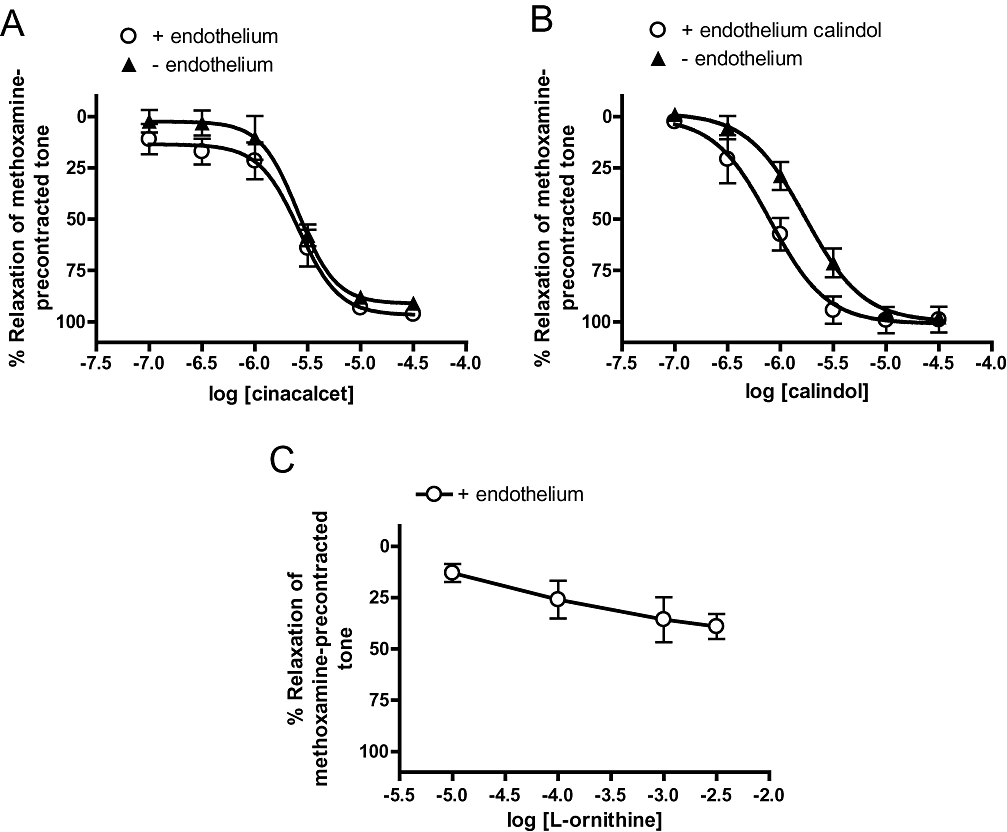
Relaxation to (A) (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) (B) (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) and (C) L-ornithine in endothelium-intact and -denuded mesenteric arteries. n = 5–6. Values are shown as means and vertical bars represent SEM.
Table 2.
Concentration-dependent relaxation to calindol in rat small mesenteric arteries
| pEC50 | Emax (%) | n | |
|---|---|---|---|
| With endothelium | 6.10 ± 0.10 | 101 ± 6 | 6 |
| Without endothelium | 5.77 ± 0.06** | 100 ± 5 | 6 |
| +endothelium | |||
| Methoxamine-precontracted | 6.20 ± 0.09 | 103 ± 4 | 5 |
| KCl-precontracted | 5.66 ± 0.05** | 99 ± 5 | 5 |
| −endothelium | |||
| Methoxamine-precontracted | 5.87 ± 0.05 | 97 ± 4 | 6 |
| KCl-precontracted | 5.48 ± 0.04** | 92 ± 4 | 4 |
| +endothelium | 6.04 ± 0.05 | 96 ± 3 | 4 |
| +50 nM iberiotoxin | 6.08 ± 0.11 | 100 ± 6 | 4 |
| +10 µM capsaicin | 5.76 ± 0.05* | 97 ± 3 | 4 |
| +endothelium | 5.97 ± 0.04 | 98 ± 3 | 7 |
| +3 µM calhex 231 | 5.84 ± 0.07 | 101 ± 5 | 5 |
| +endothelium (at 0.5 mM [Ca2+]o) | 5.76 ± 0.06# | 105 ± 4 | 5 |
| +3 µM calhex 231 | 5.81 ± 0.07 | 102 ± 5 | 5 |
| +endothelium | 6.04 ± 0.06 | 102 ± 4 | 4 |
| +1 mM L-ornithine | 6.00 ± 0.04 | 98 ± 3 | 4 |
Data are given as mean ± SEM. pEC50 and Emax were obtained as stated in Methods. n indicates number of animals used.
P < 0.05
P < 0.01 significantly different from the corresponding controls. All responses were obtained at 2 mM [Ca2+]o, unless otherwise stated.
P < 0.05 compared with relaxations at 2 mM [Ca2+]o. calhex 231, 4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide; calindol, (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride.
In addition to CaR, a related G-protein coupled receptor, GPRC6A is also expressed in the endothelium of mesenteric arteries (Harno et al., 2008) although its function remains unclear. We found that L-ornithine, an amino acid that activates rat GPRC6A (EC50 = 264 µM, Wellendorph et al., 2007) induced small relaxations (% relaxation at 3 mM = 39 ± 6%; n = 6; Figure 2C).
Role of K+ channels and perivascular sensory nerves in relaxation to calcimimetics
In endothelium-denuded vessels, precontracting arteries with 60 mM KCl, instead of 10 µM methoxamine (see Methods), reduced (P < 0.01) the pEC50 for cinacalcet without affecting the maximum response (Table 1; Figure 3A). Similar results were also obtained with calindol, with or without the endothelium (Table 2; Figure 4A,B).
Figure 3.
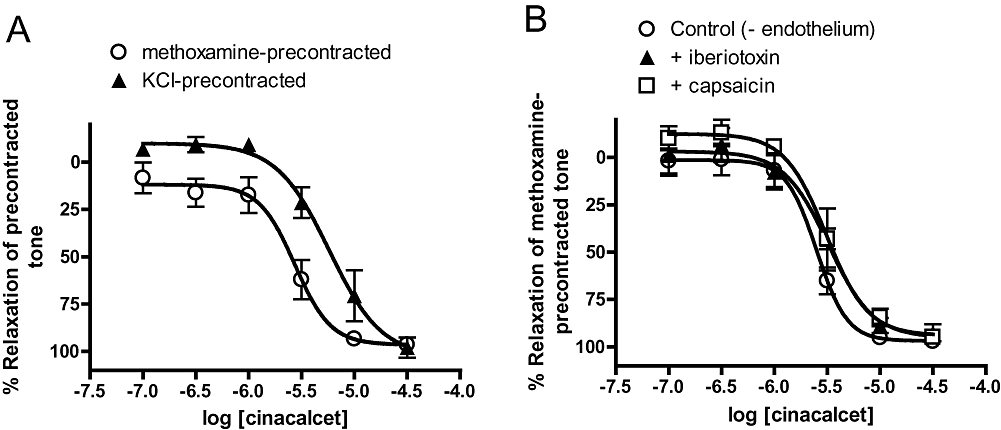
(A) Relaxation to (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) in endothelium-denuded arteries precontracted with 60 mM KCl and 10 µM methoxamine. (B) Effects of iberiotoxin (50 nM) and capsaicin (10 µM) on relaxation to cinacalcet in methoxamine-precontracted, endothelium-denuded arteries. n = 4–5. Values are shown as means and vertical bars represent SEM.
Figure 4.
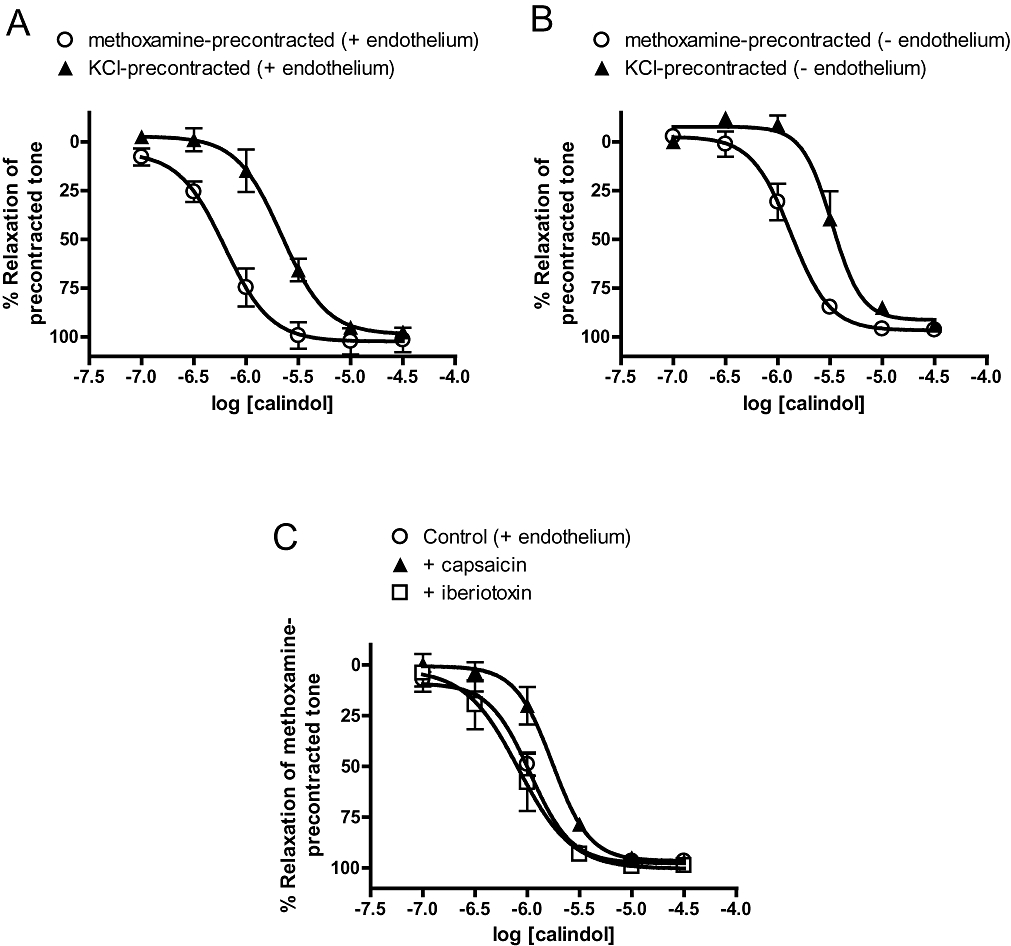
Relaxation to (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) in (A) endothelium-intact and (B) endothelium-denuded arteries precontracted with 60 mM KCl and 10 µM methoxamine. (C) Effects of iberiotoxin (50 nM) and capsaicin (10 µM) on relaxation to calindol in methoxamine-precontracted, endothelium-intact arteries. n = 4–6. Values are shown as means and vertical bars represent SEM.
Further experiments found that cinacalcet responses were not significantly attenuated by iberiotoxin, which is a selective BKCa blocker, or prolonged treatment with capsaicin, which desensitized TRPV1-expressing perivascular nerves (Table 1; Figure 3B). In contrast, the pEC50 value of calindol-induced relaxation was significantly (P < 0.05) attenuated by capsaicin treatment but not iberiotoxin (Table 2; Figure 4C). Iberiotoxin (50 nM) also had no effect on the small relaxant responses to L-ornithine (n = 4; data not shown).
Role of CaR in relaxation to calcimimetics
Interestingly, the negative modulator of CaR, calhex 231 had no significant effect on arterial relaxation induced by cinacalcet (Table 1; Figure 5A). Reducing the [Ca2+] in Krebs–Henseliet solution (from 2 to 0.5 mM) also had no effect on cinacalcet relaxations (Table 1; Figure 5A). Responses to calindol were not significantly affected by calhex 231 (Table 2; Figure 5B). Reducing [Ca2+]o slightly reduced the pEC50 (P < 0.05) of calindol-induced relaxation (Table 2), but the resultant relaxation was again not affected by calhex 231 (Table 2; Figure 5B).
Figure 5.
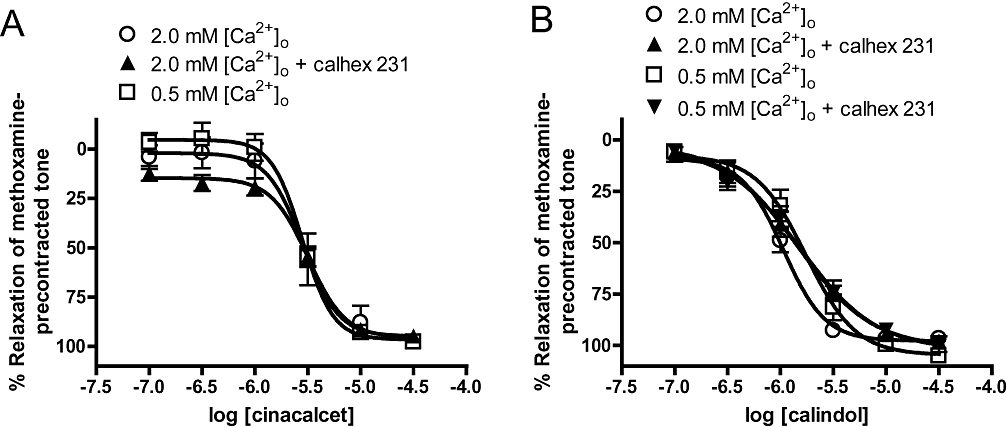
(A) Relaxation to (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) in endothelium-denuded arteries at 2 and 0.5 mM [Ca2+]o, in the absence or presence of 3 µM 4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide (calhex 231). (B) Relaxation to (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) in endothelium-intact arteries at 2 and 0.5 mM [Ca2+]o, in the absence or presence of 3 µM calhex 231. Values are shown as means and vertical bars represent SEM. Note that, on the graph, responses in the presence of calhex 231 at 2 mM [Ca2+]o overlap almost entirely with those obtained at 0.5 mM [Ca2+]o.
In endothelium-intact, methoxamine-precontracted vessels under 0.5 mM [Ca2+]o, incremental additions of CaCl2 (1–5 mM) elicited contractions followed by relaxations (Figure 6A). In contrast to the cases for cinacalcet and calindol, the Ca2+-induced relaxation was inhibited by 3 µM calhex 231 (Figure 6A), 50 nM iberiotoxin (Figure 6B) or 10 µM capsaicin (Figure 6B). It was noted that while iberiotoxin induced a rightward displacement of the CaCl2 relaxation curve, capsaicin resulted in a reduced Emax (Figure 6B). On the other hand, pretreatment of vessels with cinacalcet (1 µM) or calindol (0.3 µM) did not potentiate the responses to CaCl2; in fact, 1 µM cinacalcet significantly reduced responses to CaCl2 at 1 and 2 mM (Figure 6C). Increasing the concentration of calindol to 1 µM also had no effect on CaCl2-induced relaxation (data not shown).
Figure 6.
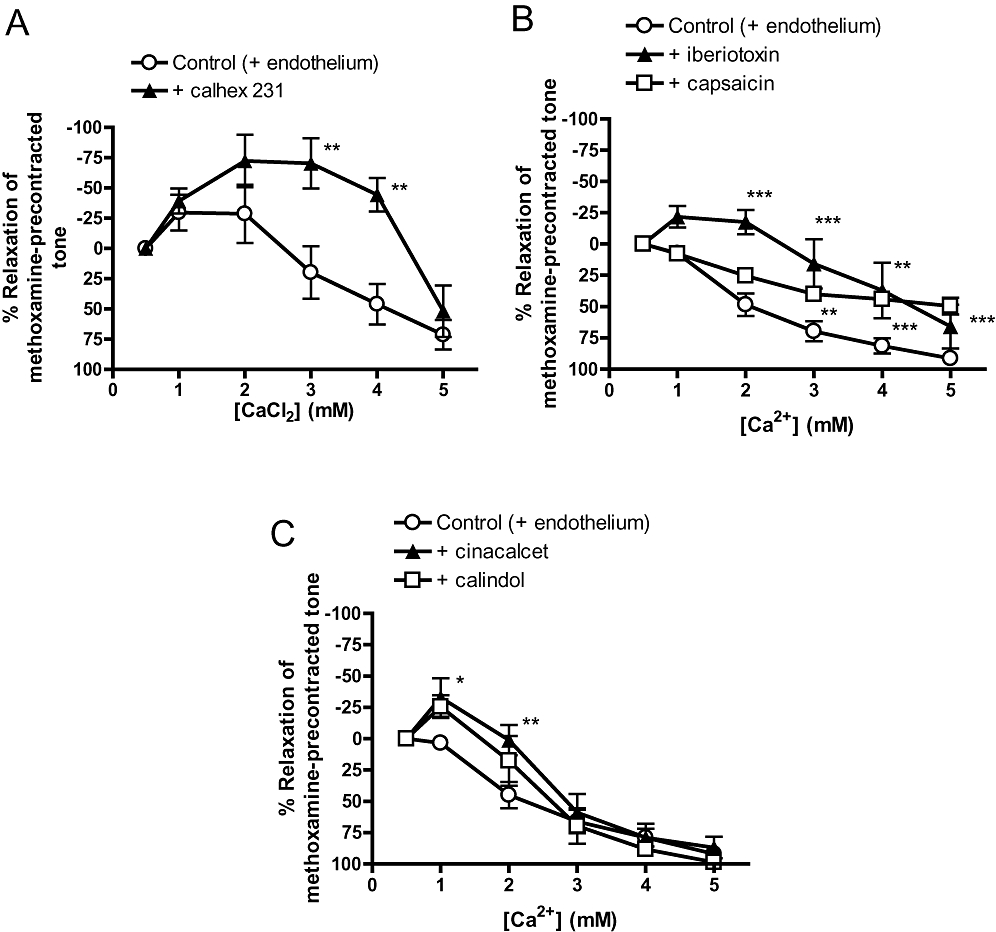
Relaxation to incremental additions of CaCl2 (1–5 mM) in endothelium-intact, methoxamine-precontracted vessels under 0.5 mM [Ca2+]o. (A) Effects of 4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide (calhex 231) (3 µM) on CaCl2-induced relaxation. (B) Effects of iberiotoxin (50 nM) or capsaicin (10 µM) on CaCl2-induced relaxation. (C) Effects of (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) (1 µM) or (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) (0.3 µM) on CaCl2-induced relaxation. n = 4–8. *P < 0.05, **P < 0.01, ***P < 0.001 versus control; two-way analysis of variance followed by Bonferroni post hoc tests. Values are shown as means and vertical bars represent SEM.
To explore if GPRC6A was involved in arterial relaxations to the calcimimetics, the effect of a brief treatment (for 5 min) with L-ornithine, which can potentiate agonist activity at GPRC6A (Harno et al., 2008), was tested. L-ornithine, at 1 mM, had no effect on cinacalcet (Table 1) or calindol responses (Table 2).
Effects of calcimimetics on CaCl2-induced contractions in the presence of methoxamine
All experiments were performed in endothelium-denuded arteries depleted of intracellular Ca2+ and then exposed to methoxamine (10 µM) in the absence of extracellular Ca2+. Under these conditions, addition of CaCl2 (0.01–10 mM) caused concentration-dependent contractions up to 1–3 mM and produced relaxation at higher concentrations. This protocol is frequently used to examine the effect of pharmacological agents on Ca2+ entry in vascular smooth muscle (e.g. Ho and Hiley, 2003). Figure 7A shows that cinacalcet, in a concentration-dependent manner, inhibited CaCl2-induced contractions; these contractions were abolished by 10 µM cinacalcet. These effects were mimicked by calindol (Figure 7B), which abolished the CaCl2-induced contractions at 3 µM.
Figure 7.
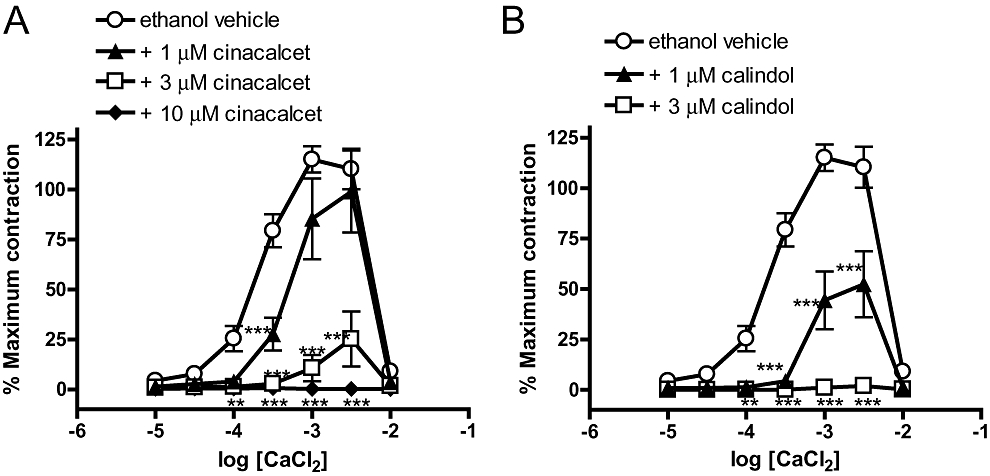
Concentration–response curves for CaCl2-induced contractions of methoxamine-stimulated, endothelium-denuded mesenteric arteries depleted of intracellular Ca2+ stores with EGTA as described in Methods. (A) Responses to CaCl2 in the presence of ethanol vehicle (0.1% vv−1) or (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) (at 1, 3, 10 µM). (B) Responses to CaCl2 in the presence of ethanol vehicle (0.1% vv−1) or (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) (at 1 or 3 µM). n = 4 for all. **P < 0.01, ***P < 0.001 versus vehicle; two-way analysis of variance, followed by Bonferroni post hoc tests. Values are shown as means and vertical bars represent SEM.
Effects of calcimimetics on contractions induced by BayK8644
Activation of voltage-gated, L-type Ca2+ channels by BayK8644 (3 µM) induced contractions of endothelium-denuded vessels previously exposed to 15 mM KCl (see Methods). These contractions were significantly inhibited by 10 µM cinacalcet, 3 µM calindol or the phenylalkaylamine L-type Ca2+ channel blocker, verapamil (at 10 µM; Figure 8A).
Figure 8.
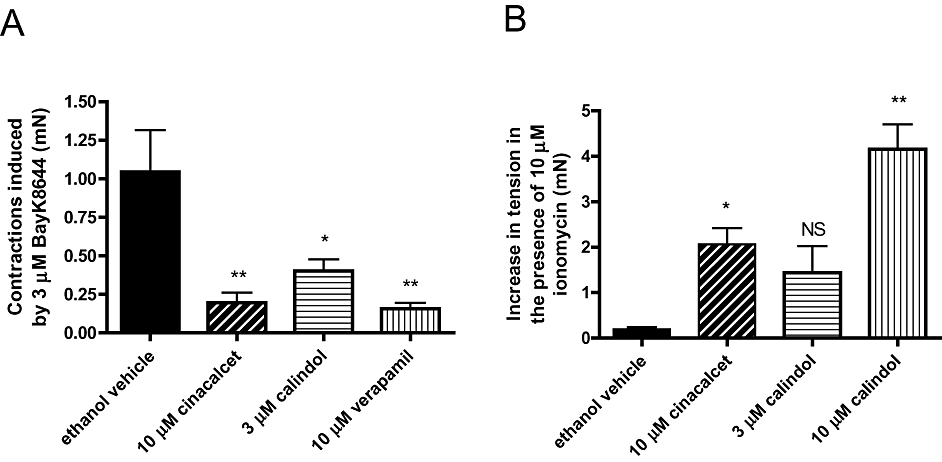
(A) Effects of ethanol vehicle (0.1% vv−1), (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) (10 µM) or (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) (3 µM) on contractions to 3 µM BayK8644 in endothelium-denuded mesenteric arteries as described in Methods. (B) Effects of ethanol vehicle (0.1% vv−1), cinacalcet (10 µM) or calindol (3 µM) on precontracted tone induced by 2 mM CaCl2 in ionomycin-stimulated, endothelium-denuded mesenteric arteries depleted of intracellular Ca2+ stores with EGTA, as described in Methods. n = 4–5. *P < 0.05, **P < 0.01 versus vehicle; one-way analysis of variance, followed by Dunnett's post hoc tests. Values are shown as means and vertical bars represent SEM. NS, not significant.
Effects of calcimimetics on CaCl2-induced contractions in the presence of ionomycin
As described in Methods, contractions were evoked by ionomycin in arteries that were denuded of endothelium, depleted of intracellular Ca2+ stores and initially maintained in the absence of extracellular Ca2+. Intriguingly, under these conditions, addition of cinacalcet (10 µM) and calindol (3 and 10 µM) induced contractions (Figure 8B). In contrast, verapamil (10 µM) had no significant effect on ionomycin-evoked contractions (0.1 ± 0.4 mN; n = 6). It was noted that contractions to the calcimimetics developed more slowly (time taken to attain maximum response ∼15 min) than their relaxations seen under methoxamine-precontracted tone (∼5 min).
Relaxation to the S-enantiomers of cinacalcet and calindol in mesenteric arteries
S-Cinacalcet, the S-enantiomer of cinacalcet that is inactive at CaR (Brown and MacLeod, 2001), also caused concentration-dependent relaxation (with endothelium, S-cinacalcet, pEC50 = 5.66 ± 0.07; Emax = 100 ± 5%; n = 6; R-cinacalcet, pEC50 = 5.46 ± 0.15; Emax = 101 ± 14%; n = 5; Figure 9A). Similar results were also obtained for the S-enantiomer of calindol, except pEC50 for S-calindol was significantly (P < 0.05) lower than that for calindol (with endothelium, S-calindol, pEC50 = 5.90 ± 0.05; Emax = 101 ± 3%; n = 4; R-calindol, pEC50 = 5.66 ± 0.15; Emax = 100 ± 5%; n = 5; Figure 9B).
Figure 9.
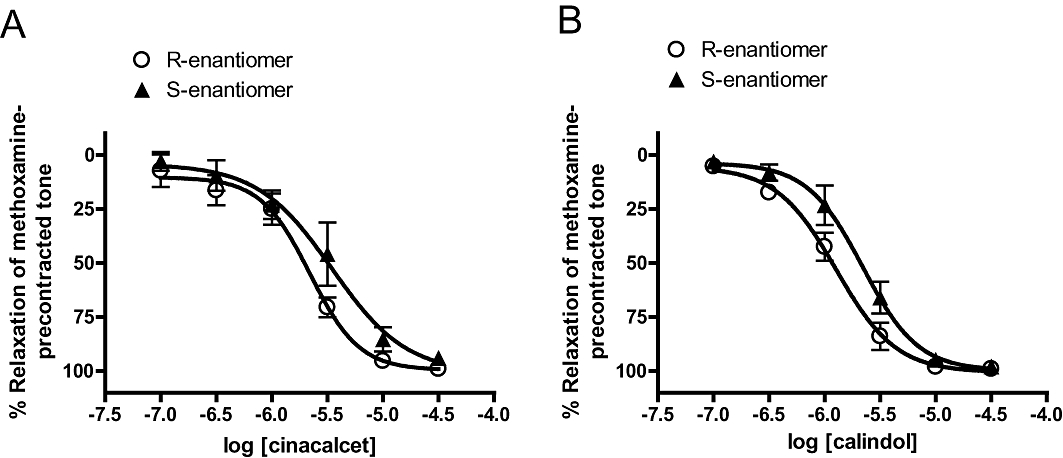
Relaxation to S-and R-enantiomers of (A) (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) and (B) (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) in endothelium-intact mesenteric arteries. n = 4–6. Values are shown as means and vertical bars represent SEM.
Relaxation to cinacalcet and calindol in mesenteric veins
In contrast to results obtained in mesenteric arteries, calindol induced small relaxations in endothelium-denuded, 60 mM KCl-precontracted mesenteric veins (Figure 10). An even smaller effect was detected for cinacalcet (Figure 10, P < 0.05); significant relaxation was only observed at 30 µM.
Figure 10.
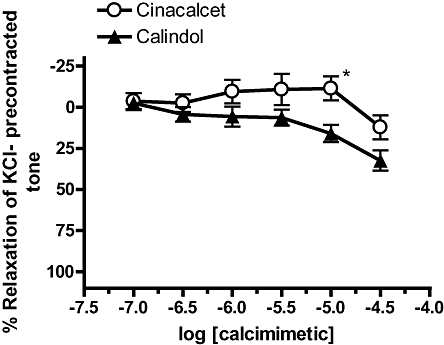
Relaxation to (R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride (cinacalcet) and (R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride (calindol) in endothelium-denuded mesenteric veins precontracted with 60 mM KCl. n = 5 for all. *P < 0.05 versus calindol, two-way analysis of variance, followed by Bonferroni post hoc tests. Values are shown as means and vertical bars represent SEM.
Discussion and conclusions
Calcimimetics, such as cinacalcet and calindol, are known to activate CaR by potentiating the agonist effects of Ca2+ ions (Jensen and Brauner-Osborne, 2007). Functional CaRs are thought to be expressed in the mesenteric arteries (Wang and Bukoski, 1998; Weston et al., 2005) and indeed we found that both cinacalcet and calindol were potent (pEC50– 5.58 and 6.10 respectively) vasorelaxants in rat small mesenteric arteries. CaR has been detected in perivascular sensory nerves (Wang and Bukoski, 1998), endothelial cells (Weston et al., 2005) and, in some cases, smooth muscle cells (Molostvov et al., 2007). In particular, Bukoski and co-workers have proposed that increasing extracellular [Ca2+] within the physiological range (from 1 to 5 mM) relaxes rat mesenteric arteries through excitation of capsaicin-sensitive nerves and subsequent release of endocannabinoids and activation of BKCa in vascular smooth muscle (Bukoski et al., 1997; Ishioka and Bukoski, 1999; Awumey et al., 2008). The authors also reported that the Ca2+-induced relaxation was independent of a functional endothelium (Bukoski et al., 1997). In contrast, we found that, at 2 mM [Ca2+]o, cinacalcet relaxations were not affected by prolonged treatment with capsaicin, which is an agonist of transient receptor potential vanilloid type 1 (TRPV1) receptors and causes desensitization of TRPV1-expressing sensory nerves. BKCa blockade with iberiotoxin or endothelial removal also had no effect. While iberiotoxin did not affect calindol responses, the pEC50 though not Emax of calindol was attenuated in denuded or capsaicin-treated arteries. This is somewhat surprising because the two calcimimetics are thought to have similar potencies and efficacies on CaR, as shown in cells stably expressing rat CaR (Kessler et al., 2004).
It should be pointed out that our data with calindol appear at odds with the recent report that relaxation to calindol is only observed after BKCa blockade (Weston et al., 2008). The authors, using isolated mesenteric arteries mounted in a pressure myograph, found that calindol hyperpolarized vascular smooth muscle cells via endothelial intermediate- conductance Ca2+-activated K+ channels (IKCa), an effect which could be prevented by an iberiotoxin-sensitive ‘K+ cloud’ in precontracted vessels (Weston et al., 2008; see also Edwards and Weston, 2004). The basis of these discrepancies remains unclear; perhaps under the current experimental conditions, relaxation mechanisms independent of IKCa activity are exaggerated. We did observe a small endothelium-dependent component of calindol responses. However, because precontraction with 60 mM KCl, which abolished K+ efflux via K+ channels, attenuated relaxation to calindol in both endothelium-intact and -denuded arteries, the participation of endothelial K+ channels remains unclear. High [K+]o also attenuated cinacalcet-induced relaxation in denuded vessels, suggesting the involvement of K+ channels in vascular smooth muscle. Our data argue against BKCa activation but the precise subtypes of K+ channels will require further investigation.
Notably, vascular actions of the two calcimimetics were insensitive to calhex 231, a negative modulator of CaR (Jensen and Brauner-Osborne, 2007), suggesting the lack of involvement of CaR. Calhex 231 tended to reduce the steepness the concentration–response curves to calindol (cf. Figure 5B); however, there was no significant change in pEC50 values. In this study, calhex 231 was used at a concentration (3 µM) that has previously been shown to abolish CaR-mediated responses in transfected cells (Petrel et al., 2003) and mesenteric arteries (Weston et al., 2008). One theoretical possibility is that, in methoxamine-precontracted arteries, CaR is maximally active and addition of a calcimimetic has no further effects on CaR. However, reducing [Ca2+]o from 2 to 0.5 mM only slightly shifted the concentration–response curve to calindol and had no effect on cinacalcet responses. Importantly, calhex 231 greatly inhibited relaxation to Ca2+ itself, confirming the effectiveness of the calcilytic in our preparation. Moreover, Ca2+-induced relaxation was also attenuated by iberiotoxin and capsaicin. Our results support the proposal that Ca2+ activates CaR on perivascular nerves, leading to the release of a diffusible substance, which in turn activates BKCa in vascular smooth muscle (Bukoski et al., 1997). On the other hand, the presence of calcimimetics (at ≤1 µM) also failed to potentiate Ca2+-induced mesenteric relaxation. We therefore conclude that CaR plays a minor role, if any, in mesenteric relaxation to the calcimimetics. This would suggest that CaR has a minimal contribution to the activation by calcimimetics of K+ channels, and endothelium-dependent or capsaicin-sensitive pathways.
Given that cinacalcet and calindol exert similar actions on CaR, it is curious to observe that relaxation to calindol, but not cinacalcet, was sensitive to endothelial removal or capsaicin treatment. One possible explanation is that calindol also positively modulates GPRC6A, which has recently been reported in the endothelium of rat mesenteric arteries (Harno et al., 2008). GPRC6A is a novel receptor that is sensitive to Ca2+ and closely related to CaR (Wellendorph et al., 2007). In contrast to cinacalcet and calindol, the GPRC6A agonist L-ornithine (up to 3 mM) caused small relaxations. While L-ornithine can potentiate the activity of other agonists or modulators on GPRC6A (Wellendorph et al., 2007; Harno et al., 2008), it had no effect on calcimimetic-induced relaxation in mesenteric arteries. At present, the effect of calindol on GPRC6A is not established (both agonist (Harno et al., 2008) and antagonist actions (Faure et al., 2009) have been reported) and no information is available regarding cinacalcet at GPRC6A. Nonetheless, our data are not consistent with a role of GPRC6A in relaxant responses to the calcimimetics.
The ability of the calcimimetics to fully relax mesenteric arteries precontracted with 60 mM KCl suggests that they might interfere with Ca2+ influx via voltage-gated Ca2+ channels, or processes downstream, in vascular smooth muscle. Ca2+ influx through L-type, voltage-gated Ca2+ channels is crucial for the contractile effects of methoxamine, and almost entirely mediates precontraction with depolarizing K+ solution (Karaki et al., 1997; Ho and Hiley, 2003). Of particular interest, both cinacalcet and calindol bear structural similarities to phenylalkylamines, for example, verapamil (IC50– 1 µM; Johnson et al., 1996), which are L-type Ca2+ channel blockers (Jensen and Brauner-Osborne, 2007). To further test the hypothesis that the calcimimetics block Ca2+ influx in mesenteric smooth muscle, we examined their effects on contractions induced by re-additions of Ca2+ to endothelium-denuded arteries that had been depleted of intracellular Ca2+ stores and stimulated with methoxamine. Using this protocol, Ca2+-induced contraction has been demonstrated to occur mainly through activation of voltage-gated Ca2+ channels (White and Hiley, 1998). Both cinacalcet and calindol potently inhibited the contractions to Ca2+, achieving 100% inhibition at 10 µM and 3 µM respectively. Moreover, the calcimimetics inhibited contractions elicited by BayK8644, an activator of L-type Ca2+ channels. Stereoisomers of cinacalcet and calindol also induced similar relaxations, consistent with a receptor-independent mechanism of relaxation (Nemeth et al., 2004). Interestingly, we also found that the calcimimetics relax mesenteric arteries to a much greater extent than mesenteric veins. This parallels the preferential relaxant effects of L-type Ca2+ channel blockers, e.g. verapamil, on the arterial circulation (Thakali et al., 2010). Taken together, we propose that cinacalcet and calindol induce mesenteric relaxation primarily by inhibiting Ca2+ influx, probably via L-type Ca2+ channels. It is conceivable that cinacalcet and calindol, which share structural similarities (cf. Figure 1), directly interact with L-type Ca2+ channels; however, this remains to be confirmed. It should be emphasized that the calcimimetics might also inhibit other Ca2+ influx pathways, for instance receptor-operated or store-operated cation channels, which are involved in agonist-induced contractions. We noted that when Ca2+ influx mechanisms were ‘by-passed’ by contracting mesenteric arteries with ionomycin, the calcimimetics, but not verapamil, elicited contractions. These results indicate that calcimimetics might somehow enhance Ca2+-sensitivity in vascular smooth muscle, an effect that is expected to oppose the predominant vasorelaxant action of the compounds. At present, the involvement of CaR in the contractile effects of calcimimetics remains unclear and is the subject of ongoing investigations.
Our data suggest that CaR-independent, vasorelaxant effects of cinacalcet and calindol should be considered when investigating the direct cardiovascular actions of calcimimetics. In this study, the estimated EC50 values (0.6–3 µM) are similar to those obtained from cellular studies of CaR (e.g. Hammerland et al., 1998; Kessler et al., 2004). A recent in vivo study has also reported that higher doses of NPS R-568, a structural analogue of cinacalcet, and its enantiomer NPS S-568 (both at 2 mg·kg−1) induced very similar hypotension responses in rats (Nakagawa et al., 2009). However, it is unclear if such off-target effects of cinacalcet are clinically relevant as cinacalcet is more effective in suppressing parathyroid hormone levels in humans (EC50, 0.01–0.03 µM; Messa et al., 2008; Serra et al., 2008) and that a lower plasma concentration of cincacalcet has been reported in clinical studies (generally ranges from 0.03 to 0.3 µM; Ohashi et al., 2004; Kumar et al., 2004; Padhi et al., 2007; Serra et al., 2008). Therefore, the relevance of L-type Ca2+ channels or other potential off-targets in the therapeutic effects of calcimimetics remains to be determined.
To conclude, our data indicate that cinacalcet and calindol act as potent arterial relaxants primarily through inhibiting Ca2+ influx into the vascular smooth muscle. Other relaxation mechanisms involve the endothelium, K+ channels and capsaicin-sensitive, perivascular nerves. However, the established CaR and the novel Ca2+-sensitive receptor, GPRC6A appear to have little role in relaxation induced by the calcimimetics.
Acknowledgments
None.
Glossary
Abbreviations
- BayK8644
(4R)- and (4S)-1,4-dihydro-2,6-dimethyl-5-nitro-4-[2-trifluoromet hyl)phenyl]-3-pyridinecarboxylic acid methyl ester
- calhex 231
4-chloro-N-[(1S,2S)-2-[[(1R)-1-(1-naphthalenyl)ethyl]amino]cyclohexyl]benzamide
- calindol
(R)-2-[[[1-(1-naphthyl)ethyl]amino]methyl]-1H-indole hydrochloride
- cinacalcet
(R)-N-(3-(3-(trifluoromethyl)phenyl)propyl)-1-(1-napthyl)ethylamine hydrochloride
Conflict of interest
The authors state no conflict of interest.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Alexander SP, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl. 2):S1–209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awumey EM, Hill SK, Diz DI, Bukoski RD. Cytochrome P-450 metabolites of 2-arachidonoylglycerol play a role in Ca2+-induced relaxation of rat mesenteric arteries. Am J Physiol Heart Circ Physiol. 2008;294:H2363–H2370. doi: 10.1152/ajpheart.01042.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EM, MacLeod RJ. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- Bukoski RD, Bian K, Wang Y, Mupanomunda M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension. 1997;30:1431–1439. doi: 10.1161/01.hyp.30.6.1431. [DOI] [PubMed] [Google Scholar]
- Edwards G, Weston AH. Potassium and potassium clouds in endothelium-dependent hyperpolarizations. Pharmacol Res. 2004;49:535–541. doi: 10.1016/j.phrs.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Faure H, Gorojankina T, Rice N, Dauban P, Dodd RH, Brauner-Osborne H, et al. Molecular determinants of non-competitive antagonist binding to the mouse GPRC6A receptor. Cell Calcium. 2009;46:323–332. doi: 10.1016/j.ceca.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Fryer RM, Segreti JA, Widomski DL, Franklin PH, Banfor PN, Koch KA, et al. Systemic activation of the calcium sensing receptor produces acute effects on vascular tone and circulatory function in uremic and normal rats: focus on central versus peripheral control of vascular tone and blood pressure by cinacalcet. J Pharmacol Exp Ther. 2007;323:217–226. doi: 10.1124/jpet.107.123901. [DOI] [PubMed] [Google Scholar]
- Hammerland LG, Garrett JE, Hung BCP, Levinthal C, Nemeth EF. Allosteric acitvation of Ca2+ receptor expressed in Xenopus laevis oocytes by NPS 467 or NPS 568. Mol Pharmacol. 1998;53:1083–1088. [PubMed] [Google Scholar]
- Harno E, Edwards G, Geraghty AR, Ward DT, Dodd RH, Dauban P, et al. Evidence for the presence of GPRC6A receptors in rat mesenteric arteries. Cell Calcium. 2008;44:210–219. doi: 10.1016/j.ceca.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Ho WS, Hiley CR. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WS, Randall MD. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br J Pharmacol. 2007;150:641–651. doi: 10.1038/sj.bjp.0707141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishioka N, Bukoski RD. A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther. 1999;289:245–250. [PubMed] [Google Scholar]
- Jensen AA, Brauner-Osborne H. Allosteric modulation of the calcium-sensing receptor. Curr Neuropharmacol. 2007;5:180–186. doi: 10.2174/157015907781695982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Hockerman GH, Scheuer T, Catterall WA. Distinct effects of mutations in transmembrane segment IVS6 on block of L-type calcium channels by structurally similar phenylalkylamines. Mol Pharmacol. 1996;50:1388–1340. [PubMed] [Google Scholar]
- Karaki H, Ozaki H, Hori M, Mitsui-Saito M, Amano K, Harada K, et al. Calcium movements, distribution, and functions in smooth muscle. Pharmacol Rev. 1997;49:157–230. [PubMed] [Google Scholar]
- Kessler A, Faure H, Petrel C, Ruat M, Dauban P, Dodd RH. N2-benzyl-N1-(1-(1-naphthyl)ethyl)-3-phenylpropane-1,2-diamines and conformationally restrained indole analogues: development of calindol as a new calcimimetic acting at the calcium sensing receptor. Bioorg Med Chem Lett. 2004;14:3345–3349. doi: 10.1016/j.bmcl.2004.03.056. [DOI] [PubMed] [Google Scholar]
- Kumar GN, Sproul C, Poppe L, Turner S, Gohdes M, Ghoborah H, et al. Metabolism and disposition of calcimimetic agent cinacalcet HCl in humans and animal modesl. Drug Metab Dispos. 2004;32:1491–1500. doi: 10.1124/dmd.104.000604. [DOI] [PubMed] [Google Scholar]
- McCarty MF. Concurrent treatment with an ACE inhibitor may amplify the utility of calcium supplementation for control of hypertension. Med Hypotheses. 2004;63:818–822. doi: 10.1016/j.mehy.2002.11.001. [DOI] [PubMed] [Google Scholar]
- Messa P, Alfieri C, Brezzi B. Cinacalcet: pharmacological and clinical aspects. Expert Opin Drug Metab Toxicol. 2008;4:1551–1560. doi: 10.1517/17425250802587017. [DOI] [PubMed] [Google Scholar]
- Molostvov G, James S, Fletcher S, Bennett J, Lehnert H, Bland R, et al. Extracellular calcium-sensing receptor is functionally expressed in human artery. Am J Physiol Renal Physiol. 2007;293:F946–F955. doi: 10.1152/ajprenal.00474.2006. [DOI] [PubMed] [Google Scholar]
- Nakagawa K, Raprekh N, Koleganova N, Ritz E, Schaefer F, Schmitt CP. Acute cardiovascular effects of the calcimimetic R-568 and its enantiomer S-568 in rats. Pediatr Nephrol. 2009;24:1385–1389. doi: 10.1007/s00467-009-1153-6. [DOI] [PubMed] [Google Scholar]
- Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther. 2004;308:627–635. doi: 10.1124/jpet.103.057273. [DOI] [PubMed] [Google Scholar]
- Odenwald T, Nakagawa K, Hadtstein C, Roesch F, Gohlke P, Ritz E, et al. Acute blood pressure effects and chronic hypotensive action of calcimimetics in uremic rats. J Am Soc Nephrol. 2006;17:655–662. doi: 10.1681/ASN.2005090914. [DOI] [PubMed] [Google Scholar]
- Ogata H, Ritz E, Odoni G, Amann K, Orth SR. Beneficial effects of calcimimetics on progression of renal failure and cardiovascular risk factors. J Am Soc Nephrol. 2003;14:959–967. doi: 10.1097/01.asn.0000056188.23717.e5. [DOI] [PubMed] [Google Scholar]
- Ohashi N, Uematsu T, Nagashima S, Kanamaru M, Togawa A, Hishida A, et al. The calcimimetic agent KRN 1493 lowers plasma parathyroid hormone and ionized calcium concentrations in patients with chronic renal failure on haemodialysis both on the day of haemodialysis and on the day without haemodialysis. Br J Clin Pharmacol. 2004;57:726–734. doi: 10.1111/j.1365-2125.2004.02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padhi D, Salfi M, Harris RZ. The pharmacokinetics of cinacalcet are unaffected following consumption of high- and low-fat meals. Am J Ther. 2007;14:235–240. doi: 10.1097/01.mjt.0000212703.71625.26. [DOI] [PubMed] [Google Scholar]
- Petrel C, Kessler A, Maslah F, Dauban P, Dodd RH, Rognan D, et al. Modeling and mutagenesis of the binding site of Calhex 231, a novel negative allosteric modulator of the extracellular Ca(2+)-sensing receptor. J Biol Chem. 2003;278:49487–49494. doi: 10.1074/jbc.M308010200. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Kass RS. Regulation of cardiac calcium channel current and contractile activity by the dihydropyridine Bay K 8644 is voltage-dependent. J Mol Cell Cardiol. 1984;16:667–670. doi: 10.1016/s0022-2828(84)80631-8. [DOI] [PubMed] [Google Scholar]
- Serra AL, Braun SC, Starke A, Savoca R, Hersberger M, Russmann S, et al. Pharmacokinetics and pharmacodynamics of cinacalcet in patients with hyperparathyroidism after renal transplantation. Am J Transplant. 2008;8:803–810. doi: 10.1111/j.1600-6143.2007.02136.x. [DOI] [PubMed] [Google Scholar]
- Smajilovic S, Hansen JL, Christoffersen TE, Lewin E, Sheikh SP, Terwilliger EF, et al. Extracellular calcium sensing in rat aortic vascular smooth muscle cells. Biochem Biophys Res Commun. 2006;348:1215–1223. doi: 10.1016/j.bbrc.2006.07.192. [DOI] [PubMed] [Google Scholar]
- Steddon SJ, Cunningham J. Calcimimetics and calcilytics – fooling the calcium receptor. Lancet. 2005;365:2237–2239. doi: 10.1016/S0140-6736(05)66782-7. [DOI] [PubMed] [Google Scholar]
- Thakali KM, Kharade SV, Sonkusare SK, Rhee SW, Stimers JR, Rusch NJ. Intracellular Ca2+ silences L-type Ca2+ channels in mesenteric veins: mechanism of venous smooth muscle resistance to calcium channel blockers. Circ Res. 2010;106:739–747. doi: 10.1161/CIRCRESAHA.109.206763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Bukoski RD. Distribution of the perivascular nerve Ca2+ receptor in rat arteries. Br J Pharmacol. 1998;125:1397–1404. doi: 10.1038/sj.bjp.0702195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellendorph P, Burhenne N, Christiansen B, Walter B, Schmale H, Brauner-Osborne H. The rat GPRC6A: cloning and characterization. Gene. 2007;396:257–267. doi: 10.1016/j.gene.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Ward DT, Ohanian J, Dodd RH, Dauban P, et al. Evidence in favor of a calcium-sensing receptor in arterial endothelial cells: studies with calindol and Calhex 231. Circ Res. 2005;97:391–398. doi: 10.1161/01.RES.0000178787.59594.a0. [DOI] [PubMed] [Google Scholar]
- Weston AH, Absi M, Harno E, Geraghty AR, Ward DT, Ruat M, et al. The expression and function of Ca(2+)-sensing receptors in rat mesenteric artery; comparative studies using a model of type II diabetes. Br J Pharmacol. 2008;154:652–662. doi: 10.1038/bjp.2008.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R, Hiley CR. The actions of some cannabinoid receptor ligands in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:533–541. doi: 10.1038/sj.bjp.0702111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonneberger K, Scofield MA, Wangemann P. Evidence for a calcium-sensing receptor in the vascular smooth muscle cells of the spiral modiolar artery. J Membr Biol. 2000;175:203–212. doi: 10.1007/s00232001068. [DOI] [PubMed] [Google Scholar]
- Zhang RZ, Gashev AA, Zawieja DC, Davis MJ. Length-tension relationships of small arteries, veins, and lymphatics from the rat mesenteric microcirculation. Am J Physiol Heart Circ Physiol. 2007;292:H1943–H1952. doi: 10.1152/ajpheart.01000.2005. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


