Abstract
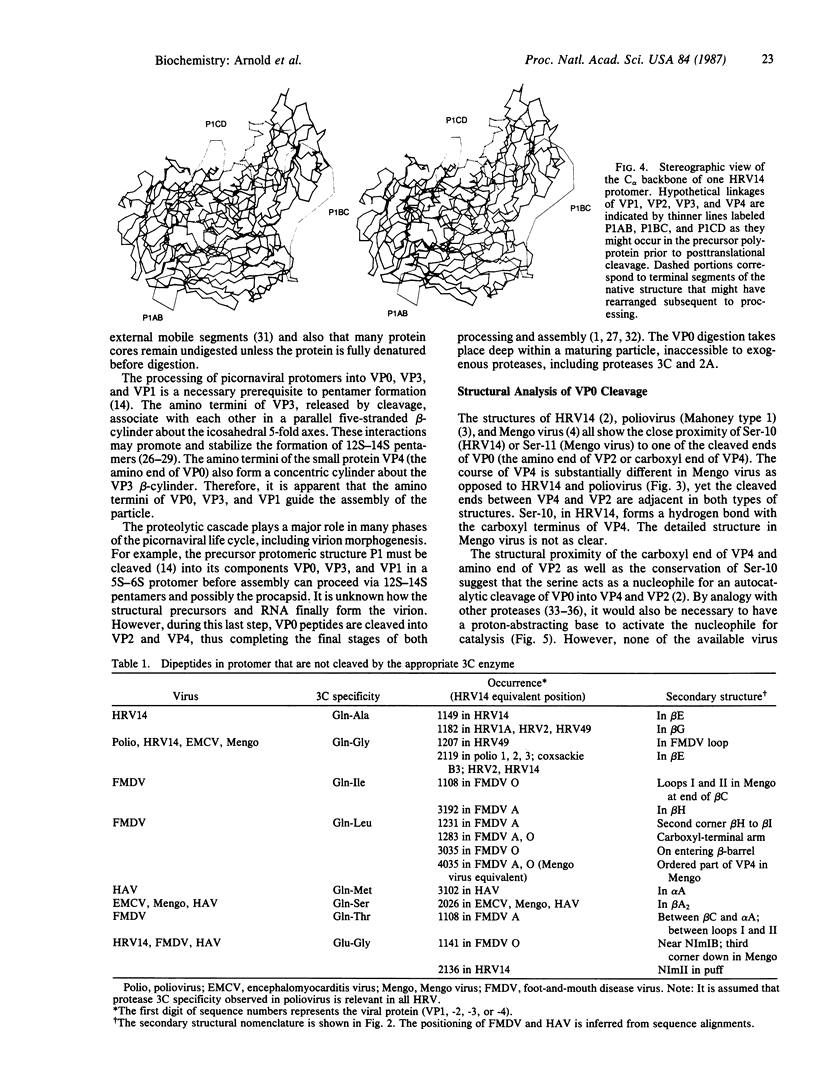
Mature picornaviral proteins are derived by progressive, posttranslational cleavage of a precursor polyprotein. These cleavages play a role in the control of virus functions. Although the processed termini are separated by as much as 75 A in the native virus capsid, the fold and arrangement of polypeptide chains in a protomer before proteolysis are likely to be similar to that found in the mature virus. The three-dimensional structures of rhinovirus and Mengo virus suggest that the cleavage sites within the protomeric precursor are in structurally flexible regions. The final proteolytic processing event, maturation of the virion peptide VP0 (also called peptide 1AB) appears to occur by an unusual autocatalytic serine protease-type mechanism possibly involving viral RNA basic groups that would serve as proton-abstractors during the cleavage reaction.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman S. Aspects of biochemical catalysis. Cell. 1984 Feb;36(2):237–239. doi: 10.1016/0092-8674(84)90216-2. [DOI] [PubMed] [Google Scholar]
- Argos P., Garavito R. M., Eventoff W., Rossmann M. G., Brändén C. I. Similarities in active center geometries of zinc-containing enzymes, proteases and dehydrogenases. J Mol Biol. 1978 Dec 5;126(2):141–158. doi: 10.1016/0022-2836(78)90356-x. [DOI] [PubMed] [Google Scholar]
- Argos P., Kamer G., Nicklin M. J., Wimmer E. Similarity in gene organization and homology between proteins of animal picornaviruses and a plant comovirus suggest common ancestry of these virus families. Nucleic Acids Res. 1984 Sep 25;12(18):7251–7267. doi: 10.1093/nar/12.18.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C., Highfield P. E., Cross G. A., Rowlands D. J., Lowe P. A., Brown F., Harris T. J. Molecular cloning of foot and mouth disease virus genome and nucleotide sequences in the structural protein genes. Nature. 1981 Apr 30;290(5809):800–802. doi: 10.1038/290800a0. [DOI] [PubMed] [Google Scholar]
- Butterworth B. E., Rueckert R. R. Kinetics of synthesis and cleavage of encephalomyocarditis virus-specific proteins. Virology. 1972 Nov;50(2):535–549. doi: 10.1016/0042-6822(72)90405-9. [DOI] [PubMed] [Google Scholar]
- Callahan P. L., Mizutani S., Colonno R. J. Molecular cloning and complete sequence determination of RNA genome of human rhinovirus type 14. Proc Natl Acad Sci U S A. 1985 Feb;82(3):732–736. doi: 10.1073/pnas.82.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenth J., Enzing C. M., Kalk K. H., Vessies J. C. Structure of porcine pancreatic prephospholipase A2. Nature. 1976 Nov 25;264(5584):373–377. doi: 10.1038/264373a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Tomas C. B., Guttman N., Baltimore D. Morphogenesis of poliovirus 3. Formation of provirion in cell-free extracts. J Virol. 1973 Nov;12(5):1181–1183. doi: 10.1128/jvi.12.5.1181-1183.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana A., Fassina G., Vita C., Dalzoppo D., Zamai M., Zambonin M. Correlation between sites of limited proteolysis and segmental mobility in thermolysin. Biochemistry. 1986 Apr 22;25(8):1847–1851. doi: 10.1021/bi00356a001. [DOI] [PubMed] [Google Scholar]
- Fout G. S., Medappa K. C., Mapoles J. E., Rueckert R. R. Radiochemical determination of polyamines in poliovirus and human rhinovirus 14. J Biol Chem. 1984 Mar 25;259(6):3639–3643. [PubMed] [Google Scholar]
- Gorbalenya A. E., Svitkin Y. V., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 is involved in the processing of the viral precursor polypeptides. FEBS Lett. 1979 Dec 1;108(1):1–5. doi: 10.1016/0014-5793(79)81164-3. [DOI] [PubMed] [Google Scholar]
- Grubman M. J., Baxt B. Translation of foot-and-mouth disease virion RNA and processing of the primary cleavage products in a rabbit reticulocyte lysate. Virology. 1982 Jan 15;116(1):19–30. doi: 10.1016/0042-6822(82)90399-3. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Ariga H., Anderson C. W., Wimmer E. Expression of a cloned gene segment of poliovirus in E. coli: evidence for autocatalytic production of the viral proteinase. Cell. 1984 Jul;37(3):1063–1073. doi: 10.1016/0092-8674(84)90441-0. [DOI] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Kester W. R., Matthews B. W. Comparison of the structures of carboxypeptidase A and thermolysin. J Biol Chem. 1977 Nov 10;252(21):7704–7710. [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kraut J., Robertus J. D., Birktoft J. J., Alden R. A., Wilcox P. E., Powers J. C. The aromatic substrate binding site in subtilisin BPN' and its resemblance to chymotrypsin. Cold Spring Harb Symp Quant Biol. 1972;36:117–123. doi: 10.1101/sqb.1972.036.01.017. [DOI] [PubMed] [Google Scholar]
- Mak T. W., Colter J. S., Scraba D. G. Structure of the Mengo virion. II. Physicochemical and electron microscopic analysis of degraded virus. Virology. 1974 Feb;57(2):543–553. doi: 10.1016/0042-6822(74)90193-7. [DOI] [PubMed] [Google Scholar]
- McGregor S., Rueckert R. R. Picornaviral capsid assembly: similarity of rhinovirus and enterovirus precursor subunits. J Virol. 1977 Feb;21(2):548–553. doi: 10.1128/jvi.21.2.548-553.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najarian R., Caput D., Gee W., Potter S. J., Renard A., Merryweather J., Van Nest G., Dina D. Primary structure and gene organization of human hepatitis A virus. Proc Natl Acad Sci U S A. 1985 May;82(9):2627–2631. doi: 10.1073/pnas.82.9.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C. In vitro synthesis and assembly of picornaviral capsid intermediate structures. J Virol. 1982 Dec;44(3):900–906. doi: 10.1128/jvi.44.3.900-906.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Rueckert R. R. Evidence for intramolecular self-cleavage of picornaviral replicase precursors. J Virol. 1982 Jan;41(1):244–249. doi: 10.1128/jvi.41.1.244-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnak J. R., Phillips B. A. Picornaviral structure and assembly. Microbiol Rev. 1981 Jun;45(2):287–315. doi: 10.1128/mr.45.2.287-315.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanway G., Hughes P. J., Mountford R. C., Minor P. D., Almond J. W. The complete nucleotide sequence of a common cold virus: human rhinovirus 14. Nucleic Acids Res. 1984 Oct 25;12(20):7859–7875. doi: 10.1093/nar/12.20.7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y. V., Gorbalenya A. E., Kazachkov Y. A., Agol V. I. Encephalomyocarditis virus-specific polypeptide p22 possessing a proteolytic activity: preliminary mapping on the viral genome. FEBS Lett. 1979 Dec 1;108(1):6–9. doi: 10.1016/0014-5793(79)81165-5. [DOI] [PubMed] [Google Scholar]
- Toyoda H., Nicklin M. J., Murray M. G., Anderson C. W., Dunn J. J., Studier F. W., Wimmer E. A second virus-encoded proteinase involved in proteolytic processing of poliovirus polyprotein. Cell. 1986 Jun 6;45(5):761–770. doi: 10.1016/0092-8674(86)90790-7. [DOI] [PubMed] [Google Scholar]




