Abstract
The recruitment of human subjects for clinical trials research is a critically important step in the discovery of new cures for diseases. However, the current recruitment methodologies are inherently inefficient. Considerable resources are expended in efforts to recruit adequate numbers of patient volunteers who meet the inclusion/exclusion criteria for clinical trials. Recruitment is particularly challenging for trials involving vulnerable, psychiatrically disordered groups. We have developed a prototype system, called MindTrial, that is based on an online model to enhance the efficiency and quality of recruitment of patients with psychiatric disorders for clinical research. The intelligent component of the MindTrial system can facilitate highly specific matches between clinical trial criteria and volunteers for self-enrollment of sufficient numbers of patient volunteers. We believe this system is particularly valuable in optimizing recruitment for clinical trial studies for development of new drugs.
Introduction
The recruitment of human subjects for clinical trials research is a critically important step in the discovery of new cures for diseases. Recruitment via the traditional methods of phone-based and face-to-face interviews is inefficient. There is considerable scope for improving on the current paradigm for recruiting. Volunteers are often subjected to redundant questioning. While this might sometimes be useful in double-checking critical criteria for a particular study, a lot of other information that is repetitive is often included as well. This redundancy is one of the factors resulting in the high recruitment cost of clinical trials. Ideally, one should be able to eliminate unsuitable patients or volunteers before initiating expensive screening and evaluation. However, this is often discovered only after considerable time and effort have been invested by both the volunteer subject and the clinical trial personnel. Currently, subjects are recruited for clinical trials on a just-in-time basis. Ideally, one should have prescreened subjects available for trials who have already indicated an interest in volunteering. If detailed information regarding their medical condition is already available, and can be searched with a high degree of specificity, it would increase the probability of obtaining appropriate subjects for clinical trials.
The Volunteer for Vanderbilt Research Program [1] is a good illustration of the benefits of using a website for volunteer initiated recruitment. The system was able to enroll a large number of potential subjects in a relatively short period of time. Minimal information (volunteers choose up to 3 out of 17 disease categories they are interested in) is captured by the website and searches by recruiters are limited to general criteria like age, race, gender and BMI. Since then, several online attempts have emerged [2], some of which focus on specific conditions [3] or demographics [4]. A recent review [5] highlights the emergence of online recruiting as a viable paradigm. There is a major ongoing effort in standardizing the clinical trials BRIDG model [6]. Fink et al [7] introduced an interactive web-based system which helps physicians in finding cancer patients and match them to relevant clinical trials. Embi et al [8] describe a clinical trial alert system which notifies the physician when it finds an eligible patient for an ongoing clinical trial.
We have developed a prototype online system, called MindTrial, that seeks to enhance the efficiency and quality of recruitment of patients with psychiatric disorders for clinical research. Our system relies on existing ontologies like the Medical Subject Headings (MeSH) [9] and SNOMED [10] and our own ontologies that model the clinical trial domain. MindTrial will not only allow one to interface directly with potential volunteers, but also allow interested but busy and research naïve practices to offer access to clinical trials for their patients. We also envision that it might spur interest for such practices to become involved in clinical research. The proposed approach to proactive recruiting is essentially an attempt to include semantics and machine intelligence to facilitate clinical trial recruitment. As a first step towards validating the system, the initial version of the system has been implemented with a focus on Generalized Anxiety Disorder.
Mental illnesses rank as the second most common disease category in which volunteers express interest. Generalized Anxiety Disorder (GAD) is a well-characterized psychiatric disorder defined by excessive anxiety and worry about a number of events or activities, occurring more days than not, for a period of at least six months. This common disorder is found in children/adolescents as well as adults. A search on the US Clinical Trials website (clinicaltrials.gov, Mar 14, 2010) retrieved 734 studies concerning Anxiety Disorders.
The MindTrial System
The key features of the system and the consequent advantages are summarized as follows:
Proactive Recruiting: Groups of potential volunteers with expressed interest in being notified about clinical trials for which they might qualify are characterized in a manner which facilitates matching with appropriate trials.
Personalized Online Recruiting: Factoring in differences in preferences and tendencies of volunteers is likely to lead to higher recruitment.
Retention Modeling: The system design includes the capability to model the probability of successful completion of a clinical trial for each subject.
Comprehensive Modeling of Inclusion and Exclusion Criteria: We dynamically retrieve criteria from diverse sources to maintain an extensive and evolving knowledge base: This will facilitate rapid and accurate assessment of potential matches between trials and subjects
Educational Interface: Ongoing education leading to optimal and updated informed consent.
Community: Forum and chatting features to connect people who have common interests, help share information between them, and build a community.
Personalized Online Recruiting
The MindTrial system provides multiple interfaces (patients/volunteers, recruiters, physicians) and dynamically maps a personalized interface to prospective users depending on their roles and responsibilities. For patients and volunteers, it facilitates proactive recruitment registration and helps recruiters to review comprehensive recruitment status including the number of contacts made, associated demographic information and the number of qualified/disqualified participants for a given study. A secondary goal is to support the design of eligibility criteria and questionnaires for a new study by modifying existing questions.
- Patient Manager: The online screening interface is based on a well defined patient model consisting of demographic information including educational, occupational, socioeconomic and residential information. It can
- Demonstrate that the information provided has been regularly refreshed
- Provide automated and personalized follow-up with both the patient and recruiters
- Provide online registration that is interactive and personalized
- Screen, evaluate, and report results
- Prompt patients regarding follow-up
- Recruiter Manager: The interface aids recruiters in searching for qualified patients/volunteers for a particular study and for reviewing a comprehensive report of recruitment status including the number of qualified/disqualified participants. It can
- Generate a list of potential patients in response to a query containing inclusion/exclusion criteria for a particular study
- Automatically forward information on subjects matching study criteria so that they may be contacted by the appropriate clinical trial team
- Provide data indicating the efficiency of recruitment
- Help to determine most appropriate sites for a given study based on local population of potential patients
Physician/Research Manager: Clinical research personnel can view the questionnaires for a clinical study, update existing criteria or questions, and publish new criteria or questions through the online interface to the question database.
Clinical Trial Ontologies
The knowledge management component will facilitate all stages of the recruitment process including search, analysis and planning. The goal is to support adaptive and intelligent search for potential participants, design of eligibility criteria, and questionnaires for a clinical study. This is made possible by the development of a suite of study ontologies. These ontologies serve to annotate and standardize protocols, and guide criteria and questionnaire design by helping to identify common versus study-specific features. The major categories include demographic information, written consent, substance use history, birth control verification, and previous trial participation. The ontology framework is modular as some subsets (such as demographic data and previous trial participation) are common to the majority of clinical trials while others (such as alcohol-specific questions of substance abuse history) may be specific to a smaller number of studies.
Comprehensive Modeling of Inclusion and Exclusion Criteria
We have used a sample of several hundred clinical trials on GAD from the NIH ClinicalTrials website to derive a canonical set of eligibility criteria. Relevant criteria can be selected from this for a particular study.
Study of unintended/unexpected consequences of inclusion/exclusion criteria can guide the adjustment of the criteria for a potentially better fit to available subjects for clinical research. For instance, if the majority of subjects fail eligibility based on a single criterion, it might be worthwhile to study the criterion and see if it can be altered without affecting the scientific integrity of the study. The intelligent search engine will facilitate the systematic design of the search criteria by suggesting adjustments to eligibility criteria of inclusion and exclusion depending upon extensive analysis of patients, clinical studies and research sites. It is also important to identify differences between those who are eligible and enrolled and those who do not enroll either through attrition during screening or ineligibility. The inclusion and exclusion criteria retrieved from this module will be used for more robust semantic matching in clinical study recruitment.
Intelligent Matchmaking
The MindTrial system provides intelligent matchmaking features for discovering potential participants in clinical trials. Two kinds of search interfaces are designed for selecting patients or potential volunteers. One is based on a detailed fine-grained checklist view where fields identical to those in the questionnaire can be selected as inclusion (desired) or exclusion (NOT desired) criteria. In addition to exact matches for the query, near matches are also displayed, ranked by semantic and information theoretic considerations. Some important inclusion and exclusion criteria for a given domain that are used for semantic matchmaking will be continuously retrieved from existing clinical trial resources such as clinicaltrials.gov. The second kind of query interface is based on summarized queries and reasoning that are expanded by clinical trial ontologies and computed on a subset of volunteer responses. For example, if a recruiter types in “Generalized Anxiety Disorder”, this will be mapped to the appropriate set of questions (e.g., self-reported diagnosis, medical record diagnosis, suggestive answers to relevant questions) and the matching subjects reported together with corresponding confidence scores. The rules to calculate the confidence scores will depend on the subset of questions (ranging from linear sums to decision trees specified by the clinical expert Investigators).
Personalized Requirement Process
Personalized recruitment facilitates intelligent selection of appropriate strategies for recruitment, at the level of a trial or for individual subjects. Ideally, each subject interacting with the system should have a personalized and optimized experience. For instance, some might prefer a graphical interface and others a textual/chart interface; some might prefer Spanish to English. Some, while wishing to participate in clinical trials, may not have the patience to complete detailed questionnaires. It might be a better strategy to obtain minimal information by dynamically switching to an abbreviated questionnaire that still gathers useful input. In turn, questionnaire modules that are either left incomplete or marked in an inconsistent fashion might point to a need to redesign the questionnaire. This level of dynamic optimization is achievable by the meticulous compilation of statistics, not just on subject data, but also interaction patterns of potential subjects with the system. The accruing data can subsequently be mined by algorithms to improve the overall effectiveness of the clinical trial recruitment process. For example, the system will have the capacity to learn from negative “dropout” and positive “survivor” cases.
Disease-specific Interactive Questionnaire
A general set of introductory questions common to all volunteers shall contain a subset of disease-specific probing questions which launch appropriate sets of detailed questions. These will be based on standard criteria like the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV). The generation of the questionnaire shall be interactive to ensure that i) questions are not redundant and ii) sufficient details are elicited on the specific medical background of the volunteer. General questions will cover basic demographic information (e.g., name, address), medical corroboration information (e.g., names of psychiatrists, physicians and facilities visited, past tests performed), trigger questions to suggest detailed questionnaires to be used (e.g., known diagnoses, history of suicidal behavior or disorderly conduct), and questions related to consent for being contacted and/or access to clinical records. Appropriate disease-specific questions will be triggered based on the responses to the general questionnaire. These will be framed as checklists and multiple choice questions with the aim of capturing a rich amount of detail without resorting to free text input. Further, this will allow the detection of inconsistencies in volunteer responses to reduce the probability of having the system corrupted by spurious data. Internally, volunteer responses will be mapped to the Clinical Trial ontologies to validate eligibility for participating in a clinical study. Ideally, the online administration of well-validated psychometric testing could be performed. These would be routinely and frequently administered to samples of the given diagnostic group and therefore provide a statistically-reliable measure of psychometric characteristics of the diagnostic group populating the database at any given time point. It is important to note that in contrast to many of the psychometric instruments that are meant to be administered by a professional, MindTrial aims only to arrive at a tentative diagnosis of the mental health status, and instead focuses on the detailed characterization of typical eligibility criteria.
Education Component
Patient and caregiver education is an important part of efforts for optimizing recruitment, retention and meeting the highest standards of informed consent. This is an ongoing process with the MindTrial system and over time should also lead to better compliance and knowledge of therapeutic options for patients and their families. A number of health related websites are available, but none that we are aware of are dedicated to integrating education regarding drug development research matters with helping to connect patients and families with participation in appropriate clinical trials. This module will be composed of interactive material with an emphasis on the nature of the new drug development process including the benefits derived by society, the essential participation of normal healthy and patient volunteers, a generic “walk-through” regarding what volunteers can expect when involved in CTs and a general discussion of risks/benefits. Specifically, educational material in multiple formats (text, animation, and video) shall be made available on the website to adapt to differing learning styles followed by a self-administered test to demonstrate comprehension of what participation in clinical trials in general and the MindTrial system in particular entails.
Development of Prototype
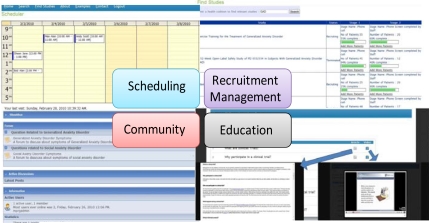
A prototype system has been developed that can be accessed [11]. The primary objectives of the MindTrial System are: 1) Semantic searching based on the Clinical Trial Ontologies and 2) Presenting search results using multiple views such as text, map, and chart views. Figure 1 shows the search interface with results in all 3 views. The text view provides detailed information, the map view shows the search results mapped on the Google map interface as markers, with each marker sub-grouped according to age group. Figure 2(a) shows an event calendar which helps the recruiter to check the subject’s schedule and assign an appointment time for an interview. The system provides means through which a subject can get information about clinical trials. Information is provided not only in the form of text articles but also through YouTube videos. Figure 2(b) shows the web interface which a recruiter can use to find information about ongoing studies and assign patients to, and to view various phases for a particular study. Figure 2(c) shows our forum interface. With the help of forums, participants can interact with others and ask questions about an ongoing clinical study with the recruiters, etc. Our main goal in implementing forums is to create a social networking community of our system users where users can help each other. Figure 2(d) shows a screen shot of our education interface.
Figure 1.
Screenshots of the MindTrial Search Interfaces
Figure 2.
MindTrial Interfaces (a) Scheduling (b) Recruitment Management (c) Community (d) Education
The MindTrial system is implemented as Web services with Microsoft SQL on the .NET Platform. We also have used existing web services such as GoogleMaps and Microsoft chat controls. For security, user authentication and security role registration for application is implemented. The clinical trial ontologies are modeled using the Protégé Ontology editor. The Semantic Web Rule Language (SWRL) is used to specify questionnaire constraints described in the protocol ontologies and SPARQL for query interfaces based on the ontologies. The use of ontologies allows for reasoning over data and integrating heterogeneous data sources like eligibility criteria and patient profiles. Additional components will provide for frequent updating of the knowledge base and ensure that industry IT security and HIPAA confidentiality standards are met.
Discussion
Unlike existing online systems like the Vanderbilt Program and IAN, the MindTrial system is a more sophisticated and subscription-based approach to enhance the quality and efficiency of recruitment for clinical trials. The online nature and personalized interface of the system will facilitate recruitment and retention of populations which may be under-represented, or particularly difficult to recruit/retain for clinical trials, such as racial minorities, women and pediatric groups. From a technical perspective, it might be hard to get sufficient numbers of volunteers enrolled. This is a critically important issue that needs careful planning. Since our system is implemented in a service fashion, customized to each individual, and minimally redundant, we expect that most volunteers will find it a relatively pleasant experience.
Conclusions
In this paper, we presented the MindTrial system that focuses on enhancing recruitment and providing an ontology based system for clinical trials on mental disorders. We believe that potential clients will find this system to be of use in determining the feasibility design and execution of trials, based on the availability of populations likely to meet inclusion/exclusion criteria. We also feel that the public profile of clinical research will be elevated by a proactive and well-structured process with an emphasis on patient education and the informed consent process, in contrast to the current paradigm which is heavily dependent upon mass media advertising typically performed ad hoc as the need to recruit for a new study arises.
Acknowledgments
This work is funded by NIH grant 1R43MH085372-01A1.
References
- [1].Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program. J Am Med Inform Assoc. 2005;12(6):608–13. doi: 10.1197/jamia.M1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chintan Patel, Sharib Khan, Karthik Gomadam. TrialX : Using semantic technologies to match patients to relevant clinical trials based on their Personal Health Records. Proc. of the International Semantic Web Conference (ISWC) 2009.
- [3].Autism Network (IAN) accessible at http://www.ianproject.org/
- [4].Army of Women accessible at http://www.armyofwomen.org/
- [5].Malorye Allison. Can web 2.0 reboot clinical trials? Feature. Nature Biotechnology. 2009;27:895–902. doi: 10.1038/nbt1009-895. [DOI] [PubMed] [Google Scholar]
- [6].BRIDG model accessible at http://www.bridgproject.org
- [7].Fink E, Kokku PK, Nikiforou S, Hall LO, Goldgof DB, Krischer JP. Selection of patients for clinical trials: an interactive Web-based system. Artif Intell Med. 2004;31:241–254. doi: 10.1016/j.artmed.2004.01.017. [DOI] [PubMed] [Google Scholar]
- [8].Embi PJ, Jain A, Clark J, Harris CM. Development of an electronic health record-based Clinical Trial Alert system to enhance recruitment at the point of care. AMIA Annu Symp Proc. 2005:231–235. [PMC free article] [PubMed] [Google Scholar]
- [9].Nelson SJ, Schopen M, Savage AG, et al. The MeSH Translation Maintenance System: Structure, Interface Design, and Implementation. Medinfo. 2004;2004:67–9. [PubMed] [Google Scholar]
- [10].Spackman KA, Campbell KE, Cote RA. SNOMED RT: a reference terminology for health care. Proc AMIA Annu Fall Symp. 1997:640–4. [PMC free article] [PubMed] [Google Scholar]
- [11].MindTrial System accessible at www.mindtrial.com