Abstract
BACKGROUND
The objective of this study was to review the outcome of women with breast cancer with known receptor status who were treated with whole brain radiotherapy for brain metastases and to determine factors that impact survival.
METHODS
A total of 223 women with breast cancer and brain metastases, who received whole brain radiotherapy, were identified. All women with HER-2–positive disease had received trastuzumab. Kaplan-Meier prodct limit method was used to determine overall survival (OS) estimates. Cox proportional hazards models were then fitted to explore the association of OS with various patient and tumor characteristics.
RESULTS
Median age at brain metastases diagnosis was 50 years. Sixty-seven (30.2%) patients had hormone receptor-positive/HER-2–negative disease, 101 (45.50%) had HER-2–positive disease, and 54 (24.3%) had triple receptor-negative disease. Median OS from brain metastases was 6 months, with 1-year survival of 30% (95% confidence interval [CI], 23%-36%). Women with hormone receptor-positive/HER-2–negative, HER-2–positive, and triple-negative tumors had median survivals of 5, 9, and 5 months, respectively (P =.0069). In the multivariate model, women with HER-2–positive disease had a significantly decreased risk of death compared with women with hormone receptor-positive/HER-2–negative disease (hazard ratio, 0.63; 95%CI, 0.42-0.94; P =.02). The risk of death among women with triple-negative disease compared with hormone receptor-positive/HER-2–negative disease was not significantly different (P =.54). Lower recursive partitioning analysis class and ≥30-gray brain radiation dose were also significantly associated with a decreased risk of death.
CONCLUSIONS
Breast tumor subtype has a significant prognostic role among women with breast cancer and brain metastases. In addition, in the trastuzumab era factors such as recursive partitioning analysis and adequate radiation dose continue to be important prognostic factors.
Keywords: breast cancer, subtype, brain metastases, radiotherapy, trastuzumab
Brain metastasis, the most common cause of malignancy in the brain, is a serious cause of comorbidity, afflicting approximately 100,000 to 170,000 patients per year in the United States.1,2 Breast cancer is the second most common cause of brain metastases, being diagnosed in approximately 10% to 20% of breast cancer patients; however, autopsy data suggest that the true prevalence of brain metastases among patients suffering from breast cancer could be as high as 30%.3-5 The incidence of brain metastases among women with breast cancer is likely increasing because of factors such as improved detection of disease, as well as the introduction of chemotherapeutic and biological agents that result in improved control of systemic disease but do not cross the blood-brain barrier.
The mainstay of treatment of brain metastases is whole brain radiotherapy, with the most common fractionation pattern for this treatment in the United States being 30 gray (Gy) in 10 once daily doses (fractions). Median survival of untreated patients with brain metastases is approximately 1 month.6 After whole brain radiotherapy, median survival of approximately 4 to 5 months has been reported.7,8 However, the prognoses of women with breast cancer and treated brain metastases vary. Several studies have reported factors that can predict for better prognosis, including younger age at brain metastases diagnosis, fewer lesions, and higher Karnofsky performance score (KPS).9,10 In addition, stratification by Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis, a classification that is based on age, KPS, and disease status, has consistently been shown to predict for prognosis, with best survival rates observed in recursive partitioning analysis class I patients and worst among recursive partitioning analysis class III patients.11
A limited number of studies have explored important prognostic factors such as HER-2 and hormone receptor status after a diagnosis of brain metastases. In addition, we have previously shown that the administration of trastuzumab among women with HER-2–positive disease and brain metastases prolongs survival compared with a comparable group not receiving trastuzumab.12 Thus, an important question that arises is whether the prognostic significance of previously described factors persists in the trastuzumab era. The goal of this retrospective study was to determine survival after a diagnosis of brain metastases in a cohort of women with breast cancer who had received whole brain radiation. We sought to confirm the prognostic significance of HER-2 status in this cohort, and in addition explore factors that would have a prognostic impact on these patients.
MATERIALS AND METHODS
Patient Population
A prospectively collected database, maintained at the Breast Medical Oncology Department of The University of Texas M. D. Anderson Cancer Center, was used to identify a cohort of women with histologically confirmed breast cancer who had developed brain metastases and received first-line whole brain radiotherapy at The University of Texas M. D. Anderson Cancer Center. As our previous work has shown the importance of the impact of trastuzumab on outcome in women with breast cancer and brain metastases,12 our prespecified study criteria required all women with HER-2–positive disease to have received trastuzumab either before or after diagnosis of brain metastases. Excluded from the analyses were patients who were male, had >1 primary or bilateral disease, or whose tumors had unknown HER-2 status. In addition, patients with leptomeningeal disease at the time of brain irradiation were excluded. Variables recorded for analyses included, but were not limited to, patient demographics, tumor characteristics, number of brain metastases, dose of radiation given for whole brain radiotherapy, performance status at the time of brain metastases diagnosis, and presence or absence of extracranial metastases. Medical charts for all patients were then cross-checked to verify the accuracy of recorded information. This retrospective study was approved by the institutional review board of The University of Texas M. D. Anderson Cancer Center.
Pathology
The grade of primary tumor specimens was classified according to the World Health Organization modified Black nuclear grading system.13 HER-2 status was determined using immunohistochemistry (IHC) and/or fluorescent in situ hybridization (FISH) technique. Tumors demonstrating no staining by IHC and/or demonstrating no gene amplification by FISH were considered to be HER-2 negative. Tumors demonstrating 3+ staining by IHC and/or gene amplification by FISH were considered to be HER-2 positive. Tumors that demonstrated 2+ staining by IHC required FISH confirmation for classification as HER-2 positive or negative. Hormone receptor-positive status was assigned to those tumors that exhibited positivity for either estrogen or progesterone receptors. Hormone receptor-negative status was assigned to those tumors that exhibited negative staining for both estrogen and progesterone receptors. Tumors were classified as triple-negative if they exhibited both HER-2–negative and hormone receptor-negative status.
Outcome Measures and Statistical Analyses
Patient characteristics were categorized by HER-2 status and compared between groups with the chi-square test and Wilcoxon rank sum test as appropriate. Breast tumor subtype was classified as follows: 1) hormone receptor positive/HER-2 negative, 2) HER-2 positive, or 3) triple receptor negative. The primary endpoint of this study was survival after diagnosis of brain metastases, which was computed from the date of diagnosis of brain metastases to the date of death from any cause or last follow-up. This endpoint was estimated by the Kaplan-Meier product limit method and compared between groups with the logrank statistic. All patients alive at the time of analysis were censored with date of last follow-up. Several covariates were examined, including HER-2 status, breast tumor subtype, age at brain metastases diagnosis, initial KPS, radiation dose, recursive partitioning analysis class, stage of disease, additional surgery/radiosurgery treatment of brain metastases, number of involved lymph nodes of the primary breast tumor, and grade and lymphovascular invasion of the primary breast tumor.
Cox proportional hazards models were fitted to explore the association of covariates and survival subsequent to the diagnosis of brain metastases. Covariates with a P value of <.05 at univariate analysis were chosen for the Cox models. We further stratified the multivariate models based on HER-2 status. All statistical tests were 2-sided, and P values <.05 were considered statistically significant. Analyses were performed using the SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Patient and Tumor Characteristics
Two hundred twenty-three women with breast cancer who developed brain metastases with known HER-2 status of their tumors were identified. Table 1 summarizes patient and tumor characteristics stratified by HER-2 status. Median age at diagnosis of brain metastases was 50 years (range, 26-79 years). Sixty-seven (30.2%) patients had hormone receptor-positive/HER-2–negative disease, 101 (45.50%) had HER-2–positive disease, and 54 (24.3%) had triple receptor-negative disease. Among women with HER-2–positive disease, 22 (21.8%) received trastuzumab before developing brain metastases, and 79 (78.2%) received trastuzumab after a diagnosis of brain metastases. Forty-six (21.1%) women had de novo stage IV disease, whereas 172 (78.9%) women had recurrent disease after a diagnosis of stage I to III breast cancer. Seventeen (8%) women presented with brain metastases as the first site of distant disease. All women received whole brain radiotherapy for their brain metastases. The median dose of radiation received was 30 Gy (range, 7.5-40 Gy), and the median number of fractions was 10 (range, 3-20 fractions). Fifteen (6.73%) women received <30 Gy, 167 (74.9%) received 30 Gy, and 41 (18.4%) received >30 Gy of radiation, including a tumor bed boost in 6 cases. In addition 33 (15%) and 8 (3.6%) women underwent surgery and radiosurgery, respectively, for their brain metastases as a component of their initial treatment.
Table 1.
Characteristics of Women With Brain Metastases Who Have Undergone Whole Brain Radiation Therapy
| Characteristics | HER-2 Positive | HER-2 Negative |
P | ||
|---|---|---|---|---|---|
| No.(%) | 101 | (45.3) | 122 | (54.7) | |
| Median age at CNS metastases diagnoses, y (range) | 49 (26-79) | – | 50.5 (27-77) | – | – |
| Median time to brain metastases, mo (range) | 12 (0-134) | – | 11.5 (0-91) | – | – |
| Menopausal status at primary breast cancer diagnosis, No. (%) | |||||
| Premenopausal | 53 | (53.54) | 59 | (49.2) | |
| Postmenopausal | 46 | (46.5) | 61 | (50.8) | .52 |
| Trastuzumab, No. (%) | |||||
| Before brain metastases | 22 | (21.8) | – | – | |
| After brain metastases | 79 | (78.2) | – | – | – |
| Race, No. (%) | |||||
| White | 70 | (48.0) | 76 | (62.3) | |
| Black | 14 | (13.9) | 24 | (19.7) | |
| Other | 17 | (16.8) | 22 | (18.0) | .46 |
| T classification, No. (%) | |||||
| T1 | 19 | (18.8) | 32 | (26.2) | |
| T2 | 36 | (35.6) | 57 | (46.7) | |
| T3 | 9 | (8.9) | 15 | (12.3) | |
| T4 | 32 | (31.7) | 18 | (14.7) | |
| TX | 5 | (4.9) | 0 | 0 | .002 |
| N classification, No. (%) | |||||
| N0 | 20 | (19.8) | 47 | (38.5) | |
| N1 | 43 | (42.6) | 47 | (38.5) | |
| N2 | 14 | (13.9) | 14 | (11.5) | |
| N3 | 20 | (19.8) | 10 | (8.2) | |
| NX | 4 | (4.0) | 4 | (4.3) | .022 |
| Stage of disease, No. (%) | |||||
| I | 9 | (9.3) | 21 | (17.4) | |
| II | 30 | (30.9) | 43 | (35.5) | |
| III | 38 | (39.2) | 31 | (25.6) | |
| IV | 20 | (20.6) | 26 | (21.4) | .11 |
| No. of positive lymph nodes, No. (%) | |||||
| <10 | 69 | (85.2) | 93 | (92.1) | |
| >10 | 12 | (14.8) | 8 | (7.9) | .14 |
| Hormone receptor (ER/PR), No. (%) | |||||
| Negative | 55 | (54.5) | 54 | (44.6) | |
| Positive | 46 | (45.5) | 67 | (55.4) | .14 |
| Grade of primary breast tumor, No. (%) | |||||
| 1/2 | 16 | (17.8) | 17 | (14.8) | |
| 3 | 74 | (82.2) | 98 | (85.2) | .56 |
| Lymphovascular invasion of primary breast tumor, No. (%) | |||||
| No | 45 | (53.6) | 73 | (68.2) | |
| Yes | 39 | (46.4) | 34 | (31.8) | .039 |
| Site of first metastases, No. (%) | |||||
| Visceral only | 35 | (34.6) | 34 | (27.9) | |
| Bone only | 7 | (31.8) | 15 | (12.3) | |
| Brain only | 8 | (7.9) | 9 | (7.4) | |
| Multiple | 40 | (39.6) | 38 | (31.2) | |
| Other | 11 | (10.9) | 26 | (21.3) | .13 |
| Extracranial metastases, No. (%) | |||||
| No | 8 | (7.9) | 7 | (5.7) | |
| Yes | 93 | (92.1) | 115 | (94.3) | 052 |
| No. of brain metastases, No. (%) | |||||
| 1 | 9 | (8.9) | 20 | (16.4) | |
| 2 | 5 | (5.0) | 15 | (12.3) | |
| 3 | 5 | (5.0) | 8 | (6.6) | |
| >3 | 82 | (81.2) | 79 | (64.8) | .046 |
| WBRT, No. (%) | |||||
| Alone | 82 | (81.2) | 100 | (82.0) | |
| Plus surgery | 14 | (13.9) | 19 | (15.6) | |
| Plus radiosurgery | 5 | (5.0) | 3 | (2.5) | .59 |
| Radiation dose, Gy, No. (%) | |||||
| <30 | 4 | (4.0) | 11 | (9.0) | |
| 30 | 79 | (78.2) | 88 | (72.1) | |
| <30 | 18 | (17.8) | 23 | (18.9) | .30 |
| KPS, No. (%) | |||||
| <70 | 34 | (34.0) | 49 | (42.2) | |
| ≥70 | 66 | (66.0) | 67 | (57.8) | .21 |
| RPA class, No. (%) | |||||
| I | 4 | (4.0) | 4 | (3.5) | |
| II | 62 | (62.0) | 63 | (54.3) | |
| III | 34 | (34.0) | 49 | (42.2) | .46 |
|
| |||||
CNS indicates central nervous system; ER, estrogen receptor; PR, progesterone receptor; WBRT, whole brain radiotherapy; Gy, grays; KPS, Karnofsky performance status; RPA, recursive partitioning analysis.
Survival From Brain Metastases
At the time of this analysis, 184 (82.5%) of women with breast cancer and brain metastases had died. Median time to brain metastases for the whole cohort was 12 months (range, 0-134 months). Median survival after a diagnosis of brain metastases was 6 months (range, 0-93 months). One-year survival of the whole cohort was 30% (95% CI [CI], 23%-36%). Fifty-three women survived >12 months after a diagnosis of brain metastases, of whom 33 (62.3%) survived between 12 months and 24 months, 14 (26.4%) survived between 24 months and 36 months, and 6 (11.3%) survived >36 months.
Table 2 summarizes the 1-year survival estimates after a diagnosis of brain metastases. On univariate analysis, several factors were found to be significantly associated with survival (Fig. 1). Breast tumor subtype was significantly associated with survival, with patients with hormone receptor-positive/HER-2–negative disease having a median survival of 5 months, those with HER-2–positive disease having a median survival of 9 months, and those with triple receptor-negative disease having a median survival of 5 months (P = .0069). Older age at brain metastases diagnoses, black race, >10 lymph nodes involved with disease at primary breast cancer diagnosis, receiving <30 Gy of whole brain radiotherapy, lower KPS, and triple receptor-negative status were all associated with poor survival outcome (Fig. 1). Women with brain metastases receiving whole brain radiotherapy alone had worse outcome compared with those who had additional treatment in the form of surgery or radiosurgery of the brain metastases (P < .0001). Women with recursive partitioning analysis of 3 had poorer survival outcomes compared with those with recursive partitioning analysis of 1 or 2 (P < .0001).
Table 2.
Overall Survival Subsequent to Brain Metastases
| Characteristics | Median, mo |
1-Year Survival |
Lower 95% CI |
Upper 95% CI |
P |
|---|---|---|---|---|---|
| Overall survival | 6 | 30% | 23% | 36% | – |
| HER-2 | |||||
| Negative | 5 | 17% | 11% | 25% | |
| Positive | 9 | 45% | 35% | 54% | .002 |
| Subtypes | .0069 | ||||
| Hormone positive/HER-2 negative | 5 | 23% | 14% | 35% | |
| HER-2 positive | 9 | 45% | 35% | 54% | |
| Triple receptor negative | 5 | 29% | 17% | 42% | |
| Age, y | |||||
| <50 | 8 | 34% | 25% | 43% | |
| ≥50 | 5 | 25% | 17% | 34% | .078 |
| Race | |||||
| White | 8 | 30% | 23% | 38% | |
| Black | 3.5 | 17% | 7.4% | 31% | |
| Other | 6 | 40% | 24% | 56% | .007 |
| Stage of disease | |||||
| I/II/III | 8 | 47% | 32% | 61% | |
| IV | 6 | 30% | 23% | 37% | .049 |
| No. of positive lymph nodes | |||||
| <10 | 6 | 29% | 22% | 37% | |
| >10 | 4 | 21% | 5.8% | 42% | .045 |
| Grade of primary breast tumor | |||||
| 1/2 | 8 | 32% | 17% | 49% | |
| 3 | 6 | 30% | 22% | 37% | .722 |
| Lymphovascular invasion of primary breast tumor | |||||
| No | 6 | 31% | 22% | 40% | |
| Yes | 6 | 28% | 18% | 39% | .563 |
| Site of first metastases | |||||
| Visceral only | 4 | 21% | 12% | 32% | |
| Bone only | 6 | 31% | 13% | 50% | |
| Brain only | 9 | 40% | 22% | 72% | |
| Multiple | 9 | 40% | 28% | 50% | |
| Other | 6 | 16% | 5.5% | 31% | .0705 |
| No. of brain metastases | |||||
| <3 | 8 | 34% | 22% | 46% | |
| ≥3 | 6 | 28% | 21% | 36% | .064 |
| WBRT | |||||
| Alone | 5 | 24% | 18% | 31% | |
| Plus surgery or radiosurgery | 14 | 53% | 35% | 67% | <.0001 |
| Radiation dose, Gy | |||||
| <30 | 1 | – | – | – | |
| ≥30 | 7 | 31% | 25% | 38% | <.0001 |
| RPA class | |||||
| I/II | 11 | 45% | 36% | 54% | |
| III | 2 | 6% | 20% | 14% | <.0001 |
| KPS | |||||
| <70 | 2 | 16% | 19.1% | 25% | |
| ≥70 | 11 | 45% | 36% | 53% | <.0001 |
| Extracranial metastases | |||||
| No | 9 | 50% | 19% | 74% | |
| Yes | 6 | 28% | 22% | 35% | .117 |
|
| |||||
CI indicates confidence interval; WBRT, whole brain radiotherapy; Gy, grays; RPA, recursive partitioning analysis; KPS, Karnofsky performance status.
Figure 1.
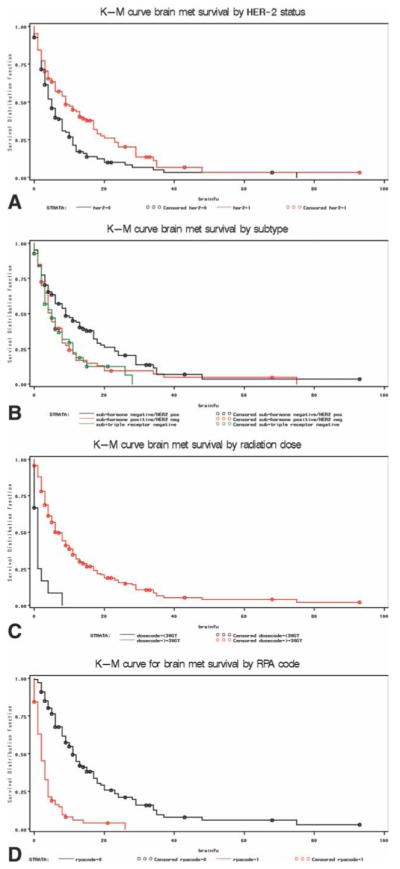
Kaplan-Meier (K-M) curves for survival after a diagnosis of central nervous system metastases (met) are shown stratified by (A) HER-2 status, (B) breast cancer subtype, (C) radiation dose, and (D) recursive partitioning analysis (RPA). brainfu indicates brain function; GY, grays.
Table 3 summarizes the multivariate model results for the whole cohort. Variables in the model included breast tumor subtype, stage of disease, recursive partitioning analysis, radiation dose, treatment, number of positive lymph nodes, and race. Age and KPS variables were not included in the model, as they formed the components used to calculate recursive partitioning analysis. Women with HER-2–positive disease and brain metastases had a lower risk of death compared with those with hormone receptorpositive/HER-2–negative disease (hazard ratio [HR], 0.63; 95% CI, 0.42-0.94; P = .02). The risk of death was not significantly different among women with triple receptor-negative disease compared with those with hormone receptor–positive/HER-2–negative disease (HR, 0.87; 95%CI, 0.55-1.37; P = .54). In addition, women with a recursive partitioning analysis score of 3 had a higher risk of death compared with those with recursive partitioning analysis scores of 1 or 2 (HR, 3.47; 95% CI, 2.35-5.14; P < .0001), and those receiving whole brain radiation doses of <30 Gy had a higher risk of death compared with those receiving a dose of≥30 Gy (HR, 3.41; 95% CI, 1.56-7.50; P = .002). Other factors significantly associated with survival after a diagnosis of brain metastases included initial stage of disease and number of involved lymph nodes.
Table 3.
Multivariable Model for Survival After Brain Metastases (n=176)
| Characteristics | HR | Lower 95% CI |
Upper 95% CI |
P |
|---|---|---|---|---|
| HER-2 positive vs hormone positive/HER-2 negative | 0.63 | 0.42 | 0.94 | .02 |
| Triple negative vs hormone positive/HER-2 negative | 0.87 | 0.55 | 1.37 | .54 |
| Stage I/II/III vs stage IV | 2.50 | 1.24 | 5.04 | .01 |
| WBRT alone vs WBRT and surgery or radiosurgery | 0.67 | 0.40 | 1.13 | .13 |
| No. of positive lymph nodes (>10 vs <10) | 1.99 | 1.15 | 3.42 | .01 |
| Black race vs white/other | 1.41 | 0.85 | 2.34 | .18 |
| RPA class III vs RPA class I/II | 3.47 | 2.35 | 5.14 | <.001 |
| Radiation dose (<30 vs ≥30 Gy) | 3.41 | 1.56 | 7.50 | .002 |
|
| ||||
HR indicates hazard ratio; CI, confidence interval; WBRT, whole brain radiotherapy; RPA, recursive partitioning analysis; Gy, grays.
DISCUSSION
The purpose of this study was to determine survival after a diagnosis of brain metastases and to examine factors that impact this survival in a contemporary cohort of women with breast cancer treated with whole brain radiotherapy. This study is unique in that all patients had known receptor status and received trastuzumab for HER-2+ disease. Median survival of the whole cohort was 6 months, which is consistent with previously published studies. Factors that were found to be associated with a better prognostic outcome on both univariate and multivariate analyses were positive HER-2 status of breast tumors, lower recursive partitioning analysis class, and higher dose of radiotherapy.
By using gene expression profiling, several distinct intrinsic breast tumor subtypes have been identified, including hormone receptor-positive subtypes (ie, luminal A and B), basal-like subtype (triple negative), and hormone receptor-negative/HER-2–positive subtype.14,15 Each subtype is associated with a distinct prognostic outcome. Surrogates of these subtypes using IHC have also been studied classifying tumors based on hormone receptor, HER-2, epidermal growth factor receptor (EGFR), and cytokeratin 5/6.16 By using IHC surrogates to classify breast tumors, Carey et al16 reported on the correlation between survival and breast tumor subtype in a cohort of 469 women with early stage breast cancer. Patients in this cohort who had HER-2–positive disease did not receive trastuzumab in the adjuvant setting. The authors observed that survival was poorest among the women with HER-2–positive/hormone receptor-negative tumors, followed by those with basal-like tumors, with the best outcome observed among women with luminal A and B tumors, which essentially concurred with the findings of the original gene expression profiling studies. Apart from various prognostic outcomes, research has also focused on determining the risk of developing brain metastases that is attached to each subtype. In an earlier study, Pestalozzi et al17 examined a cohort of 9524 women with early stage breast cancer who were enrolled in the International Breast Cancer Study Group clinical trials during the pretrastuzumab era. The authors reported that estrogen receptor-negative disease and HER-2–positive disease were independently predictive for the development of brain metastases as the site of first recurrence. In a more recent study, Heitz et al18 reported that compared with women with other breast tumor subtypes, women with triple receptor-negative disease (odds ratio [OR], 4.16; 95% CI, 2.26-7.64; P < .001) had the highest odds of developing brain metastases, followed by those with hormone receptor-negative/HER-2–positive disease (OR, 3.43; 95% CI, 1.46-8.05; P .005).
With data pointing to the finding that breast tumor subtype influences risk of developing future brain metastases, the next question was to evaluate whether these tumor subtypes influenced survival after the development of brain metastases. Nam et al19 recently reported on prognostic outcome of 126 women with breast cancer and brain metastases stratified by breast tumor subtype. Excluding women who had received trastuzumab after a diagnosis of brain metastases, the authors reported a median survival of 4.0, 7.3, 3.1, and 3.4 months among women with luminal A, luminal B, hormone receptor-negative/HER-2–positive, and triplenegative disease, respectively (P .0448). Studying a cohort of 222 women with breast cancer and brain metastases, Niwinska and Murawska20 reported median overall survivals of 3.7, 9, and 15 months among women with triple-negative, HER-2–positive, and estrogen receptor/progesterone receptor-positive HER-2–negative disease, respectively. Our group previously reported on a cohort of 598 women with breast cancer and brain metastases looking specifically at the effect of the introduction of trastuzumab on the survival outcome after a diagnosis of brain metastases.12 We reported median survivals of 6.3, 6.1, and 11.6 months, respectively, among women with HER-2–negative disease, HER-2–positive disease who had never received trastuzumab, and HER-2–positive disease who had received trastuzumab either before or at the time of diagnosis of brain metastases (P < .0001).With these results, we hypothesized that the prognostic outcome based on breast tumor subtypes may have changed with the introduction of trastuzumab.
In our present study, we attempted to partially answer this question. Given that whole brain radiation is the most common treatment for brain metastases, our study cohort was restricted to women with breast cancer and brain metastases who had received whole brain radiotherapy at The University of Texas M. D. Anderson Cancer Center, and those with HER-2–positive disease had to have received trastuzumab. We then stratified women into 3 groups based on the receptor status of their tumors. Among women with hormone receptor-positive/HER-2–negative (surrogate for luminal A tumors), HER-2–positive (a surrogate for a combination of luminal B and HER-2–positive/hormone receptor-negative tumors), and triple-negative disease (a surrogate for basal-like tumors), we observed a median survival of 5, 9, and 5 months, respectively, after a diagnosis of brain metastases (P = .0069). These results were confirmed in the multivariate analysis, where compared with women with triple-negative disease, women with HER-2–positive disease had a 37% decreased risk of death after a diagnosis of brain metastases compared with women with HER-2–negative disease, which was statistically significant, whereas among women with triple-negative disease, no significant difference was observed. Our limited data provide provocative evidence for the finding that at least within the boundaries of the cohort studied, prognostic outcome of breast tumor subtypes has changed with the introduction of trastuzumab, with HER-2–positive tumors now no longer associated with the poorest outcome. This is somewhat surprising, given that most patients received trastuzumab after the diagnosis of brain metastasis, and penetration across the blood-brain barrier has been questioned for this drug. To truly assess this hypothesis, a larger sample size would be required, including breast tumors undergoing gene expression profiling or additional IHC staining for cytokeratin 5/6 and EGFR, so that true surrogates of luminal A, luminal B, HER-2, and basal-like subtypes can be obtained.
In the pretrastuzumab era, several studies identified several additional factors that predict prognostic outcome among women with breast cancer and brain metastases who had undergone whole brain radiotherapy.7,9-11 Some of the most important ones that have been identified include KPS, age, number of brain metastases, and the presence of active extracranial disease. Examining data derived from a database of 3 randomized RTOG trials and using recursive partitioning analysis, Gaspar et al11 developed a 3-tiered recursive partitioning analysis prognostic index that was based on KPS, primary tumor status, age of the patient, and presence of extracranial metastases. The authors observed median survival rates of 7.1, 4.2, and 2.3 months for patients who were categorized as recursive partitioning analysis class I, II, and III, respectively. Since its development, this prognostic system has been widely validated.10,21,22 In our study, women categorized as recursive partitioning analysis class I/II and III had median survival rates of 11 and 2 months, respectively (P < .0001). After adjusting for several factors, including breast tumor subtype, recursive partitioning analysis remained a statistically significant prognostic factor, with a higher risk of death associated with recursive partitioning analysis class III compared with recursive partitioning analysis class I/II.
We acknowledge that our study has several important limitations, including it being a retrospective study and thus subject to all the biases that are inherent to this type of study. First, all patients received whole brain radiotherapy, which may itself reflect a bias either toward patients with more brain metastases who were selected for whole brain radiotherapy or toward patients with better performance status able to undergo radiation. In addition, patients who received <30 Gy to the brain had outcomes similar to those with untreated brain metastases based on historical studies. This appears to be an inadequate dose independent of performance status. However, details as to why 30 Gy was not received are not available. Patient choice, toxicity, or deteriorating performance status are likely and would bias this result. Similarly, although whole brain radiotherapy without surgery or radiosurgery was associated with worse outcome on univariate analysis, this was not significant on multivariate analysis, likely reflecting a larger disease burden in patients who received only whole brain radiotherapy. However, despite these limitations we made several important observations. First, we observed that breast tumor subtype still plays an important prognostic role after the development of brain metastases, and we provided provocative evidence that indicates that the prognostic profile of these breast tumor subtypes may have changed with the introduction of trastuzumab. The best prognostic group in our cohort was the group of women who had HER-2–positive disease and had received trastuzumab. Future studies will need to focus on this important group to identify additional prognostic factors specific to this cohort. Second, we observed that factors such as recursive partitioning analysis and adequate radiation dose continue to be important prognostic factors among women with breast cancer and brain metastases in the trastuzumab era.
CONFLICT OF INTEREST DISCLOSURES
Supported in part by the Susan G. Komen Foundation and the Nellie B. Connally Fund for Breast Cancer Research.
REFERENCES
- 1.Weil RJ, Palmieri DC, Bronder JL, et al. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wen PY, Black PM, Loeffler JS. Treatment of metastatic breast cancer: metastatic brain cancer. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 6th ed. Lippincott, William, & Wilkins; Philadelphia, PA: 2001. pp. 2655–2670. [Google Scholar]
- 3.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–180. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 4.DiStefano A, Yong Yap Y, Hortobagyi GN, et al. The natural history of breast cancer patients with brain metastases. Cancer. 1979;44:1913–1918. doi: 10.1002/1097-0142(197911)44:5<1913::aid-cncr2820440554>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 5.Posner JB. Back pain and epidural spinal cord compression. Med Clin North Am. 1987;71:185–205. doi: 10.1016/s0025-7125(16)30865-3. [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom JT, Minn H, Lertola KK, et al. Prognosis of patients treated for intracranial metastases with whole-brain irradiation. Ann Med. 1998;30:296–299. doi: 10.3109/07853899809005858. [DOI] [PubMed] [Google Scholar]
- 7.Lagerwaard FJ, Levendag PC, Nowak PJ, Eijkenboom WM, Hanssens PE, Schimitz PI. Identification of prognostic factors in patients with brain metastases: a review of 1292 patients. Int J Radiat Oncol Biol Phys. 1999;43:795–803. doi: 10.1016/s0360-3016(98)00442-8. [DOI] [PubMed] [Google Scholar]
- 8.Van den Bent MJ. The diagnosis and management of brain metastases. Curr Opin Neurol. 2001;14:717–723. doi: 10.1097/00019052-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Saito EY, Viani GA, Ferrigno R, et al. Whole brain radiation therapy in management of brain metastasis: results and prognostic factors. Radiat Oncol. 2006;1:20. doi: 10.1186/1748-717X-1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu MT, Hsieh CY, Wang AY, et al. Prognostic factors affecting the outcome of brain metastases from breast cancer. Support Care Cancer. 2007;15:349. doi: 10.1007/s00520-006-0164-0. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 12.Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol. 2008;19:1242–1248. doi: 10.1093/annonc/mdn036. [DOI] [PubMed] [Google Scholar]
- 13.Fisher ER, Redmond C, Fisher B. Histologic grading of breast cancer. Pathol Annu. 1980;15:239–251. [PubMed] [Google Scholar]
- 14.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 15.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carey LA, Perou CM, Livasy CA, et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295:2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 17.Pestalozzi BC, Zahrieh D, Price KN, et al. Identifying breast cancer patients at risk for central nervous system (CNS) metastases in trials of the International Breast Cancer Study Group (IBCSG) Ann Oncol. 2006;17:935–944. doi: 10.1093/annonc/mdl064. [DOI] [PubMed] [Google Scholar]
- 18.Heitz F, H P, Traut A, et al. Cerebral metastases (CM) in breast cancer (BC) with focus on triple-negative tumors [abstract] J Clin Oncol. 2008;26 Abstract 1010. [Google Scholar]
- 19.Nam BH, Kim SY, Han HS, et al. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niwinska A, Murawska M. Brain metastases in breast cancer patients: differences in survival depending on biological subtype and RPA RTOG prognostic class [abstract] J Clin Oncol. 2008;26 doi: 10.1093/annonc/mdp407. Abstract 1056. [DOI] [PubMed] [Google Scholar]
- 21.Broadbent AW, Hruby G, Tin MM, Jackson M, Firth I. Survival following whole brain radiation treatment for cerebral metastases: an audit of 474 patients. Radiother Oncol. 2004;71:259–265. doi: 10.1016/j.radonc.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Mahmoud-Ahmed AS, Suh JH, Lee SY, Crownover RL, Barnett GH. Results of whole brain radiotherapy in patients with brain metastases from breast cancer: a retrospective study. Int J Radiat Oncol Biol Phys. 2002;54:810–817. doi: 10.1016/s0360-3016(02)02967-x. [DOI] [PubMed] [Google Scholar]


