Abstract
Purpose
Young women with breast cancer have higher locoregional recurrence (LRR) rates than older patients. The goal of this study is to determine the impact of locoregional treatment strategy, breast-conserving therapy (BCT), mastectomy alone (M), or mastectomy with adjuvant radiation (MXRT), on LRR for patients 35 years or younger.
Methods and Materials
Data for 668 breast cancers in 652 young patients with breast cancer were retrospectively reviewed; 197 patients were treated with BCT, 237 with M, and 234 with MXRT.
Results
Median follow-up for all living patients was 114 months. In the entire cohort, 10-year actuarial LRR rates varied by locoregional treatment: 19.8% for BCT, 24.1% for M, and 15.1% for MXRT (p = 0.05). In patients with Stage II disease, 10-year actuarial LRR rates by locoregional treatment strategy were 17.7% for BCT, 22.8% for M, and 5.7% for MXRT (p = 0.02). On multivariate analysis, M (hazard ratio, 4.45) and Grade III disease (hazard ratio, 2.24) predicted for increased LRR. In patients with Stage I disease, there was no difference in LRR rates based on locoregional treatment (18.0% for BCT, 19.8% for M; p = 0.56), but chemotherapy use had a statistically significant LRR benefit (13.5% for chemotherapy, 27.9% for none; p = 0.04).
Conclusions
Young women have high rates of LRR after breast cancer treatment. For patients with Stage II disease, the best locoregional control rates were achieved with MXRT. For patients with Stage I disease, similar outcomes were achieved with BCT and mastectomy; however, chemotherapy provided a significant benefit to either approach.
Keywords: Breast cancer, Breast-conserving therapy, Mastectomy, Radiation, Young age
INTRODUCTION
Despite optimal therapies, clinical studies have suggested that young patients with breast cancer consistently have worse outcomes than those who develop the disease later in life. Definitions of “young age” have been controversial, with some studies suggesting that cutoff points at age 30, 35, and 40 years all segregate a “young” population that does more poorly than similarly staged “older” patients. However, many series found that patients 35 years or younger consistently have higher locoregional recurrence (LRR) rates when treated with breast-conserving therapy (BCT) (1–9), and some have reported that this translates into decreased overall survival (OS) (7).
Breast cancer in young patients has been hypothesized to be more biologically aggressive than that occurring in older cohorts based on histopathologic features, including higher grade, more lymphovascular space invasion (LVSI), more extensive intraductal component, and more estrogen receptor (ER) negativity (1, 6, 10, 11). However, many studies have shown that young age remains a powerful predictor of poor outcome even after controlling for these features (7, 12, 13). In this study, we examine the impact of locoregional management, BCT, mastectomy alone (M), or mastectomy with postoperative radiation (MXRT) on LRR, distant metastasis (DM), and OS in women 35 years or younger. We report that maximal locoregional therapy benefits most subgroups of young women with breast cancer.
METHODS AND MATERIALS
Patient selection
We retrospectively reviewed the records of young women (age ≤35 years) diagnosed with breast cancer and treated at the University of Texas M. D. Anderson Cancer Center, Houston, TX, from 1973 to 2006. This cohort included 668 breast cancers in 652 women. Patients with a diagnosis of inflammatory breast cancer, ductal carcinoma in situ, sarcoma, and unknown primary cancer were excluded from analysis, as were those who refused breast irradiation after breast-conservation surgery, those with metastatic disease within 6 months of diagnosis, and those who did not receive a definitive surgery. Patients were staged according to the 2002 American Joint Committee on Cancer (AJCC) guidelines (14). For patients who received neoadjuvant chemotherapy, the most advanced stage (initial clinical and/or pathologic stage) was assigned; for patients who did not receive neoadjuvant chemotherapy, pathologic stage was used.
Standard treatment
All patients received definitive locoregional treatment with BCT, M, or MXRT. Decisions about locoregional treatment strategy, chemotherapy, and hormonal therapy were based on clinical staging, physician discretion, and patient preference. All patients underwent surgical evaluation of the axilla with either full Level I/II axillary dissection or sentinel lymph node biopsy followed by axillary dissection in the case of one or more positive sentinel lymph nodes. In the entire population, a median of 16 lymph nodes was removed (including those treated with sentinel lymph node biopsy alone; n = 26). Patients treated with MXRT received postmastectomy radiation prescribed to the chest wall and undissected draining lymphatics to a median dose of 50 Gy in 25 fractions, followed by a sequential boost to the chest wall flaps and any site of initially gross disease. A 10-Gy boost was prescribed if margins were negative (n = 188), 14 Gy if margins were less than 2 mm (n = 22), and 16 Gy if margins were positive and no reexcision was performed (n = 10). Patients treated with BCT received external beam radiation prescribed to the entire breast to a median dose of 50 Gy in 25 fractions followed by a boost to the tumor bed and scar with a 2-cm margin for an additional 10 Gy if margins were negative (n = 160), 14 Gy if margins were less than 2 mm (n = 21), and 16 Gy if margins were positive and no reexcision was performed (n = 7). Chemotherapy regimens were generally doxorubicin based, and patients with positive lymph nodes generally were treated with taxane-based therapy after its introduction (n = 162). The majority of patients (73.1%) were not evaluated for Her-2/neu receptor status, and only 1.0% of patients received trastuzumab.
Statistical analysis
The frequencies of pathologic and clinical factors between the groups of patients were compared using chi-squared statistic. End points were calculated as the interval between pathologic diagnosis of the primary cancer and the event of interest. Local recurrence (LR) was defined as recurrence in the ipsilateral breast, chest wall, or overlying skin. The LRR was defined as ipsilateral local or regional nodal recurrence (including axillary, supraclavicular, infraclavicular, or internal mammary nodal beds). Any other site of recurrence was coded as DM. All LRRs were considered events regardless of their relation to DM in time. Ten-year actuarial rates of LRR, DM, and OS were calculated using the Kaplan-Meier statistic, and comparisons between groups were calculated using log-rank test. All p value calculations were two sided, and only p ≤ 0.05 was considered statistically significant. Multivariate analysis was performed using forward stepwise Cox regression; all variables on univariate analysis with p ≤ 0.1 were used. Unknown variables were included as a separate category for the purpose of multivariate analysis.
RESULTS
Patient characteristics
Median follow-up for all patients was 91 months (range, 2–411 months), and for all living patients, 114 months (range, 7–411 months). Median age was 33 years (range, 16–35 years). Table 1 lists patient, tumor, and treatment characteristics.
Table 1.
Patient and tumor characteristics for the entire population and by treatment strategy
| Total No. (%) | No. with BCT (%) | No. with M (%) | No. with MXRT (%) | p value* | |
|---|---|---|---|---|---|
| All patients | 652 | 196 (29.5) | 237 (35.5) | 234 (35.0) | |
| Age at diagnosis (y) | 0.59 | ||||
| ≤19 | 2 (0.3) | 0 (0) | 1 (0.4) | 1 (0.4) | |
| 20–24 | 18 (2.7) | 3 (1.5) | 8 (3.4) | 7 (3.0) | |
| 25–29 | 137 (20.5) | 36 (18.3) | 46 (19.4) | 55 (23.5) | |
| 30–35 | 511 (76.5) | 158 (80.2) | 182 (76.8) | 171 (73.1) | |
| Race | 0.06 | ||||
| White/Caucasian | 414 (62.0) | 112 (56.9) | 159 (67.1) | 143 (61.1) | |
| Black/African American | 99 (14.8) | 40 (20.3) | 29 (12.2) | 30 (12.8) | |
| Hispanic | 135 (20.2) | 36 (18.3) | 45 (19.0) | 54 (23.1) | |
| Other | 20 (3.0) | 9 (4.6) | 4 (1.7) | 7 (3.0) | |
| Side | 0.17 | ||||
| Left | 339 (50.7) | 99 (50.3) | 131 (55.3) | 109 (46.6) | |
| Right | 329 (49.3) | 98 (49.7) | 106 (44.7) | 125 (53.4) | |
| Family history | 0.78 | ||||
| Negative | 395 (59.1) | 113 (57.4) | 137 (57.8) | 145 (62.0) | |
| Positive distant | 176 (26.3) | 56 (28.4) | 62 (26.2) | 58 (24.8) | |
| Positive first degree | 88 (13.2) | 27 (13.7) | 34 (14.3) | 27 (11.5) | |
| Unknown | 9 (1.3) | 1 (0.5) | 4 (1.7) | 4 (1.7) | |
| Decade of treatment | <0.001 | ||||
| 1973–1979 | 42 (6.3) | 1 (0.5) | 8 (3.4) | 33 (14.1) | |
| 1980–1989 | 154 (23.1) | 21 (10.7) | 89 (37.6) | 44 (18.8) | |
| 1990–1999 | 357 (53.4) | 124 (62.9) | 132 (55.7) | 101 (43.2) | |
| 2000–2006 | 115 (17.2) | 51 (25.9) | 8 (3.4) | 56 (23.9) | |
| T stage | <0.001 | ||||
| T1 | 201 (30.1) | 88 (44.7) | 80 (33.8) | 33 (14.1) | |
| T2 | 273 (40.9) | 86 (43.7) | 110 (46.4) | 77 (32.9) | |
| T3 | 123 (8.4) | 16 (8.1) | 36 (15.2) | 71 (30.3) | |
| T4 | 57 (8.5) | 7 (3.6) | 6 (2.5) | 44 (18.8) | |
| TX | 14 (2.1) | 0 (0) | 5 (2.1) | 9 (3.9) | |
| N stage | <0.001 | ||||
| N0 | 222 (33.2) | 107 (54.3) | 93 (39.2) | 22 (9.4) | |
| N1 | 233 (34.9) | 54 (27.4) | 103 (43.5) | 76 (32.5) | |
| N2 | 129 (19.3) | 23 (11.7) | 28 (11.8) | 78 (33.3) | |
| N3 | 84 (12.5) | 13 (6.6) | 13 (5.5) | 58 (24.8) | |
| AJCC Stage | <0.001 | ||||
| I | 101 (15.1) | 53 (26.9) | 42 (17.7) | 6 (2.6) | |
| IIA | 164 (24.6) | 75 (38.1) | 72 (30.4) | 17 (7.3) | |
| IIB | 132 (19.8) | 25 (12.7) | 63 (26.6) | 44 (33.3) | |
| IIIA | 138 (20.7) | 26 (13.2) | 39 (16.5) | 73 (31.2) | |
| IIIB | 40 (6.0) | 5 (2.5) | 4 (1.7) | 31 (13.2) | |
| IIIC | 84 (12.6) | 13 (6.6) | 13 (5.5) | 58 (24.8) | |
| Unknown (TX, N0-2) | 9 (1.3) | 0 (0) | 4 (1.7) | 5 (2.1) | |
| Stage Group | <0.001 | ||||
| I | 101 (15.1) | 53 (26.9) | 42 (17.7) | 6 (2.6) | |
| II | 296 (44.4) | 101 (51.3) | 153 (57.0) | 61 (26.1) | |
| III | 262 (39.2) | 43 (21.8) | 56 (23.6) | 162 (69.2) | |
| Unknown (TX, N0-2) | 9 (1.3) | 0 (0) | 4 (1.7) | 5 (2.1) | |
| Histologic type | 0.04 | ||||
| Invasive ductal | 622 (93.1) | 188 (95.4) | 222 (93.7) | 212 (90.6) | |
| Invasive lobular | 14 (2.1) | 1 (0.5) | 8 (3.4) | 5 (2.1) | |
| Invasive mixed | 14 (2.1) | 2 (1.0) | 2 (0.8) | 10 (4.3) | |
| Unknown/other | 18 (2.6) | 6 (3.0) | 5 (2.1) | 7 (3.0) | |
| Grade | 0.01 | ||||
| MBNG I | 9 (1.3) | 2 (1.0) | 5 (2.1) | 2 (0.9) | |
| MBNG II | 207 (31.0) | 66 (33.5) | 78 (32.9) | 63 (26.9) | |
| MBNG III | 389 (58.2) | 122 (61.9) | 130 (54.9) | 137 (58.5) | |
| Unknown | 63 (9.4) | 7 (3.6) | 24 (10.1) | 32 (13.7) | |
| Final margin status | 0.03 | ||||
| Negative | 564 (84.4) | 160 (81.2) | 216 (91.1) | 188 (80.3) | |
| Close | 52 (7.8) | 21 (10.7) | 9 (3.8) | 22 (9.4) | |
| Positive | 23 (3.4) | 7 (3.6) | 6 (2.5) | 10 (4.3) | |
| Unknown | 63 (9.4) | 9 (4.6) | 6 (2.5) | 14 (6.0) | |
| ER status | 0.02 | ||||
| Negative | 274 (41.0) | 97 (49.2) | 95 (40.1) | 82 (35.0) | |
| Positive | 268 (40.1) | 75 (38.1) | 91 (38.4) | 102 (43.6) | |
| Unknown | 126 (18.9) | 25 (12.7) | 51 (21.5) | 50 (21.4) | |
| PR status | 0.05 | ||||
| Negative | 283 (42.4) | 93 (47.2) | 96 (40.5) | 94 (40.2) | |
| Positive | 216 (32.3) | 70 (35.5) | 75 (31.6) | 71 (30.3) | |
| Unknown | 169 (25.3) | 34 (17.3) | 666 (27.8) | 69 (29.5) | |
| LVSI | <0.001 | ||||
| Absent | 447 (66.9) | 149 (75.6) | 167 (70.5) | 131 (56.0) | |
| Present | 221 (33.1) | 48 (24.4) | 70 (29.5) | 103 (44.0) | |
| Chemotherapy | <0.001 | ||||
| No | 69 (10.3) | 32 (16.2) | 29 (12.2) | 8 (3.4) | |
| Yes | 599 (89.7) | 165 (83.8) | 208 (87.8) | 226 (96.6) | |
| Hormone therapy | 0.002 | ||||
| No | 504 (75.4) | 149 (75.6) | 195 (82.3) | 160 (68.4) | |
| Yes | 164 (24.6) | 48 (24.4) | 42 (17.7) | 74 (31.6) |
Abbreviations: BCT = breast-conserving therapy; M = mastectomy alone; MXRT = mastectomy with postoperative radiation; AJCC = American Joint Committee on Cancer; MBNG = Modified Black's Nuclear Grade; ER = estrogen receptor; PR = progesterone receptor; LVSI = lymphovascular space invasion.
p value represents comparison of the three treatment groups (BCT, M, and MXRT).
Patients were treated with one of three treatment strategies: BCT, M, or MXRT. As expected, more aggressive local therapy was associated with worse prognostic features (Table 1). Patients treated with MXRT had more advanced T stage, N stage, AJCC stage, and stage group; greater LVSI; and more frequent use of chemotherapy than those treated with BCT or M (all p < 0.001). They also had more frequent use of hormonal therapy (p = 0.002). Differences in histologic type, margin status, ER status, and progesterone receptor (PR) status were largely driven by differences in the “unknown” populations. There were no statistically significant differences with respect to age at diagnosis, race, laterality of disease, or family history of breast or ovarian cancer.
LR and LRR for the entire population
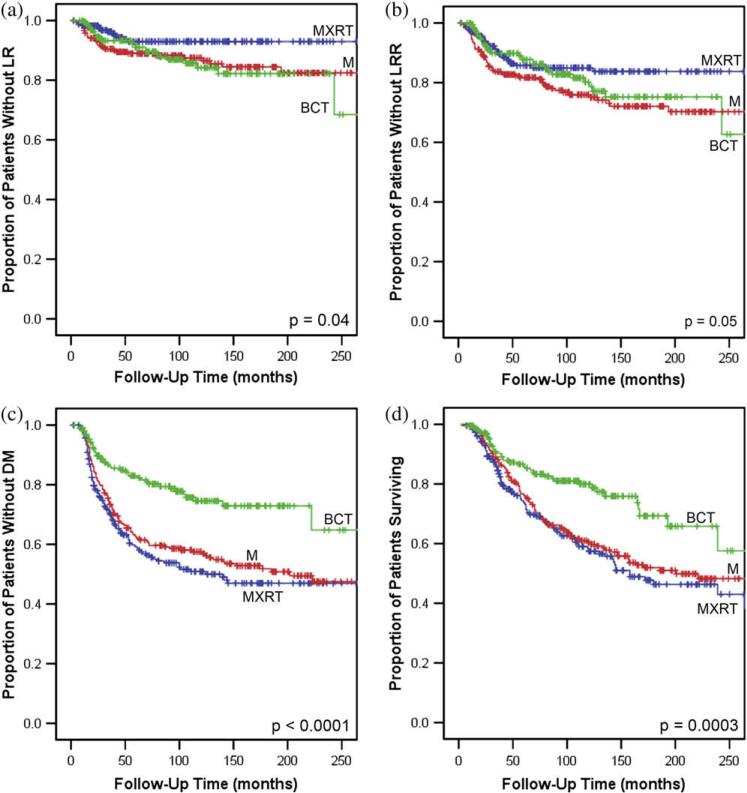
For all patients, the 10-year actuarial LR rate was 11.6%, and the LRR rate was 19.8%. Ten-year actuarial rates of LR differed by treatment strategy: 15.8% for BCT, 12.5% for M, and 7.0% for MXRT (p = 0.04; Fig. 1a). Ten-year actuarial rates of LRR also differed: 19.8% for BCT, 24.1% for M, and 15.0% for MXRT (p = 0.05; Fig. 1b; Table 2).
Fig. 1.
Kaplan-Meier curves for (a) local recurrence (LR)-free survival (b) locoregional recurrence (LRR)-free survival, (c) distant metastasis (DM)-free survival, and (d) overall survival for all patients with breast cancer diagnosed at 35 years and younger. BCT = breast-conserving therapy (green); M = mastectomy alone (red); MXRT = mastectomy with postoperative radiation (blue).
Table 2.
Ten-year actuarial rates of LRR, DM, and OS according to clinical and pathologic features
| n | LRR (%) | p value | DM (%) | p value | OS (%) | p value | |
|---|---|---|---|---|---|---|---|
| All patients | 668 | 19.8 | 39.9 | 64.6 | |||
| Type of treatment | 0.05 | <0.0001 | 0.0003 | ||||
| BCT | 197 | 19.8 | 25.5 | 80.0 | |||
| M | 237 | 24.1 | 42.5 | 60.4 | |||
| MXRT | 234 | 15.0 | 49.1 | 57.5 | |||
| Age at diagnosis (y) | <0.0001 | 0.006 | <0.0001 | ||||
| ≤19 | 2 | 100.0 | 100.0 | 0.0 | |||
| 20–24 | 18 | 17.4 | 30.2 | 75.1 | |||
| 25–29 | 137 | 24.1 | 47.1 | 60.0 | |||
| 30–35 | 511 | 18.5 | 38.0 | 65.6 | |||
| Race | 0.48 | 0.92 | 0.53 | ||||
| White/Caucasian | 414 | 17.9 | 40.8 | 63.2 | |||
| Black/African-American | 99 | 22.8 | 39.8 | 63.8 | |||
| Hispanic | 135 | 22.9 | 38.2 | 69.1 | |||
| Other | 20 | 29.2 | 33.6 | 75.1 | |||
| Side | 0.23 | 0.70 | 0.37 | ||||
| Left | 339 | 21.9 | 40.3 | 65.5 | |||
| Right | 329 | 17.6 | 39.6 | 63.7 | |||
| Family history | 0.52 | 0.89 | 0.74 | ||||
| Negative | 395 | 17.7 | 40.3 | 65.9 | |||
| Positive distant | 176 | 20.7 | 40.3 | 62.2 | |||
| Positive first degree | 88 | 25.7 | 36.9 | 60.8 | |||
| Unknown | 9 | 41.7 | 44.4 | 87.5 | |||
| Decade of treatment | 0.38 | 0.006 | 0.01 | ||||
| 1973–1979 | 42 | 22.0 | 61.9 | 48.9 | |||
| 1980–1989 | 154 | 16.7 | 44.8 | 57.2 | |||
| 1990–1999 | 357 | 19.8 | 34.1 | 70.3 | |||
| 2000–2006 | 115 | 22.8 | 43.8 | 49.5 | |||
| T Stage | 0.69 | <0.0001 | 0.0002 | ||||
| T1 | 201 | 15.9 | 34.2 | 73.1 | |||
| T2 | 273 | 19.4 | 36.6 | 67.1 | |||
| T3 | 123 | 26.1 | 40.5 | 60.0 | |||
| T4 | 57 | 19.6 | 66.4 | 41.0 | |||
| TX | 14 | 32.3 | 74.3 | 32.1 | |||
| N Stage | 0.004 | <0.0001 | <0.0001 | ||||
| N0 | 222 | 18.6 | 28.4 | 78.4 | |||
| N1 | 233 | 18.3 | 38.6 | 67.1 | |||
| N2 | 129 | 16.4 | 44.5 | 57.3 | |||
| N3 | 84 | 34.4 | 68.3 | 30.6 | |||
| AJCC Stage | 0.02 | <0.0001 | <0.0001 | ||||
| I | 101 | 19.5 | 29.0 | 80.8 | |||
| IIA | 164 | 14.0 | 32.0 | 71.4 | |||
| IIB | 132 | 22.7 | 32.1 | 74.0 | |||
| IIIA | 138 | 17.7 | 41.2 | 62.4 | |||
| IIIB | 40 | 18.9 | 58.3 | 49.3 | |||
| IIIC | 84 | 34.4 | 68.3 | 30.6 | |||
| Unknown (TX, N0-2) | 9 | 0 | 70.4 | 26.7 | |||
| Stage Group | 0.38 | <0.0001 | <0.0001 | ||||
| I | 101 | 19.5 | 29.0 | 80.8 | |||
| II | 296 | 17.8 | 32.0 | 72.7 | |||
| III | 262 | 23.1 | 52.3 | 50.5 | |||
| Unknown (TX, N0-2) | 9 | 0 | 70.4 | 26.7 | |||
| Histologic type | 0.98 | 0.68 | 0.61 | ||||
| Invasive ductal | 622 | 20.0 | 39.7 | 65.0 | |||
| Invasive lobular | 14 | 14.3 | 51.8 | 52.8 | |||
| Invasive mixed | 14 | 17.5 | 54.9 | 41.3 | |||
| Unknown/other | 18 | 16.7 | 26.3 | 81.9 | |||
| Grade | 0.43 | 0.14 | 0.04 | ||||
| MBNG I | 9 | 0 | 60.0 | 75.0 | |||
| MBNG II | 207 | 16.9 | 38.9 | 70.4 | |||
| MBNG III | 389 | 21.3 | 38.0 | 64.1 | |||
| Unknown | 63 | 22.6 | 53.2 | 48.8 | |||
| Final margin status | 0.41 | 0.05 | 0.02 | ||||
| Negative | 564 | 19.2 | 37.9 | 66.2 | |||
| Close | 52 | 32.1 | 51.2 | 51.7 | |||
| Positive | 23 | 13.0 | 64.1 | 41.0 | |||
| Unknown | 29 | 21.5 | 49.4 | 68.8 | |||
| ER status | 0.12 | 0.87 | 0.59 | ||||
| Negative | 274 | 22.7 | 37.4 | 64.8 | |||
| Positive | 268 | 19.9 | 43.1 | 64.3 | |||
| Unknown | 126 | 13.7 | 40.1 | 63.1 | |||
| PR status | 0.06 | 0.77 | 0.48 | ||||
| Negative | 283 | 24.0 | 39.1 | 65.2 | |||
| Positive | 216 | 20.1 | 39.9 | 64.2 | |||
| Unknown | 169 | 12.9 | 41.7 | 62.0 | |||
| LVSI | 0.23 | 0.004 | 0.009 | ||||
| Absent | 447 | 18.2 | 36.7 | 68.2 | |||
| Present | 221 | 23.3 | 46.4 | 56.9 | |||
| Chemotherapy | 0.14 | 0.005 | 0.06 | ||||
| No | 69 | 22.4 | 27.9 | 76.6 | |||
| Yes | 599 | 19.5 | 41.2 | 63.1 | |||
| Hormone therapy | 0.77 | 0.97 | 0.64 | ||||
| No | 504 | 20.4 | 39.2 | 65.1 | |||
| Yes | 164 | 16.7 | 44.3 | 59.9 |
Abbreviations: BCT = breast-conserving therapy; M = mastectomy alone; MXRT = mastectomy with postoperative radiation; AJCC = American Joint Committee on Cancer; MBNG = Modified Black's Nuclear Grade; ER = estrogen receptor; PR = progesterone receptor; LVSI = lymphovascular space invasion; LRR = locoregional recurrence; DM = distant metastasis; OS = overall survival.
On univariate analysis of factors impacting on LRR, locoregional treatment strategy (p = 0.05), N stage (p = 0.004), AJCC stage (p = 0.02), and age group (p < 0.0001) all had a statistically significant impact on outcome (Table 2; Fig. 1b). Other factors, including T stage, grade, histologic type, LVSI, margin status, ER and PR status, decade of treatment, family history of breast and ovarian cancer, race, and use of chemotherapy or hormonal therapy, had no statistically significant impact on LRR.
DM for the entire population
For all patients, the 10-year actuarial rate of DM was 39.9%. The DM rates differed by locoregional treatment strategy: 25.5% for BCT, 42.5% for M, and 49.1% for MXRT (p < 0.0001; Fig. 1c). On univariate analysis, locoregional treatment approach (p < 0.0001), age group at diagnosis (p = 0.006), decade of treatment (p = 0.006), T stage (p < 0.0001), N stage (p < 0.0001), AJCC stage (p < 0.0001), LVSI (p = 0.004), and use of chemotherapy (p = 0.005) had a statistically significant impact on DM rates (Table 2). Other factors, including race, family history of breast or ovarian cancer, histologic type, grade, margin status, ER status, PR status, and use of hormonal therapy, did not have a statistically significant impact (Table 2).
OS for the entire population
For all patients, the 10-year actuarial overall survival rate was 64.6%. Ten-year OS rates differed by locoregional treatment strategy: 80.0% for BCT, 60.4% for M, and 57.5% for MXRT (p = 0.0003; Table 2; Fig. 1d). On univariate analysis, there were statistically significant differences in OS based on locoregional treatment strategy (p = 0.0003), age group at diagnosis (p < 0.0001), decade of treatment (p = 0.01), T stage (p = 0.0002), N stage (p < 0.0001), AJCC stage (p < 0.0001), grade (p = 0.04), margin status (p = 0.02), and LVSI (p = 0.009; Table 2). There was a borderline significant difference based on the use of chemotherapy (p = 0.06). There were no differences observed based on race, family history of breast or ovarian cancer, histologic type, ER status, PR status, or the use of hormonal therapy.
Patients with Stage I disease
The majority of the patients with Stage I disease (n = 101) were treated with BCT (n = 53) or M (n = 42). There was a small number of patients (n = 6) treated with MXRT; these patients were not considered in the analysis. Patients with Stage I disease treated with BCT and M did not have statistically significant differences in tumor size, ER positivity, PR positivity, LVSI, grade, histologic type, or margin status (all p > 0.05). There was no statistically significant difference in 10-year actuarial LRR rates based on locoregional treatment strategy (18.0% for BCT, 19.8% for M; p = 0.56) (Table 3).
Table 3.
Ten-year actuarial rates of LRR according to clinical and pathologic features by treatment strategy
| 10-year LRR (%) |
|||||
|---|---|---|---|---|---|
| n | BCT | M | MXRT | p value | |
| All patients | 668 | 19.8 | 24.1 | 15.0 | 0.05 |
| Age at diagnosis (y) | |||||
| ≤19 | 2 | N/A | 100.0 | 100.0 | 0.32 |
| 20–24 | 18 | 0 | 37.5 | 0 | 0.13 |
| 25–29 | 137 | 12.7 | 33.0 | 24.1 | 0.33 |
| 30–35 | 511 | 22.0 | 20.9 | 12.7 | 0.15 |
| Race | |||||
| White/Caucasian | 414 | 18.3 | 22.1 | 12.8 | 0.17 |
| Black/African-American | 99 | 25.0 | 21.0 | 23.1 | 0.88 |
| Hispanic | 135 | 18.6 | 28.8 | 18.6 | 0.41 |
| Other | 20 | 20.0 | 62.5 | 0 | 0.21 |
| Side | |||||
| Left | 339 | 21.2 | 26.7 | 13.4 | 0.06 |
| Right | 329 | 15.6 | 20.9 | 16.5 | 0.42 |
| Family history | |||||
| Negative | 395 | 16.1 | 20.8 | 14.3 | 0.38 |
| Positive distant | 176 | 24.5 | 26.4 | 13.3 | 0.23 |
| Positive first degree | 88 | 25.6 | 29.2 | 18.7 | 0.53 |
| Unknown | 9 | 0 | 66.7 | 25.0 | 0.55 |
| Decade of treatment | |||||
| 1973–1979 | 42 | 0 | 27.1 | 22.0 | 0.77 |
| 1980–1989 | 154 | 4.8 | 20.6 | 14.6 | 0.43 |
| 1990–1999 | 357 | 19.7 | 25.3 | 12.2 | 0.08 |
| 2000–2006 | 115 | 26.4 | 37.5 | 16.7 | 0.29 |
| T Stage | |||||
| T1 | 201 | 13.5 | 19.0 | 14.4 | 0.72 |
| T2 | 273 | 21.6 | 21.5 | 14.1 | 0.43 |
| T3 | 123 | 30.7 | 39.1 | 16.3 | 0.12 |
| T4 | 57 | 42.2 | 50.0 | 10.2 | 0.02 |
| TX | 14 | N/A | 20.0 | 40.0 | 0.94 |
| N Stage | |||||
| N0 | 222 | 16.1 | 23.3 | 9.9 | 0.10 |
| N1 | 233 | 22.3 | 20.4 | 12.2 | 0.35 |
| N2 | 129 | 20.1 | 32.7 | 10.0 | 0.02 |
| N3 | 84 | 63.9 | 44.6 | 29.0 | 0.64 |
| AJCC Stage | |||||
| I | 101 | 18.0 | 19.8 | 37.5 | 0.56 |
| IIA | 164 | 12.4 | 18.8 | 0 | 0.15 |
| IIB | 132 | 36.7 | 27.3 | 7.8 | 0.05 |
| IIIA | 138 | 13.8 | 28.0 | 13.8 | 0.20 |
| IIIB | 40 | 33.3 | 50.0 | 10.8 | 0.07 |
| IIIC | 84 | 63.9 | 44.6 | 29.0 | 0.64 |
| Unknown (TX, N0-2) | 9 | N/A | 0 | 0 | N/A |
| Stage Group | |||||
| I | 101 | 18.0 | 19.8 | 37.5 | 0.56 |
| II | 296 | 17.7 | 22.8 | 5.7 | 0.02 |
| III | 262 | 28.4 | 23.9 | 18.4 | 0.13 |
| Unknown (TX, N0-2) | 9 | N/A | 0 | 0 | N/A |
| Histologic type | |||||
| Invasive ductal | 622 | 19.6 | 24.6 | 14.8 | 0.04 |
| Invasive lobular | 14 | 0 | 12.5 | 20.0 | 0.88 |
| Invasive mixed | 14 | 0 | 50.0 | 16.7 | 0.26 |
| Unknown/other | 18 | 33.3 | 0 | 14.3 | 0.31 |
| Grade | |||||
| MBNG I | 9 | 0 | 0 | 0 | N/A |
| MBNG II | 207 | 11.8 | 22.1 | 13.5 | 0.53 |
| MBNG III | 389 | 25.2 | 25.5 | 13.8 | 0.07 |
| Unknown | 63 | 14.3 | 25.0 | 22.8 | 0.80 |
| Final margin status | |||||
| Negative | 564 | 19.1 | 22.1 | 15.5 | 0.23 |
| Close | 52 | 41.2 | 44.4 | 16.9 | 0.37 |
| Positive | 23 | 14.3 | 58.3 | 10.0 | 0.72 |
| Unknown | 29 | 12.5 | 66.7 | 7.7 | 0.001 |
| ER status | |||||
| Negative | 274 | 26.0 | 25.1 | 15.9 | 0.45 |
| Positive | 268 | 11.6 | 29.1 | 16.2 | 0.07 |
| Unknown | 126 | 18.4 | 13.8 | 12.2 | 0.66 |
| PR status | |||||
| Negative | 283 | 28.4 | 29.0 | 12.6 | 0.07 |
| Positive | 216 | 10.6 | 28.6 | 19.4 | 0.06 |
| Unknown | 169 | 13.4 | 12.2 | 13.7 | 0.93 |
| LVSI | |||||
| Absent | 447 | 17.0 | 23.3 | 13.0 | 0.03 |
| Present | 221 | 29.5 | 35.6 | 17.8 | 0.60 |
| Chemotherapy | |||||
| No | 69 | 22.3 | 27.6 | 0 | 0.17 |
| Yes | 599 | 19.3 | 23.6 | 15.5 | 0.18 |
| Hormone therapy | |||||
| No | 504 | 21.9 | 24.2 | 14.4 | 0.04 |
| Yes | 164 | 11.7 | 22.4 | 16.7 | 0.73 |
Abbreviations: N/A = not applicable; LRR = locoregional recurrence; BCT = breast-conserving therapy; M = mastectomy alone; MXRT = mastectomy with postoperative radiation; AJCC = American Joint Committee on Cancer; MBNG = Modified Black's Nuclear Grade; ER = estrogen receptor; PR = progesterone receptor; LVSI = lymphovascular space invasion.
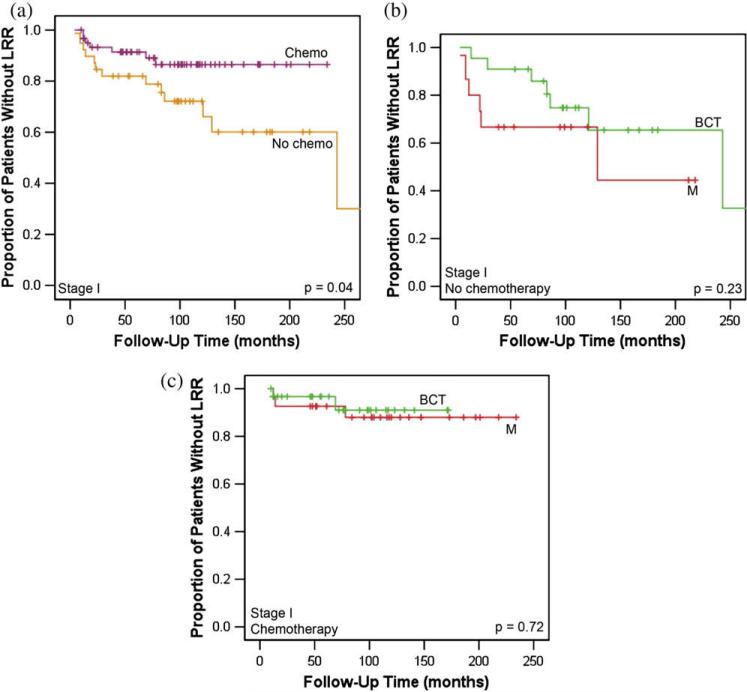
On univariate analysis, patients with Stage I disease who received chemotherapy had improved LRR compared with those who did not (13.5% vs. 27.9%; p = 0.04; Fig. 2a). Patients with Stage I disease who received chemotherapy had more high-grade tumors than those who did not (p = 0.005). There were no statistically significant differences in ER positivity, PR positivity, LVSI, margin status, histologic type, or tumor size between these groups (all p > 0.05). In patients with Stage I disease who did not receive chemotherapy, there was no statistically significant difference in LRR rates between those treated with M and BCT (33.3% vs. 25.3%; p = 0.23; Fig. 2b). In those who received chemotherapy, there was no statistically significant difference in LRR rates between those treated with M and BCT (9.0% vs. 12.0%; p = 0.72; Fig. 2c). On multivariate analysis of patients with Stage I disease, lack of adjuvant chemotherapy had a statistically significant impact on LRR (hazard ratio [HR], 2.73; p = 0.037), and the use of hormonal therapy had a borderline impact on LRR (HR, 2.54; p = 0.055; Table 4).
Fig. 2.
Kaplan-Meier curves for locoregional recurrence (LRR)-free survival for young patients with Stage I breast cancer treated with (a) chemotherapy (chemo) vs. no chemotherapy, (b) no chemotherapy by treatment approach, and (c) chemotherapy by treatment approach (breast-conserving treatment [BCT] vs. mastectomy alone [M]).
Table 4.
Multivariate analyses by stage
| Hazard ratio | 95% Confidence interval | p value | |
|---|---|---|---|
| Stage I: LRR | |||
| No adjuvant chemotherapy | 2.73 | 1.06–7.04 | 0.037 |
| Hormonal therapy | 2.54 | 0.98–6.56 | 0.055 |
| Stage I: OS | |||
| Hormonal therapy | 2.17 | 0.90–5.23 | 0.084 |
| Stage II: LRR | |||
| Mastectomy alone | 4.45 | 1.36–14.6 | 0.014 |
| BCT | 3.40 | 0.99–11.7 | 0.052 |
| Grade 3 | 2.24 | 1.19–4.23 | 0.012 |
| Stage II: OS | |||
| Mastectomy alone | 1.72 | 1.11–2.67 | 0.015 |
| Positive nodes | 1.52 | 0.97–2.37 | 0.067 |
| Stage III: LRR | |||
| Mastectomy alone | 2.37 | 1.24–4.51 | 0.009 |
| ≥10 positive nodes | 2.50 | 1.36–4.60 | 0.003 |
| Younger age | 0.91 | 0.85–0.99 | 0.021 |
| No neoadjuvant chemotherapy | 0.59 | 0.31–1.09 | 0.094 |
| Stage III: OS | |||
| Mastectomy alone | 1.58 | 1.02–2.45 | 0.041 |
| ≥4 positive nodes | 2.29 | 1.42–3.67 | 0.001 |
| ≥10 positive nodes | 1.47 | 0.95–2.26 | 0.082 |
| No adjuvant chemotherapy | 1.70 | 1.00–2.87 | 0.049 |
| ER negativity | 1.92 | 1.28–2.87 | 0.001 |
| BCT | 0.54 | 0.29–0.99 | 0.046 |
| No neoadjuvant chemotherapy | 0.41 | 0.25–0.66 | < 0.0001 |
Abbreviations: LRR = locoregional recurrence; OS = overall survival; BCT = breast-conserving therapy; ER = estrogen receptor.
For patients with Stage I disease, there was no statistically significant difference in 10-year actuarial DM rates based on locoregional treatment (27.4% for BCT, 27.7% for M; p = 0.15). In addition, there was no statistically significant difference in OS rates based on locoregional treatment approach (92.4% for BCT, 72.0% for M; p = 0.19). On multivariate analysis of patients with Stage I disease, only use of hormonal therapy had a borderline impact on OS (HR, 2.17; p = 0.084; Table 4).
Patients with Stage II disease
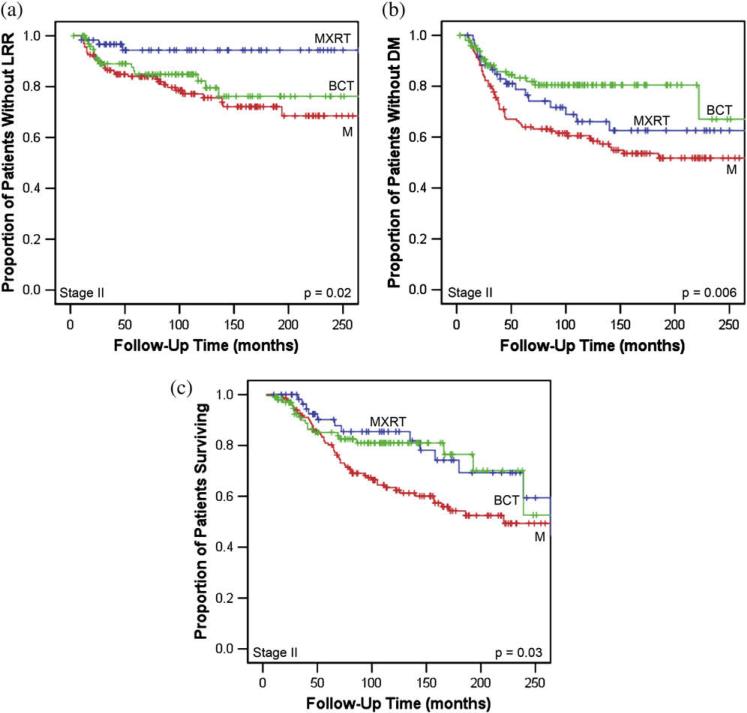
In patients with Stage II disease (n = 296), locoregional treatment approach had a significant impact on LRR rates (17.7% for BCT, 22.8% for M, and 5.7% for MXRT; p = 0.02; Table 3; Fig. 3a). The improvement in outcome in patients treated with MXRT was observed despite more advanced N stage (p = 0.004) and increased use of neoadjuvant chemotherapy (p < 0.0001) in this group compared with M or BCT. There were no statistically significant differences among treatment groups in terms of T stage, LVSI, histologic type, margin status, grade, ER positivity, or PR positivity. On multivariate analysis, M (HR, 4.45; p = 0.014) and Grade III disease (HR, 2.24; p = 0.012) were found to be predictive of LRR (Table 4). Use of BCT was found to be of borderline significance (HR, 3.40; p = 0.052). For patients treated with BCT, 38 patients received neoadjuvant chemotherapy and 63 did not; there was no difference in LRR rates between these groups (p = 0.29).
Fig. 3.
Kaplan-Meier curves for young patients with Stage II breast cancer describing (a) locoregional recurrence (LRR)-free survival, (b) distant metastasis (DM), and (c) overall survival by treatment approach (breast-conserving treatment [BCT] vs. mastectomy alone [M] vs. mastectomy with adjuvant radiation therapy [MXRT]).
For patients with Stage II disease, there was a statistically significant difference in 10-year actuarial DM rates based on locoregional treatment approach (19.5% for BCT, 33.9% for M, and 39.4% for MXRT; p = 0.006; Fig. 3b). Furthermore, for patients with Stage II disease, patients treated with M had a statistically significant decrease in 10-year OS rate (63.4%) compared with those treated with BCT (81.0%) or MXRT (85.4%; p = 0.03; Fig. 3c). On multivariate analysis of patients with Stage II disease, M (HR, 1.72; p = 0.015) was found to be predictive of inferior OS (Table 4). Lymph node positivity (HR, 1.52; p = 0.067) was of borderline significance.
Patients with Stage III disease
For patients with Stage III disease (n = 262), locoregional treatment approach had no statistically significant impact on 10-year LRR rates (28.4% for BCT, 23.9% for M, 18.4% for MXRT; p = 0.13; Table 3). On multivariate analysis, M (HR, 2.37; p = 0.009) and 10 or more positive lymph nodes (HR, 2.50; p = 0.003) were found to be predictive of higher LRR rates (Table 4). Diagnosis at an older age (HR, 0.091; p = 0.021) was found to be predictive of lower LRR rates (Table 4).
For patients with Stage III disease, there was a trend toward higher rates of DM for patients treated with MXRT (54.0%) and M (58.3%) compared with BCT (36.2%; p = 0.08). On univariate analysis of patients with Stage III disease, there was no statistically significant difference in 10-year OS rates based on locoregional approach (62.4% for BCT, 46.6% for M, and 48.4% for MXRT; p = 0.16). On multivariate analysis, M (HR, 1.58; p = 0.041), four or more positive lymph nodes (HR, 2.29; p = 0.001), no adjuvant chemotherapy (HR, 1.70; p = 0.049), and ER negativity (HR, 1.92; p = 0.001) were associated with worse OS (Table 4). Use of BCT (HR, 0.054; p = 0.046) and lack of neoadjuvant chemotherapy (HR, 0.41; p < 0.0001) were associated with improved OS.
DISCUSSION
Locoregional recurrence after optimal breast cancer treatment in young women (age ≤35 years) is a significant problem; the true LRR risk in young patients, and hence their optimal treatment, has been questioned. This study confirms high rates of LRR in young women with breast cancer. In contrast to prior reports, this study explicitly segregates locoregional treatment into MXRT, M, and BCT (rather than simply mastectomy vs. BCT). Patients treated with M consistently had the highest rates of locoregional failure compared with both MXRT and BCT. This effect was largely driven by high locoregional failure rates in women with Stage II disease treated with BCT or M. These patients may be better served by maximal locoregional treatment at diagnosis.
In young women with breast cancer, the 10-year actuarial LRR rate was almost 20%. This is consistent with prior reports; Coulombe et al. (9) noted an 18.0% rate of 10-year LRR for patients aged 20–39 years, and Elkhuizen et al. (15) reported a 28.0% rate of 10-year LRR for patients 35 years or younger. Furthermore, a pooled analysis of two European trials established that age of 35 years or younger conferred an HR for local recurrence of 9.24 compared with age older than 60 years (16). Some studies, including a consolidated analysis of the European Organisation for Research and Treatment of Cancer (EORTC) trials of patients with Stage I and II breast cancers, suggested that both young age and BCT are independent risk factors for LRR. However, the EORTC report compared BCT with mastectomy without taking the use of postmastectomy radiation into consideration (17). Given evidence that reduced rates of LRR lead to improvements in OS (18) and the increasing benefit of radiation therapy with increasing risk of LRR, it is important to define LRR risk and optimal treatment strategies for such potentially high-risk subgroups as patients diagnosed at a young age.
The most striking finding in the present study is the statistically significant improvement in LRR rates for patients with Stage II breast cancer treated with MXRT (5.7%) compared with both BCT (17.7%) and M (22.8%). This improved outcome was seen despite more advanced disease in the MXRT group. These data suggest that patients with Stage II disease who choose mastectomy should be counseled that their LRR rate could be significantly improved with postmastectomy radiation. However, adjuvant radiation must be carefully considered; it is not without risks and has a significant impact on both the type and timing of reconstruction (19, 20). Furthermore, these data suggest that young age should be a consideration in selecting appropriate patients with Stage II disease for BCT. Although it was not possible to address these issues in this study, this decision also should incorporate genetic testing and breast magnetic resonance imaging to further delineate the risks of additional disease and optimize treatment.
On multivariate analysis of the Stage II population, the most important risk factor for LRR and OS was treatment with M. Prior studies have established the link between LRR and OS (18) in large heterogeneous cohorts, and smaller studies have shown this in specific subgroups of young patients (15). The present data suggest that intensification of locoregional treatment through the use of adjuvant radiation is paramount for patients with Stage II disease.
Although there was no difference in LRR rates based on locoregional treatment approach in patients with Stage I disease, 10-year LRR rates were high. Our present data show a locoregional benefit to systemic therapy (13.5% for chemotherapy, 27.9% for none; p = 0.04). Similarly, van der Leest et al. (21) reported an analysis of women 40 years and younger with early-stage breast cancer treated with BCT. They found adjuvant chemotherapy reduced the local recurrence rate by greater than 50% (21). In our present study, there is insufficient information to determine the magnitude of benefit for patients with primary disease less than the standard threshold to recommend chemotherapy.
Compared with the pooled analysis of patients with Stages I and II breast cancers treated on EORTC Trials 10801, 10854, and 10902 (17), our data do not suggest that BCT is an independent predictor of inferior LRR. In their study, de Bock et al. (17) found that young age (HR, 2.8) and BCT (HR, 1.82) were predictive of inferior LRR. However, they did not specifically address how many patients treated with mastectomy received adjuvant radiation. Data presented in this report imply that grouping all patients treated with mastectomy regardless of the use of postmastectomy radiation represents a biased comparison in favor of the mastectomy group. Based on the data here, we suggest that BCT is not necessarily contraindicated in young patients with early-stage disease; rather, M is consistently inferior.
Both LRR and OS rates in young patients with Stage III breast cancers did not differ based on locoregional treatment strategy. This may be caused by the competing risk of DM in this subset of patients (50.5% at 10 years). However, the equivalent rates of LRR based on locoregional treatment approach within this group suggest that the use of BCT in carefully selected patients in this group is appropriate.
The differences in outcome based on locoregional treatment strategy observed in this study are intriguing. However, there are several limitations to the present investigation. Most prominently, these data are retrospective in nature. As a result, there are inherent and potentially hidden biases that have governed treatment decisions and may impact on outcome. In an attempt to control for these differences, treatment groups were evaluated for similarity, and differences between cohorts are indicated when they are significant. In many cases, the imbalances may even strengthen the benefit seen; patients treated with MXRT had more advanced disease than those treated with M or BCT and yet had superior rates of LRR. In addition, multivariate analysis was used to attempt to account for hidden biases and deduce the true relationships between variables and outcomes. Multivariate analysis was limited to patients within each stage to minimize the differences in censoring strategies and limit the likelihood of interactions with stage not considered by the model. Although our data are strengthened by multivariate analyses and a large patient population, these findings should be viewed as hypothesis-generating.
SUMMARY
In this study, we report that young women with breast cancer have high LRR rates and locoregional treatment strategy impacts on LRR rates and, possibly, OS in subsets of these young patients. For patients with Stage I, the data support consideration of chemotherapy for all patients until more definitive data are gathered regarding appropriate selection criteria. For patients with Stage II disease, for whom the balance between risk of LRR and DM leans largely toward LRR, locoregional treatment approach may have the largest benefit. Patients treated with M have inferior LRR and, possibly, OS rates compared with those treated with either BCT or MXRT. Although it is premature to recommend MXRT to all young women with Stage II breast cancers, strong consideration should be given to offering adjuvant radiation to women who choose mastectomy, and the choice of BCT should be made with an understanding of the risks and benefits. Given that the prospective randomized trial designed to compare M with MXRT for patients with Stage II of all age groups failed to complete accrual, as well as the implicit challenges in trials comparing surgical approaches, it is unlikely that a randomized trial comparing different locoregional treatment approaches in young women with breast cancer will be feasible. Careful review of these subgroups from existing randomized data sets and registries is needed to more fully understand the implications of these treatment decisions and more fully educate young patients about the potential risks and benefits of each approach.
Footnotes
Presented at the 49th Annual Meeting of the American Society of Therapeutic Radiology and Oncology, Los Angeles, CA, Oct 28–Nov 1, 2007.
Conflict of interest: none.
REFERENCES
- 1.Nixon AJ, Neuberg D, Hayes DF, et al. Relationship of patient age to pathologic features of the tumor and prognosis for patients with stage I or II breast cancer. J Clin Oncol. 1994;12:888–894. doi: 10.1200/JCO.1994.12.5.888. [DOI] [PubMed] [Google Scholar]
- 2.Kurtz JM, Spitalier JM, Amalric R, et al. Mammary recurrences in women younger than forty. Int J Radiat Oncol Biol Phys. 1988;15:271–276. doi: 10.1016/s0360-3016(98)90004-9. [DOI] [PubMed] [Google Scholar]
- 3.Kurtz JM, Amalric R, Brandone H, et al. Local recurrence after breast-conserving surgery and radiotherapy. Helv Chir Acta. 1989;55:837–842. [PubMed] [Google Scholar]
- 4.Recht A, Connolly JL, Schnitt SJ, et al. The effect of young age on tumor recurrence in the treated breast after conservative surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1988;14:3–10. doi: 10.1016/0360-3016(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 5.Clark RM, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node-negative breast cancer: An update. Ontario Clinical Oncology Group. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 6.Kurtz JM, Jacquemier J, Amalric R, et al. Why are local recurrences after breast-conserving therapy more frequent in younger patients? J Clin Oncol. 1990;8:591–598. doi: 10.1200/JCO.1990.8.4.591. [DOI] [PubMed] [Google Scholar]
- 7.de la Rochefordiere A, Asselain B, Campana F, et al. Age as prognostic factor in premenopausal breast carcinoma. Lancet. 1993;341:1039–1043. doi: 10.1016/0140-6736(93)92407-k. [DOI] [PubMed] [Google Scholar]
- 8.Kim SH, Simkovich-Heerdt A, Tran KN, et al. Women 35 years of age or younger have higher locoregional relapse rates after undergoing breast conservation therapy. J Am Coll Surg. 1998;187:1–8. doi: 10.1016/s1072-7515(98)00114-8. [DOI] [PubMed] [Google Scholar]
- 9.Coulombe G, Tyldesley S, Speers C, et al. Is mastectomy superior to breast-conserving treatment for young women? Int J Radiat Oncol Biol Phys. 2007;67:1282–1290. doi: 10.1016/j.ijrobp.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 10.Albain KS, Allred DC, Clark GM. Breast cancer outcome and predictors of outcome: Are there age differentials? J Natl Cancer Inst Monogr. 1994:35–42. [PubMed] [Google Scholar]
- 11.Leborgne F, Leborgne JH, Ortega B, et al. Breast conservation treatment of early stage breast cancer: Patterns of failure. Int J Radiat Oncol Biol Phys. 1995;31:765–775. doi: 10.1016/0360-3016(94)00414-5. [DOI] [PubMed] [Google Scholar]
- 12.Matthews RH, McNeese MD, Montague ED, et al. Prognostic implications of age in breast cancer patients treated with tumorectomy and irradiation or with mastectomy. Int J Radiat Oncol Biol Phys. 1988;14:659–663. doi: 10.1016/0360-3016(88)90086-7. [DOI] [PubMed] [Google Scholar]
- 13.Oh JL, Bonnen M, Outlaw ED, et al. The impact of young age on locoregional recurrence after doxorubicin-based breast conservation therapy in patients 40 years old or younger: How young is “young”? Int J Radiat Oncol Biol Phys. 2006;65:1345–1352. doi: 10.1016/j.ijrobp.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Greene FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6th ed. Wiley-Liss; New York: 2002. [Google Scholar]
- 15.Elkhuizen PH, van de Vijver MJ, Hermans J, et al. Local recurrence after breast-conserving therapy for invasive breast cancer: High incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40:859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 16.Voogd AC, Nielsen M, Peterse JL, et al. Differences in risk factors for local and distant recurrence after breast-conserving therapy or mastectomy for stage I and II breast cancer: Pooled results of two large European randomized trials. J Clin Oncol. 2001;19:1688–1697. doi: 10.1200/JCO.2001.19.6.1688. [DOI] [PubMed] [Google Scholar]
- 17.de Bock GH, van der Hage JA, Putter H, et al. Isolated locoregional recurrence of breast cancer is more common in young patients and following breast conserving therapy: Long-term results of European Organisation for Research and Treatment of Cancer studies. Eur J Cancer. 2006;42:351–356. doi: 10.1016/j.ejca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the rand-omised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 19.Kronowitz SJ, Robb GL. Breast reconstruction with postmastectomy radiation therapy: Current issues. Plast Reconstr Surg. 2004;114:950–960. doi: 10.1097/01.prs.0000133200.99826.7f. [DOI] [PubMed] [Google Scholar]
- 20.Jugenburg M, Disa JJ, Pusic AL, et al. Impact of radiotherapy on breast reconstruction. Clin Plast Surg. 2007;34:29–37. doi: 10.1016/j.cps.2006.11.013. abstract v–vi. [DOI] [PubMed] [Google Scholar]
- 21.van der Leest M, Evers L, van der Sangen MJ, et al. The safety of breast-conserving therapy in patients with breast cancer aged < or = 40 years. Cancer. 2007;109:1957–1964. doi: 10.1002/cncr.22639. [DOI] [PubMed] [Google Scholar]