Abstract
Infant cues, such as smiling or crying facial expressions, are powerful motivators of human maternal behavior, activating dopamine-associated brain reward circuits. Oxytocin, a neurohormone of attachment, promotes maternal care in animals, although its role in human maternal behavior is unclear. We examined 30 first-time new mothers to test whether differences in attachment, based on the Adult Attachment Interview, were related to brain reward and peripheral oxytocin response to infant cues. On viewing their own infant’s smiling and crying faces during functional MRI scanning, mothers with secure attachment showed greater activation of brain reward regions, including the ventral striatum, and the oxytocin-associated hypothalamus/pituitary region. Peripheral oxytocin response to infant contact at 7 months was also significantly higher in secure mothers, and was positively correlated with brain activation in both regions. Insecure/dismissing mothers showed greater insular activation in response to their own infant’s sad faces. These results suggest that individual differences in maternal attachment may be linked with development of the dopaminergic and oxytocinergic neuroendocrine systems.
Keywords: Attachment, mother-infant relations, dopamine, oxytocin, reward, functional MRI, striatum, insula, maternal
INTRODUCTION
The attachment relationship between infants and their caregivers is critical for human development, ensuring infant survival and optimal social, emotional and cognitive development (Insel and Young, 2001; Sroufe et al, 2005). The relationship between a mother and her infant is particularly salient, with evidence that the biological processes of pregnancy, parturition and lactation may all contribute to the establishment of the mother-infant bond (Strathearn et al, 2009; Kinsley et al, 2008).
In both human and animal research, significant differences in early maternal caregiving have been observed—ranging from sensitive and responsive infant care to maternally perpetrated abuse or neglect (Strathearn et al, 2009; Sroufe et al, 2005), with corresponding differences in infant health and developmental outcomes (Sroufe et al, 2005; Strathearn et al, 2001; Thompson, 2008; Francis et al, 1999; Weaver et al, 2004). Understanding the neurobiological processes underlying these differences in maternal behavior may help us to identify more effective treatment and preventative strategies.
The neurobiology of attachment behavior has been studied extensively in animal models (Insel and Young, 2001; Swain et al, 2007), and more recently in humans using functional magnetic resonance imaging (fMRI) (Lorberbaum et al, 2002; Bartels and Zeki, 2004; Swain et al, 2007; Strathearn et al, 2008). Although there is likely to be a complex interaction of multiple neuroendocrine systems, two specific systems have been shown to consistently play a role in promoting and maintaining maternal behavior: 1) the dopaminergic reward processing system (Champagne et al, 2004; Strathearn et al, 2008; Ferris et al, 2005) and 2) the oxytocinergic system (Bartels and Zeki, 2004; Champagne et al, 2001; Levine et al, 2007) Oxytocin, a neuromodulatory hormone produced in the hypothalamus, has well-described central actions associated with the onset of maternal behavior, as well as peripheral actions in stimulating uterine contraction during labor and milk ejection during lactation. It is released in response to stimuli such as infant suckling, somatosensory touch, or even the sight or sound of a nursing mother’s infant (Lucas et al, 1980; McNeilly et al, 1983; Johnston and Amico, 1986; Uvnas-Moberg et al, 1993). Oxytocin release into the peripheral circulation occurs within seconds of stimulation and its half-life has been estimated to be 6 to 7 minutes (Vankrieken et al, 1983; Robinson and Verbalis, 2003). In randomized, placebo-controlled trials, intranasal oxytocin produces a broad range of social effects, including enhanced social memory, improved eye gaze when viewing faces, increased recognition and memory of facial expressions and identity, and increased manifestations of trust (Domes et al, 2007; Savaskan et al, 2008; Baumgartner et al, 2008; Kosfeld et al, 2005; Guastella et al, 2008b; Guastella et al, 2008a). Oxytocin receptors are located in the ventral striatum, a key dopaminergic brain region, and receptor binding is linked functionally to maternal behavior in the rat (Olazabal and Young, 2006a). Thus, oxytocin may link social cues, such as infant facial expressions, with dopamine-associated reinforcement pathways.
The extent to which these biological systems explain differences in the quality of human attachment between mothers and infants, is yet to be explored (Strathearn, 2006). In this study, we aimed to measure differences in maternal brain reward activation and peripheral oxytocin release in response to infant cues, based on the mother’s adult attachment classification. We hypothesized that mothers with secure patterns of adult attachment would show an increased brain response to their own infant’s face in mesocorticolimbic reward regions, including the midbrain ventral tegmental area, the ventral striatum and the medial prefrontal cortex, and that this would be true on viewing both happy and sad infant face cues. We also hypothesized that “secure” mothers would show an enhanced peripheral oxytocin response on interacting with their infants, which would correlate with maternal brain responses.
MATERIALS AND METHODS
Study setting and participants
In this cohort study, we recruited first-time pregnant women during the third trimester of pregnancy and monitored them for 14 months postnatally. Recruitment occurred in Houston, Texas, between August 2004 and April 2006 and was through prenatal clinic visits and advertisements on billboards, in magazines and on the internet. We excluded potential subjects who were on psychotropic medications, were using cigarettes during pregnancy, were left-handed or had any contraindication to MRI scanning. Research was approved by the Institutional Review Board at Baylor College of Medicine, and all subjects provided written informed consent.
Study design (Fig. 1)
Figure 1.

Study timeline and data collected at each of 4 study visits. Abbreviations: AAI, Adult Attachment Interview; PDQ, Personality Disorder Questionnaire 4+; BDI, Beck Depression Inventory; PANAS, Positive and Negative Affect Schedule; ATQ, Adult Temperament Questionnaire—Short form; IBQ, Infant Behavior Questionnaire—Revised; PSI, Parenting Stress Index; WTAR, Wechsler Test of Adult Reading.
Visit 1: Pregnancy
During this visit, each enrolled woman participated in a modified version of the Adult Attachment Interview (AAI) (Crittenden, 2004; George et al, 1996), a semi-structured 1½-2 hour-long interview involving specified questions and follow-up inquiries relating to childhood relationships with attachment figures, usually parents. The modified version was chosen because of its theoretical links with patterns of information processing in the brain (Strathearn, 2006; Crittenden, 2008). Each digitally recorded interview was transcribed (with personally identifying details altered to preserve anonymity), and coded blindly to classify each woman’s adult attachment pattern, which was not revealed until study completion.
During this visit we also collected sociodemographic data, and screening information for depression (Beck Depression Inventory, BDI) (Beck et al, 1996) and personality disorders (Personality Disorder Questionnaire 4+, PDQ) (see Supplementary Table 1 online). We repeated the BDI on each post-natal visit, and calculated a mean post-natal score.
Visit 2: Videotaping and oxytocin sampling
Approximately 7 months post-delivery, each mother and infant attended a session at the Human Neuroimaging Laboratory. We requested that mothers abstain from caffeine and tobacco for 2-3 hours prior to the visit. After separating from their infants, the mothers had an intravenous cannula inserted, and 20 minutes later had blood drawn for baseline measurements of serum oxytocin, free cortisol, epinephrine and norepinephrine. We also measured serum estradiol, progesterone and β human chorionic gonadotropin levels to exclude a current pregnancy and to assess menstrual status. During this separation period, we videotaped each infant to obtain still images for use in the subsequent fMRI visit. Smiling, neutral and crying faces were elicited in a standardized setting, as described elsewhere (Strathearn et al, 2008). The mother and infant were then reunited for a 5-minute “free-play” period in which they physically interacted on the floor, after which another blood sample was drawn. They then participated in a 6-minute modified “still face” procedure (Koos and Gergely, 2001), during which mother and infant could hear and see each other via a mirror, but not interact physically. We then obtained a third blood sample after the mother left the room, followed by a final blood draw after 20 minutes of separation. Before and after the interaction period, each mother rated their current feelings using the Positive and Negative Affect Schedule (PANAS) (Watson et al, 1988), a 5-point rating of 20 affect states, such as “interested”, “excited”, “irritable” and “nervous”.
Each mother also completed a 120 item self-report questionnaire, the Parenting Stress Index (PSI) (Abidin, 1995), designed to help identify potentially dysfunctional parent-child relations. We assessed adult and infant temperament using the self-report Adult Temperament Questionnaire—Short form (ATQ) and the Infant Behavior Questionnaire—Revised (IBQ) (Gartstein and Rothbart, 2003). The mothers also reported their breastfeeding status, which was repeated at Visit 3.
Visit 3: Scanning
At ~11 months post-delivery, a minimum of 3 months after the videotaping session, each mother underwent fMRI scanning while viewing 60 unique infant face images, 30 of her own infant and 30 of the matched unknown infant face. There were 6 face categories, each containing 10 images, namely, own-happy (OH), own-neutral (ON), own-sad (OS), unknown-happy (UH), unknown-neutral (UN) and unknown-sad (US). Each mother viewed randomly presented baby face images for 2 seconds each within a rapid event-related fMRI design, with a random inter-stimulus interval of 2, 4 or 6 seconds (Fig. 2). Visual images were generated using a computer controlled LCD projector, and presented to the mother on an overhead mirror display.
Figure 2.
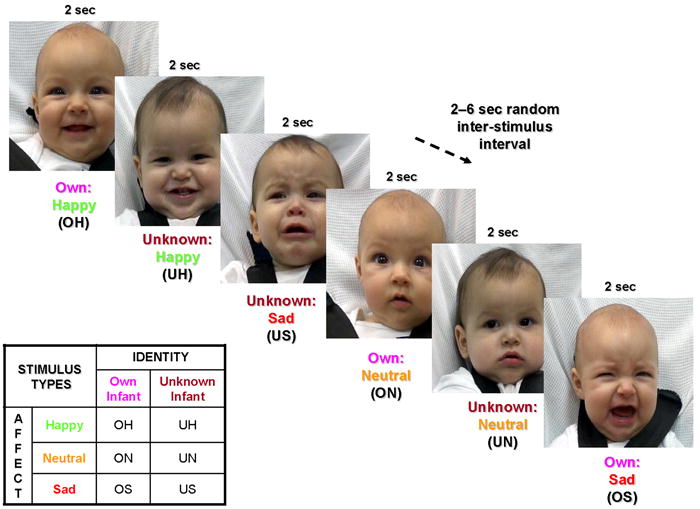
Baby face presentation paradigm in functional MRI experiment. Infant face images were presented for 2 seconds, followed by a variable 2-6 second period of a plain black screen. Six stimulus types were presented in random order: own-happy (OH), own-neutral , own-sad, unknown-happy, unknown-neutral, unknown-sad. Reproduced with permission from Pediatrics, Vol. 122, Pages 40-51, Copyright © 2008 by the AAP.
Visit 4: Child follow-up
Finally, at 14 months of age we performed a general assessment of child development using the Screening Test of the Bayley Scales of Infant and Toddler Development (Bayley, 2006).
Variables and statistical methods
Predictor variable – Adult attachment
We determined each mother’s adult attachment classification using the AAI (George et al, 1996; Fonagy et al, 1991) which categorizes the mother’s capacity to form secure attachment relationships on the basis of a narrative of her own attachment experience. Over the past 25 years, over 200 studies have reported over 10,000 AAIs (van IJzendoorn and Bakermans-Kranenburg, 2009). From both cross-sectional and prospective longitudinal studies, adult attachment has been shown to reliably predict maternal behavior patterns, the development of infant attachment (van IJzendoorn, 1995), and infant social and emotional development (Sroufe et al, 2005). We chose to measure attachment during pregnancy using a longitudinal design to preclude the possibility that the infant’s temperament or mother-infant interaction patterns might influence the way the mother discusses her own attachment experiences.
The coding is based on the subject’s coherence and consistency in describing attachment-related experiences and their effects on current functioning (Crittenden, 2004). The 3 basic styles, which parallel Ainsworth’s original classification of attachment in infancy (Ainsworth and Bell, 1970) include Type A “Insecure/Dismissing”, Type B “Secure” and Type C “Insecure/Preoccupied”. Individuals with Type B attachment styles tend to provide balanced descriptions of childhood experiences, using both temporal/causal order and affect to describe both positive and negative events and feeling states. Individuals with Type A attachment describe events or feelings in more cognitive terms, avoiding or inhibiting displays of negative affect. In contrast, Type C individuals exaggerate affective responses, with omitted or distorted cognitive processing (Crittenden, 2008). Fifty percent of the transcripts were double coded to ensure reliability, with an 87% agreement with regard to a 4-group classification (kappa=0.78). Discrepancies were resolved through conferencing between coders.
Potential confounding variables
We measured a variety of socioeconomic and behavioral factors to compare the characteristics of women in the two attachment groups (see Supplementary Table 1 online). Continuous measures were evaluated using t-tests or the Mann-Whitney U-test for nonparametric data (as determined from histogram analysis). We compared categorical variables using the Chi-Square test, or Fisher Exact test when numbers were insufficient. We used the Kendall’s tau-b test for ordinal or ranked nonparametric variables. We compared PANAS ratings of the mothers’ affect before and after contact with their infants between groups using a repeated measure analysis of variance (ANOVA).
Serial measurements of cortisol, norepinephrine and epinephrine were also compared between attachment groups using linear mixed modeling. Analyses were performed using SPSS (version 15.0) and P < 0.05 (2-tailed) was considered statistically significant.
Outcome Variables
1) Oxytocin Response
We used linear mixed modeling to assess the effects of attachment group, “mother-infant interaction” time point, breastfeeding status, and all 2-way interactions, on oxytocin response. Residual plots were used to confirm normality of distribution. Cases with missing data points were excluded (one Type B and two Type A subjects). The difference in mean oxytocin concentration between attachment groups, at each time point, was compared using a z-test (with Bonferroni correction for multiple comparisons; alpha ≤ 0.0125 was considered statistically significant). The mean oxytocin concentration from the two “mother-infant interaction” time points (which were highly correlated: rS=0.77, P < 0.001) was recomputed as a percentage change from the first baseline measure, to provide a single index for correlation with fMRI data. To determine the correlation between “% change in oxytocin” and fMRI blood-oxygen-level-dependent (BOLD) activation measured 4 months later (z-transformed beta weights), we calculated a Spearman correlation coefficient. We used a Bonferroni correction to adjust the alpha level for multiple comparisons with the beta weights for the 6 types of infant face (OH, OS, UN, etc). An alpha < 0.008 was considered statistically significant.
We measured oxytocin concentrations using a sensitive and specific liquid phase radioimmunoassay, in which oxytocin antiserum does not cross-react with arginine vasopressin or other oxytocin-like peptides (Amico et al, 1985). The lower limit for detectability of the assay is 0.5pg ml-1; inter- and intra-assay coefficients of variation are < 10%.
2) Functional MRI Brain Response
We prepared thirty standardized face images from each infant (10 happy, 10 neutral and 10 sad) for use in the fMRI scanning paradigm, along with 30 images from an “unknown” baby which were matched on age, race and independently-coded degree of affect (Fig. 2) (Strathearn et al, 2008). To ensure that the degree of infant facial affect did not vary between attachment groups, all faces were re-coded by three blinded raters using the 9-point Self-Assessment Manikin (Bradley and Lang, 1994) (ICC = 0.90). Using a mixed model three-way ANOVA, we saw no main effects for attachment group (F2,28 = 1.9, NS) or order of presentation (Wilk’s Lamda =.502, F9,20 = 2.0, NS). Similarly, none of the interactions with attachment security were significant.
Imaging was performed using a 3 Tesla Siemens Allegra head-only MRI scanner. High-resolution T1-weighted structural images (192 slices, in plane resolution 256 × 256; field of view [FOV] 245 mm; slice thickness 1 mm) were first acquired, followed by whole-brain functional runs of around 185 scans (gradient recalled echo planar imaging; 37 slices; repetition time 2000 msec; echo time 25 msec; flip angle, 90°; 64 × 64 matrix [in plane resolution]; FOV 220 mm; slice thickness 3 mm; positioned at 30 degrees in the axial plane to the anterior commissure/posterior commissure line). Imaging data for each subject were preprocessed in BrainVoyager QX, version 1.7.9 and analyzed in version 1.9.10, as previously described (Strathearn et al, 2008). Coregistration of functional and anatomical data for individual subjects confirmed that the functional data did include the hypothalamus/pituitary region (see representative image of coregistration in Supplementary Fig. 1 online).
A BrainVoyager protocol file was created for each functional run, representing the timing of each stimulus event. Each predictor was then convolved using a double-gamma hemodynamic response function. Using the General Linear Model (GLM), effects for the whole group (n = 30) were evaluated using a random effects between-subjects analysis. After specifying a particular contrast in stimulus types (e.g. OH>UH or OS>US), a group t-map was generated onto a template 3-dimensional anatomical image. An activation map threshold was determined using a false discovery rate (FDR) of 5% to control for multiple comparisons, and a cluster threshold of 4 voxels. Smaller cluster thresholds were also examined in the striatum (3 voxels) and brainstem (1 voxel) to reveal activation of smaller nuclei. Anatomical regions were identified using the automated “Talairach Daemon” (Lancaster et al, 2000), and confirmed manually using a human brain atlas (Mai et al, 2004).
Next, we compared activation patterns between attachment groups using a 2-factor random effects ANOVA model, with fixed effects analyses for 1) “infant face category” as a repeated measure within-factor variable and 2) “attachment group” as a between-factor variable. Whole brain differences in activation were assessed using a threshold of q(FDR) < 0.05. Mean beta weights were calculated and compared between the 2 attachment groups, in a priori regions of interest (midbrain, striatum, prefrontal cortex) and the hypothalamus, using the t-test and the Mann-Whitney U-test for non-parametric data.
RESULTS
Description of subjects
Of 112 women recruited during pregnancy, 61 met eligibility criteria and were enrolled in the study, with 44 participating in fMRI scanning approximately one year later. Ten women were unable to be scanned (9 due to a current pregnancy and one because of a past history of seizures) and 7 had withdrawn from the study or were lost to follow-up. Of the 44 scanned women, 15 were classified as having insecure/dismissing attachment (Type A). A further 16 women demonstrated secure patterns of attachment, without unresolved trauma or loss (Type B). A small group (n = 4) were classified as insecure/preoccupied (Type C), and the remaining 9 women had combined or atypical patterns. We specifically compared women from the two predominant attachment groups – Type A and B, and to ensure equal numbers in each group, one Type B mother was excluded.
The 30 women who were enrolled into the study were generally from middle to high socioeconomic backgrounds (based on the Four-Factor Index of Social Status [A. B. Hollingshead, PhD, working paper, 1985]: mean score 51.4 ± 9.4 at time of enrollment). Eighty percent had completed a college or graduate degree and 70% were married. The median WTAR-predicted IQ for the group was 112 (range 81-120). Sixty percent identified themselves as non-Hispanic White, one-quarter were Hispanic and one-tenth African American.
Subjects within the two attachment groups did not differ in age, race, education, socioeconomic status, marital status or predicted IQ (see Methods and Supplementary Table 1 online). Both groups were also comparable in screening measures of personality disorder risk and parenting stress (at the 7 month visit) and depression (measured at each study visit). There were no significant differences seen in temperament subscales of either the mother or child, the mothers’ ratings of emotions before and after mother-infant interaction (based on the PANAS during Visit 2) (Watson et al, 1988) or in scales of infant development (measured during Visit 4). We also found no significant difference in breastfeeding status at Visits 2 or 3, although Type B mothers tended to breastfeed longer and Type A mothers were significantly more likely to be separated from their child for longer periods of time each week (P = 0.03).
Oxytocin response to mother-infant interaction (Visit 2)
During the 7 month postpartum visit, Type B mothers showed a significantly higher peripheral oxytocin response following periods of mother-infant interaction (Fig. 3a; time point by attachment group interaction effect adjusted for breastfeeding at this visit, F = 2.9, P = 0.04). Although there were no differences between attachment groups in the two baseline measurements, after the 5-minute “free-play” interaction Type B mothers had significantly higher oxytocin levels (P = 0.01). This difference persisted into an additional mirror-based interaction period, although it was no longer statistically significant (P = 0.07). There were no significant differences in serum free cortisol, epinephrine or norepinephrine, or in baseline serum estradiol or progesterone.
Figure 3.

Peripheral oxytocin and related brain activation in response to infant cues. (a) Mothers with Type B (secure) attachment patterns show a greater peripheral oxytocin response during an episode of physical interaction with their infant (mean ± sem; Bonferroni corrected comparison at free play time point, P = 0.01). The first baseline sample was collected 20 minutes after mother-infant separation; the second immediately after a 5-minute “free-play” involving direct physical contact between the mother and infant. The third sample was after a modified still-face procedure, in which the mother was in direct visual and auditory contact with her infant (via a mirror) but was physically separated by a screen divider. The final sample was collected after a further 20-minute period of complete mother-infant separation. (b) Compared to Type A mothers, Type B mothers show greater activation of the hypothalamus/pituitary region in response to own vs. unknown infant face images (all affect groups combined) (mean beta ± sem, t = 4.2, P = 0.0003). The whole brain analysis threshold was q(FDR) < 0.05; P < 0.002. Structural brain image created from average of all subjects. Inset of magnified hypothalamic/pituitary region (single subject image to improve anatomical clarity). (c) Peripheral oxytocin response correlates with activation of hypothalamus/pituitary region in response to neutral own infant face cues (rS = 0.60, P = 0.001). A single outlying value was omitted from the graph, but not the statistical calculations.
Whole group analysis of maternal brain responses (Visit 3)
On the whole brain analysis, when mothers viewed their own infant’s happy faces, compared to unknown happy faces (OH>UH), key dopamine-associated reward processing regions were activated, overlapping previously reported regions (Strathearn et al, 2008), and including the substantia nigra, dorsal putamen and thalamic nuclei. In addition, activation was seen in various regions of the striatum, caudate nuclei, insular cortex, superior temporal gyrus and pre- and post-central gyri (P < 0.05, FDR corrected). As in the prior study, no significant activation was seen on contrasting own vs. unknown sad (OS>US) or neutral (ON>UN) infant faces, or in contrasting face affect groups, testing “own” and “unknown” faces separately or combined (e.g. OH>ON, US>UN, H>S). After combining all affect groups together and contrasting own vs. unknown faces, an activation pattern overlapping our previous study results (Strathearn et al, 2008) was seen, including both mesocorticolimbic (ventral tegmental area and ventral striatum) and nigrostriatal pathway (substantia nigra and dorsal striatum) activation, but not the prefrontal or anterior cingulate cortex.
Attachment group comparisons
We next compared own vs. unknown (O>U) infant face responses between the two attachment groups after combining all affect groups, to look specifically for hypothesized differences in activation of dopamine-associated brain reward regions (in the midbrain, striatum and forebrain) and the hypothalamus. Type B mothers showed significantly more activation in the lateral prefrontal cortex bilaterally, the left medial prefrontal cortex (mPFC) and the hypothalamus/pituitary region (O>U; P < 0.05, FDR corrected) (Table 1; Fig. 3b; Supplementary Fig. 1 online). In the hypothalamus/pituitary region, where oxytocin is produced and released peripherally, Type B mothers had a greater response to own-infant faces than did Type A mothers (median beta values 1.54 vs. -2.09; Mann-Whitney U-test, z = -2.10, P < 0.05). Furthermore, among Type B mothers, the response was greater for their own infant compared to unknown infant faces (median betas 1.54 vs. -2.50; z = -2.10, P < 0.05) (Supplementary Fig. 2 online). On further fMRI analysis of the three individual affect groups (happy, neutral and sad), only neutral faces (ON>UN) produced a similar activation pattern between attachment groups within the hypothalamic/pituitary region (P < 0.05, FDR corrected). The activation signal in response to own-neutral infant faces correlated significantly with the mother’s peripheral oxytocin response on interaction with her infant (z-transformed beta weights and % change in oxytocin; rS=0.60, P = 0.001) (Fig. 3c). When attachment groups were compared in this correlation analysis, no differences in line slope (P = 0.80) or position (P = 0.12) were detected. No correlation was seen between oxytocin response and brain activation in the mPFC, or when viewing unknown infant faces.
Table 1.
Areas of significant activation within the prefrontal cortex, striatum and midbrain, when comparing Type A and Type B attachment groups. All regions-of-interest P≤0.0001; voxel threshold=4, except as noted. Talairach coordinates (x, y, z) represent centre-of-gravity mean values for each region-of-interest.
| Region-of-Interest / Cluster (Brodmann Area, BA) | Right Hemisphere |
Left Hemisphere |
||
|---|---|---|---|---|
| x, y, z | Mean t-score | x, y, z | Mean t-score | |
| A. Own > Unknown (all affect groups combined): Secure > Insecure/Dismissing | ||||
| Prefrontal cortex | ||||
| Middle frontal gyrus (BA 10) | 44, 46, 18 | 3.42 | - | - |
| Medial frontal gyrus (BA 10) | - | - | -7, 58, 10 | 3.45 |
| Superior frontal gyrus (BA 10) | - | - | -32, 51, 25 | 3.62 |
| Insula / Frontal operculum (BA 13) | - | - | -40, 17, 11 | 3.71 |
| Hypothalamus / pituitary region | - | - | -3, 2 -16 | 4.04 |
| Insecure/Dismissing > Secure | ||||
| Dorsolateral prefrontal cortex | ||||
| Precentral gyrus (BA 6) | 44, -16, 25 | 3.67 | -24, -16, 50 | 3.35 |
| Precentral gyrus (BA 9) | - | - | -31, 5, 34 | 3.41 |
| Superior frontal gyrus (BA 9) | 39, 34, 30 | 4.23 | - | - |
| Inferior frontal gyrus (BA 9) | 36, 10 22 | 3.54 | - | - |
| Middle frontal gyrus (BA 8/6) | 24, 21, 35 | 3.58 | -24, 6, 44 | 3.67 |
| Middle frontal gyrus (BA 46) | - | - | -41, 29, 21 | 3.75 |
| Medial prefrontal cortex | ||||
| Superior frontal gyrus (BA 8) | 13, 36, 44 | 3.42 | - | - |
| Anterior Insula (BA 13) | - | - | -31, -3, 21 | 3.57 |
| B. Own-Happy Faces: Secure > Insecure/Dismissing | ||||
| Medial prefrontal cortex | ||||
| Medial frontal gyrus (BA 10) | 7, 64, 8 | 3.97* | -6, 60, 9 | 3.54 |
| Sub-gyral white matter | - | - | -22, 36, 24 | 3.52 |
| Orbitofrontal cortex | ||||
| Inferior frontal gyrus (BA 46/45) | 48, 40, 5 | 3.66 | -54, 17, 8 | 3.65* |
| Superior frontal gyrus (BA 10) | - | - | -20, 55, 5 | 3.73 |
| Striatum | ||||
| Ventral striatum / nucleus accumbens (BA 25) | - | - | -2, 10, -4 | 3.39* |
| Insecure/Dismissing > Secure | ||||
| Dorsolateral prefrontal cortex | ||||
| Middle frontal gyrus (BA 46) | 44, 30, 17 | 3.78 | -41, 37, 14 | 3.86 |
| Middle frontal gyrus (BA 9) | 28, 24, 34 | 3.82 | -40, 21, 27 | 3.57* |
| Middle frontal gyrus (BA 9) | 36, 32, 31 | 3.98 | - | - |
| Superior frontal gyrus | 21, 18, 50 | 3.54 | - | - |
| Subcallosal gyrus | 1, 13, -15 | 3.64 | - | - |
| C. Own-Sad Faces: Secure > Insecure/Dismissing | ||||
| Lateral prefrontal cortex | ||||
| Inferior frontal gyrus | - | - | -38, 41, -2 | 3.54 |
| Superior frontal gyrus (BA 9) | - | - | -26, 40, 32 | 3.74 |
| Striatum | ||||
| Ventral striatum / Nucleus accumbens | 12, 10, -3 | 3.47 | - | - |
| Insecure/Dismissing > Secure | ||||
| Dorsolateral prefrontal cortex | ||||
| Precentral gyrus (BA 44) | - | - | -53, 4, 13 | 3.65 |
| Middle frontal gyrus (BA 9) | 35, 31, 34 | 3.77 | - | - |
| Middle frontal gyrus (BA 8) | 23, 20, 42 | 3.49 | - | - |
| Medial frontal gyrus (BA 6) | 18, 6, 49 | 3.79 | - | - |
| Inferior frontal gyrus (BA 44) | 47, 12, 11 | 3.59 | - | - |
| Anterior insula | ||||
| Anterior Insula (BA 13) | 37, 19, 18 | 3.57 | -34, 27, 16 | 3.54 |
| Anterior Insula (BA 13) | 38, 17, -1 | 3.66 | - | - |
| Anterior Insula (BA 13) | 27, 18, -7 | 3.59 | - | - |
| Medial frontal lobe | ||||
| Superior frontal gyrus (BA 9) | 10, 47, 30 | 3.48 | - | - |
| Medial frontal gyrus – posterior (BA 6) | 14, -13, 55 | 3.40 | - | - |
| Uncus / Enterorhinal cortex (BA 28) | 15, -9, -24 | 3.62 | - | - |
| Anterior cingulate cortex (BA 32) | 14, 30, 7 | 3.68 | - | - |
| Medial frontal gyrus / Gyrus rectus (BA 25) | 3, 10, -15 | 3.61 | - | - |
Only seen at a threshold of 3 voxels.
In post-hoc analyses, we then directly compared own-infant faces between attachment groups, in each affect state separately (e.g. OH in Type A vs. Type B), without the inclusion of unknown infant face comparisons. From the hypothesized regions of interest, for the happy face contrast, Type B mothers showed significantly greater activation in the ventral striatum, as well as the orbitofrontal cortex (OFC) and mPFC bilaterally (Table 2). An equal but opposite BOLD response was seen in Type A mothers in the ventral striatum (Fig. 4a). In the mPFC, Type B mothers had a much larger increase in mean beta values compared with Type A mothers. In contrast, Type A mothers showed significantly more activation in the dorsolateral prefrontal cortex (dlPFC) bilaterally.
Figure 4.

Brain responses to happy and sad own-infant faces, contrasting mothers with Type A (insecure/dismissing) and B (secure) attachment classifications (mean beta values ± sem) (a) Type B mothers show greater activation of the ventral striatum (VS; t = 3.1, P < 0.005) and medial prefrontal cortex (mPFC; t = 3.0, P < 0.01) in response to happy own-infant faces. (b) Type B mothers show greater activation of the right ventral striatum (t = 3.0, P < 0.01) in response to sad own-infant faces. Type A mothers show greater activation of the right anterior insula (t = -3.9, P < 0.0005).
In response to own infant sad faces, the right ventral striatum was also more active in Type B mothers (though at a more anterior position than seen in the happy face contrast) (Table 2; Fig. 4b). Type A mothers again showed more activation of the dlPFC in response to own-sad faces, as well as a much stronger activation signal in the anterior insula bilaterally, compared with Type B mothers (Fig. 4b). Activation in the right ventral striatum in response to own-neutral infant faces was also highly correlated with peripheral oxytocin response (rS = 0.57, P = 0.002; Fig. 5). Unknown infant faces produced no such correlation. None of the contrasts, for happy or sad faces, showed significant differences across attachment groups in activation of midbrain regions.
Figure 5.
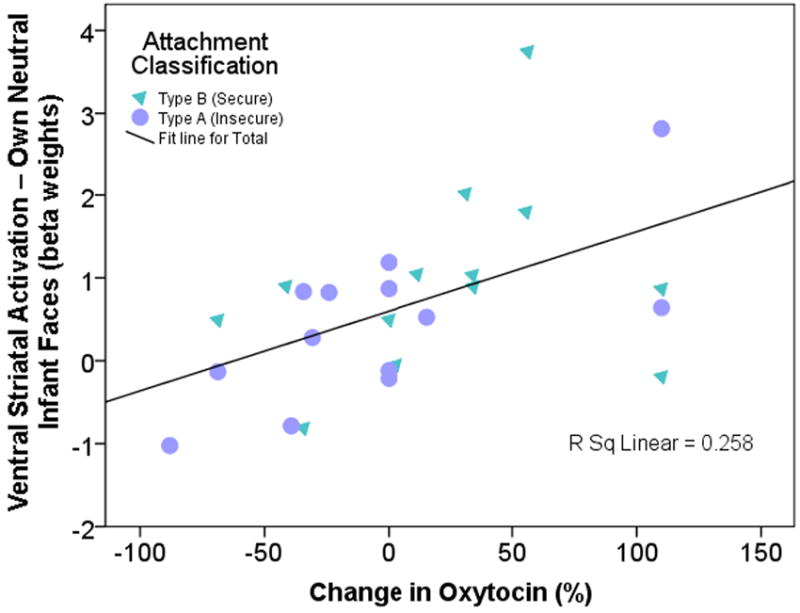
Peripheral oxytocin response after episodes of mother-infant interaction correlates with activation in the right ventral striatum (area shown in Figure 4b) in response to neutral own infant face cues (rS = 0.57, P = 0.002). Percent oxytocin change calculated from the first baseline measurement and a mean of the second and third samples, which were taken during episodes of mother-infant interaction.
Overall, mothers with Type B attachment tended to show greater left hemisphere activation, whereas Type A had predominantly right hemisphere activation, especially for happy and sad infant faces (Table 1).
DISCUSSION
This study demonstrates group differences in maternal brain and oxytocin response to infant cues, based on adult attachment patterns measured prior to the birth of the mother’s first child. As hypothesized, mothers with secure vs. insecure/dismissing attachment showed increased activation of mesocorticolimbic reward brain regions, on viewing their own infant’s smiling face. Furthermore, they showed an increased peripheral oxytocin response while interacting with their infants, which was positively correlated with activation of oxytocinergic and dopamine-associated reward processing regions of the brain (hypothalamus/pituitary and ventral striatum). Finally, striking differences in brain activation were seen in response to their own infant’s sad facial affect. Securely attached mothers continued to show greater activation in reward processing regions, while “insecure/dismissing” mothers showed increased activation of the anterior insula, a region associated with feelings of unfairness, pain and disgust (see review, Montague and Lohrenz, 2007).
The lack of “reward” activation in mothers with insecure/dismissing attachment is consistent with a recent study of brain responses to smiling adult faces and positive task feedback (Vrticka et al, 2008), where ventral striatum activation was inversely correlated with dismissing attachment scores. In linking attachment security with ventral striatal activation, our findings suggest that for securely attached mothers, infant cues (whether positive or negative in affect) may act as an important signal of “incentive salience” (Berridge, 2007), reinforcing and motivating responsive maternal care.
Striatal activation and de-activation has also been modeled to represent deviations from expectation, with regard to the timing and magnitude of predicted reward (Montague et al, 1996; Schultz et al, 1997; Daw and Doya, 2006). Specifically, an unexpected reward signal predicts an increase in dopaminergic activity and in measurable neural response at the level of the striatum, whereas the omission of an expected reward at a specific time predicts a decrease in dopamine-related response. Although the prediction error model has not been tested directly with regard to subjective feelings, our results suggest that insecure/dismissing mothers may interpret their own infant’s face (regardless of affect) as representing an omitted reward. This is consistent with the theoretical and observed nature of dismissing adult attachment, in which close interpersonal relationships are perceived as being less intrinsically rewarding (Cassidy and Shaver, 1999).
Furthermore, mothers with insecure/dismissing attachment styles showed greater activation of dlPFC and anterior insula in response to their own infant’s sad face, suggesting cognitive control over a negative affective response (Greene et al, 2004; Sanfey et al, 2003). In line with our current understanding that activation of the anterior insula may signal “norm violations” (Montague and Lohrenz, 2007), insecure/dismissing mothers may cognitively appraise their infant’s sad affect as a violation of an “expected” affect state. This may lead to avoidance or rejection of negative infant cues (Sanfey et al, 2003), rather than the “approach” responses seen in Type B secure mothers. While the ventral striatal activation seen in Type B mothers has been associated with anticipated gain, right anterior insula activation is seen in anticipation of loss (Knutson et al, 2007). These results are consistent with a previously published model of the cortical organization of the attachment system (Strathearn, 2006; Crittenden, 2008), which postulates that individuals with insecure/dismissing attachment are biased toward cognitive information processing, and tend to inhibit negative affective responses. Although anterior insula activation has also been linked with empathic responses to a loved one’s feeling of physical pain (Singer et al, 2004), dismissing individuals score much lower on a scale of emotional empathy (Sonnby-Borgstrom and Jonsson, 2004), making this interpretation less likely.
Oxytocin has long been implicated as an important neuromodulatory hormone involved in maternal behavior (Insel, 1992; Insel and Young, 2001). Synthesized in the paraventricular nucleus of the hypothalamus, there are oxytocinergic projections to the posterior pituitary gland where it is released into the blood stream. In addition, oxytocin neurons project centrally to regions important in the manifestation of social and maternal behaviors (Numan, 2006). There is some evidence to suggest that oxytocin neurons in the hypothalamus may directly project to the ventral striatum, facilitating dopamine release (Liu and Wang, 2003; Ross et al, 2009) and thus linking social and maternally-related cues to reward processing and behavioral reinforcement (Insel, 2003). Rodent studies have demonstrated that oxytocin receptor binding in the nucleus accumbens (a nucleus of the ventral striatum) facilitates the onset of maternal behavior (Olazabal and Young, 2006a; Olazabal and Young, 2006b).
While there has been some controversy surrounding the relationship between peripheral and central oxytocin production (McGregor et al, 2008), these results, while tentative, are consistent with the idea that differences in peripheral oxytocin response may reflect central oxytocin production and contribute to individual differences in maternal caregiving behavior. Other studies have shown reduced peripheral oxytocin responses in cocaine addicted mothers (Light et al, 2004) and in pregnant women with lower maternal-fetal attachment scores (Levine et al, 2007). Furthermore, reduced peripheral oxytocin levels have been seen in orphanage-adopted children with histories of early neglect, who display severe impairments in social reciprocity (Fries et al, 2005). The observation that oxytocin levels are higher in securely attached mothers following interaction with their infants suggests the importance of this neuropeptide in mediating attachment and social behaviors, as seen in human randomized placebo-controlled trials of intranasal oxytocin (Baumgartner et al, 2008; Guastella et al, 2008b), as well as in rodent studies (Insel and Young, 2001; Champagne et al, 2001; Insel, 1992; Liu and Wang, 2003). In our study, the correlation of interaction-elicited peripheral oxytocin with the activation of reward regions in the brain suggests that oxytocin may be one mechanism by which socially-relevant cues activate dopaminergic pathways and thus reinforce behavior. Mothers with secure attachment patterns when interacting with their infants may produce more oxytocin, which increases the experience of reward and in turn may contribute to the mother’s ability to provide consistent, nurturant care. However, caution is warranted in interpreting these findings. We have no independent measure of 1) the effect of oxytocin secretion on the estimated beta values in specified brain regions, nor 2) whether oxytocin is actually released during this behavioral condition. In fact, peripheral oxytocin measurements during real-time mother-infant interaction were collected 4 months prior to fMRI scanning, providing no opportunity to examine simultaneous correlations. Nevertheless, the correlation between oxytocin and hemodynamic response separated over time suggests that the oxytocin response may reflect an enduring trait difference associated with attachment security.
Numerous previous investigations have shown that mothers with insecure attachment patterns are less likely to establish secure relationships with their children, and that their children tend to have greater difficulties regulating affect, forming peer relationships and establishing secure attachment relationships themselves (Sroufe et al, 2005; van IJzendoorn, 1995). While the transgenerational transmission of attachment has been frequently observed, its mechanism is still poorly understood (van IJzendoorn, 1995). This study may help shed light on this question, with evidence that secure attachment is associated with more intense maternal reward activation to infant facial expressions, while insecure/dismissing mothers show greater insula response to negative infant cues. Additional research is needed to confirm these findings in larger cohorts of mothers, including mothers with insecure/preoccupied attachment. A randomized controlled trial of intranasal oxytocin may also help to clarify any causal relationship between oxytocin response and maternal brain activation.
In conclusion, this study is the first to examine the neuroendocrine basis of human mother-infant attachment. As such, it may help us to better understand the transmission of attachment patterns across generations and how secure maternal attachment may confer the observed developmental advantages in infants and children (Sroufe et al, 2005; van IJzendoorn, 1995).
Supplementary Material
Acknowledgments
This research was supported by National Institute of Child Health and Human Development (K23 HD43097), General Clinical Research Center (MO1 RR00188), Baylor Child Health Research Center: Pediatrics Mentored Research Program (K12 HD41648) (L. Strathearn); Kane Family Foundation, National Institute of Neurological Disorders and Stroke (NS 045790), National Institute of Drug Abuse (DA 11723) (P. R. Montague); and a Child and Family Center Program Grant from the Menninger Foundation (P. Fonagy). We would like to thank H. Cai for technical assistance in performing the radioimmunoassays of oxytocin, O. Smith for statistical advice, and technical staff at the Human Neuroimaging Laboratory for assistance with conducting the experiments.
Footnotes
DISCLOSURE / CONFLICT OF INTEREST The authors have no disclosures or conflicts of interest.
References
- Abidin RR. Parenting stress index, professional manual. Psychological Assessment Resources; Lutz, FL: 1995. [Google Scholar]
- Ainsworth MD, Bell SM. Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 1970;41:49–67. [PubMed] [Google Scholar]
- Amico JA, Ervin MG, Leake RD, Fisher DA, Finn FM, Robinson AG. A novel oxytocin-like and vasotocin-like peptide in human plasma after administration of estrogen. J Clin Endocrinol Metab. 1985;60:5–12. doi: 10.1210/jcem-60-1-5. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin Shapes the Neural Circuitry of Trust and Trust Adaptation in Humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Bayley N. Bayley Scales of Infant and Toddler Development. Harcourt Assessment; San Antonio, TX: 2006. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Berridge KC. The debate over dopamine’s role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: The self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Cassidy J, Shaver PR. Handbook of Attachment: Theory, Research, and Clinical Applications. The Guilford Press; New York: 1999. [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci U S A. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden P. Raising Parents Attachment, parenting and child safety. Willan Publishing; Devon, U.K: 2008. [Google Scholar]
- Crittenden PM. Patterns of Attachment in Adulthood: A Dynamic-Maturational Approach to Analyzing. The Adult Attachment Interview. 2004 Unpublished manuscript. [Google Scholar]
- Daw ND, Doya K. The computational neurobiology of learning and reward. Current Opinion in Neurobiology. 2006;16:199–204. doi: 10.1016/j.conb.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61:731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonagy P, Steele H, Steele M. Maternal representations of attachment during pregnancy predict the organization of infant-mother attachment at one year of age. Child Dev. 1991;62:891–905. doi: 10.1111/j.1467-8624.1991.tb01578.x. [DOI] [PubMed] [Google Scholar]
- Francis D, Diorio J, Liu D, Meaney MJ. Nongenomic transmission across generations of maternal behavior and stress responses in the rat. Science. 1999;286:1155–1158. doi: 10.1126/science.286.5442.1155. [DOI] [PubMed] [Google Scholar]
- Fries ABW, Ziegler TE, Kurian JR, Jacoris S, Pollak SD. Early experience in humans is associated with changes in neuropeptides critical for regulating social behavior. PNAS. 2005;102:17237–17240. doi: 10.1073/pnas.0504767102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behavior & Development. 2003;26:64–86. [Google Scholar]
- George C, Kaplin N, Main M. Adult Attachment Interview. (third edition) 1996 Unpublished manuscript. [Google Scholar]
- Greene JD, Nystrom LE, Engell AD, Darley JM, Cohen JD. The neural bases of cognitive conflict and control in moral judgment. Neuron. 2004;44:389–400. doi: 10.1016/j.neuron.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin Increases Gaze to the Eye Region of Human Faces. Biological Psychiatry. 2008a;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Mathews F. Oxytocin Enhances the Encoding of Positive Social Memories in Humans. Biological Psychiatry. 2008b;64:256–258. doi: 10.1016/j.biopsych.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Insel TR. Oxytocin--a neuropeptide for affiliation: evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology. 1992;17:3–35. doi: 10.1016/0306-4530(92)90073-g. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology & Behavior. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Johnston JM, Amico JA. A prospective longitudinal study of the release of oxytocin and prolactin in response to infant suckling in long term lactation. J Clin Endocrinol Metab. 1986;62:653–657. doi: 10.1210/jcem-62-4-653. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, et al. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav. 2008;37:43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural Predictors of Purchases. Neuron. 2007;53:147–156. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos O, Gergely G. A contingency-based approach to the etiology of ‘disorganized’ attachment: the ‘flickering switch’ hypothesis. Bull Menninger Clin. 2001;65:397–410. doi: 10.1521/bumc.65.3.397.19851. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–1564. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–544. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, et al. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- Lucas A, Drewett RB, Mitchell MD. Breast-feeding and plasma oxytocin concentrations. Br Med J. 1980;281:834–835. doi: 10.1136/bmj.281.6244.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. Elsevier; San Diego, CA: 2004. [Google Scholar]
- McGregor IS, Callaghan PD, Hunt GE. From ultrasocial to antisocial: a role for oxytocin in the acute reinforcing effects and long-term adverse consequences of drug use? Br J Pharmacol. 2008;154:358–368. doi: 10.1038/bjp.2008.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly AS, Robinson IC, Houston MJ, Howie PW. Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed) 1983;286:257–259. doi: 10.1136/bmj.286.6361.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci. 1996;16:1936–1947. doi: 10.1523/JNEUROSCI.16-05-01936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Lohrenz T. To detect and correct: norm violations and their enforcement. Neuron. 2007;56:14–18. doi: 10.1016/j.neuron.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Numan M. Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behav Cogn Neurosci Rev. 2006;5:163–190. doi: 10.1177/1534582306288790. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Oxytocin receptors in the nucleus accumbens facilitate “spontaneous” maternal behavior in adult female prairie voles. Neuroscience. 2006a;141:559–568. doi: 10.1016/j.neuroscience.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Olazabal DE, Young LJ. Species and individual differences in juvenile female alloparental care are associated with oxytocin receptor density in the striatum and the lateral septum. Hormones and Behavior. 2006b;49:681–687. doi: 10.1016/j.yhbeh.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Robinson AG, Verbalis JG. The Posterior Pituitary Gland. In: Larson PR, Kronenberg HM, Melmed S, Polonsky KS, editors. Williams Textbook of Endocrinology. W. B. Saunders; 2003. pp. 281–330. [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, et al. Characterization of the Oxytocin System Regulating Affiliative Behavior in Female Prairie Voles. 2009 doi: 10.1016/j.neuroscience.2009.05.055. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The Neural Basis of Economic Decision-Making in the Ultimatum Game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, SchΣchinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–374. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A Neural Substrate of Prediction and Reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Sonnby-Borgstrom M, Jonsson P. Dismissing-avoidant pattern of attachment and mimicry reactions at different levels of information processing. Scand J Psychol. 2004;45:103–113. doi: 10.1111/j.1467-9450.2004.00385.x. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson E, Collin WA. The development of the person: The Minnesota study of risk and adaptation from birth to adulthood. Guilford; New York: 2005. [Google Scholar]
- Strathearn L. Exploring the Neurobiology of Attachment. In: Mayes LC, Fonagy P, Target M, editors. Developmental Science and Psychoanalysis: Integration and Innovation. Karnac Press; 2006. [Google Scholar]
- Strathearn L, Abdullah M, Najman JM, O’Callaghan M. Does breastfeeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatrics. 2009;123:483–493. doi: 10.1542/peds.2007-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Gray PH, O’Callaghan M, Wood DO. Childhood neglect and cognitive development in extremely low birth weight infants: a prospective study. Pediatrics. 2001;108:142–151. doi: 10.1542/peds.108.1.142. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. J Child Psychol & Psychiat. 2007;48:262–287. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RA. Early Attachment and Later Development: Familiar Questions, New Answers. In: Cassidy J, Shaver PR, editors. Handbook of Attachment. Guilford Press; New York: 2008. [Google Scholar]
- Uvnas-Moberg K, Bruzelius G, Alster P, Lundeberg T. The antinociceptive effect of non-noxious sensory stimulation is mediated partly through oxytocinergic mechanisms. Acta Physiol Scand. 1993;149:199–204. doi: 10.1111/j.1748-1716.1993.tb09612.x. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH. Adult attachment representations, parental responsiveness, and infant attachment: a meta-analysis on the predictive validity of the Adult Attachment Interview. Psychol Bull. 1995;117:387–403. doi: 10.1037/0033-2909.117.3.387. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Bakermans-Kranenburg MJ. The first 10,000 Adult Attachment Interviews: Distributions of adult attachment representations in clinical and non-clinical groups. 2009 doi: 10.1080/14616730902814762. [DOI] [PubMed] [Google Scholar]
- Vankrieken L, Godart A, Thomas K. Oxytocin determination by radioimmunoassay. Gynecol Obstet Invest. 1983;16:180–185. doi: 10.1159/000299248. [DOI] [PubMed] [Google Scholar]
- Vrticka P, Andersson F, Grandjean D, Sander D, Vuilleumier P. Individual attachment style modulates human amygdala and striatum activation during social appraisal. PLoS ONE. 2008;3:e2868. doi: 10.1371/journal.pone.0002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Weaver ICG, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


