Abstract
Aims
The left ventricular phenotype of idiopathic dilated cardiomyopathy (DCM) can appear similar in paediatric and adult patients. However, the aetiology of paediatric DCM is usually idiopathic, and often leads an aggressive clinical course. A structural underpinning of DCM is extracellular matrix changes, which are determined by a balance between matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs (TIMPs). This study tested the hypothesis that different MMP/TIMP profiles occur in paediatric and adult DCM patients.
Methods and results
Left ventricular samples from paediatric (age 9 ± 5 years; n = 10) and adult (age 62 ± 3 years; n = 20) DCM (at time of transplant) were subjected to an MMP/TIMP multiplex array and immunoassay in order to measure the MMP subclasses; collagenases (MMP-8, -13), gelatinases (MMP-2, -9), stromelysin/matrilysin (MMP-3, -7), membrane type (MT1-MMP), as well as for the four known TIMPs. MMP-8 and -9 levels increased by over 150% (P < 0.05), whereas MMP-3 and -7 levels decreased by over 30% (P < 0.05) in paediatric DCM when compared with adult DCM. TIMP-1 and -2 levels increased two-fold (P < 0.05), but TIMP-3 fell by 41% (P < 0.05) in paediatric DCM. Myocardial levels of specific interleukins (IL-1beta, IL-2, IL-8) were increased by approximately 50% in paediatric DCM.
Conclusions
These unique findings demonstrated that a specific MMP/TIMP profile occurs in paediatric DCM when compared with adult DCM, and that local cytokine induction may contribute to this process. These distinct differences in the determinants of myocardial matrix structure and function may contribute to the natural history of DCM in children.
Keywords: Cardiomyopathy, Extracellular matrix, Matrix metalloproteinase, Paediatrics
Introduction
Dilated cardiomyopathy (DCM) is the most common form of cardiomyopathy in both adults and children. While sharing a similar phenotype of left ventricular (LV) dilatation and systolic dysfunction as adults, children with DCM have an ominous clinical course with aggressive progression to congestive heart failure and poor prognosis.1,2 While ischaemic heart disease, systemic hypertension, and valvular pathology are the primary aetiologies of DCM in adults, a large majority of paediatric DCMs are idiopathic. Nonetheless, the clinical features of paediatric DCM are not unlike that seen in adults: a chronic and progressive alteration in myocardial structure that leads to myocardial systolic dysfunction, arrhythmias, congestive heart failure, and sudden death.
Maladaptive myocardial remodelling is the hallmark of DCM, and significant changes in the myocardial extracellular matrix (ECM) are known to facilitate the structural and functional changes in adult DCM.3,4 The maintenance of ECM homeostasis is coordinated through the proteolytic degradation and synthesis of ECM components by the matrix metalloproteinases (MMPs) and their endogenous tissue inhibitors (TIMPs). Increased MMP and decreased TIMP levels within the myocardium, resulting in a more favourable state for ECM degradation, has been shown to exist in ischaemic, viral, and idiopathic DCM in adults.5–11 However, the magnitude and profiles of myocardial MMPs and TIMPs in paediatric DCM has not been studied. Accordingly, the objective of this study was to quantify and compare myocardial MMP and TIMP levels in children and adults with idiopathic DCM.
Methods
Patients
Human LV myocardial samples were obtained from explanted hearts of patients undergoing orthotopic heart transplantation secondary to idiopathic DCM. Twenty adults (mean age: 62 ± 3 years, range: 57–68 years) and 10 children (mean age: 9 ± 5 years, range: 2–14 years) were enrolled. The male:female distribution was 13:7 in the adult group and 6:4 in the paediatric. All patients underwent standard cardiomyopathy evaluation, including echocardiography, left and right heart catheterization, coronary angiography, and myocardial biopsy with both light and electron microscopic examinations. A comprehensive viral workup including parvoviral, enteroviral, and Epstein-Barr viral PCR was performed. In all patients, the diagnosis of idiopathic DCM was established based on normal or minimal coronary artery disease on angiography, and the absence of valvular or pericardial diseases. Patients with confirmed myocarditis, hypertrophic or restrictive cardiomyopathy, or structural heart defects were excluded. This study also did not include patients with a history of haematologic, neoplastic, or primary renal/liver diseases, or those with acute or chronic infections, autoimmune diseases, and patients receiving anti-inflammatory agents. There was no history of any familial forms of DCM, and there was no history of genetic malformations of the musculo-skeletal, cardiovascular, or other major systems. Left ventricular specimens were subjected to routine light microscopic examination using haematoxylin–eosin staining. These studies consistently revealed myocyte hypertrophy, but a normal muscle fascicle architecture and geometry. There was no evidence of significant vasculitis or a myocardial inflammatory infiltrate. Left ventricular specimens were subjected to electron microscopy and the myofibrillar structure, mitochondria and intercalated discs appeared normal, with no inclusion bodies or other abnormal intracellular or extracellular depositions. Using immunofluorescence, negative staining for IgG, IgM, C3, fibrin, C1q, and albumin was obtained. Finally, there were no abnormal staining patterns when LV specimens were subjected to immunolocalization for human leukocyte antigen – disease resistance.
The LV ejection fractions were equivalent between the two groups (adult: 19 ± 8% vs. paediatric: 24 ± 7, P = 0.2). Parental or patient consents were obtained for all myocardial samples used in the study, and the protocol was approved by the Medical University of South Carolina and Columbia University Institutional Review Boards for Human Research (HR# 8076, MUSC). At the time of the cardiac transplantation procedure, the explanted heart was immediately placed in iced saline, and full thickness sections of the LV free wall were snap frozen in liquid nitrogen and stored at −70°C until use.
Matrix metalloproteinase, tissue inhibitor of matrix metalloproteinase, and interleukin quantification
Representative classes of MMP species known to degrade ECM and basement membrane components were studied, including collagenases (MMP-8, -13), gelatinases (MMP-2, -9), stromelysin/matrilysin (MMP-3, -7), and membrane type (MT1-MMP). The four known TIMPs (TIMP-1, -2, -3, -4) were also studied. Myocardial abundance of MMP-8, -2, -9, -3 and all four TIMPs were quantified by a commercially available multiplex suspension array (MSA) using highly sensitive and specific antisera following manufacturer's recommendations (R&D Systems, Minneapolis, MN, USA).12 Due to the composition of the MSA system, MMP-7 and -13 levels were not analysed by MSA, but rather by immunoblotting. Since, MT1-MMP is a transmembrane protease,4,9 then immunoblotting was performed in myocardial extracts for this MMP type. Using the same MSA approach, interleukins 1-beta (IL-1b), IL-2, IL-6, and IL-8 (Human MAP Base Kit LUH000, R&D Systems) were measured.
Multiplex suspension array
Myocardial samples were homogenized in ice-cold extraction/homogenization buffer [buffer volume used is 1:6 w/v; containing 10 mM cacodylic acid pH 5.0, 0.15 M NaCl, 20 mM ZnCl, 1.5 mM NaN3, and 0.01% Triton X-100 (v/v)]. The homogenate was then centrifuged (800 g, 10 min, 4°C) (model 5810 or 5417c, Eppendorf, Westbury, NY, USA) and the supernatant removed to a fresh tube and stored on ice. Protein concentrations of the myocardial extracts were determined by the Bradford method (Bio-Rad Protein Assay, Hercules, CA, USA), and the extracted samples were aliquoted and stored at −80°C until used in subsequent assays. Tissue homogenate, consisting of 50 μg of total protein, were assayed for MMP-8, -2, -9, -3, TIMPs 1-4, IL-1b, and IL-2,-6,-8 using an enzyme-linked MSA (Luminex X500002Z15; Bio-Rad, Hercules, California). The identification and quantification of the analyte/bead complexes were determined by flow cytometry with dual excitation lasers. The excitation and emission wavelengths were 532 and 575 nm, respectively. Each analyte concentration was calculated from an analyte-specific five-parameter logistic calibration equation using standards that were included in each assay (Bio-Plex Manager Software 4.1.1). The values obtained using MSA fall within the linear portion of the calibrations curves, and are within the same range and coefficient of variation (less than 20%) for past immunoassay approaches.13–15 The MMP and TIMP fluorescent readings were first converted to absolute values and then reported as picograms/mg (pg/mg) of initial wet weight of myocardium. The interleukin values were reported in terms of fentograms/mg (fg/mg) of wet weight of myocardium.
Myocardial immunoblotting
The relative abundance of MMP-7, 13, and MT1-MMP were examined by quantitative immunoblotting, as described previously.6 Briefly, following electrophoretic separation, the proteins were transferred to a nitrocellulose membrane and probed with antisera (0.4 µg/mL) corresponding to MMP-7 (AB8117, Chemicon), MMP-13 (AB8120, Chemicon), and MT1-MMP (AB815, Chemicon). After incubation with a secondary antibody, immunoreactive signals were detected by chemiluminescence (Western Lightning Chemiluminescence Reagent Plus, Perkin Elmer). Negative controls, which omit the specific antisera, were utilized in order to ensure specificity of the immunoreactive signal. Molecular weight markers were used to determine correct weight of the MMP immunoreactive signal. The immunoblots were analysed by densitometric methods, and relative myocardial MMP abundance reported as integrated optical density.
Myocardial collagen content
Left ventricular samples of known weight were homogenized in ice-cold extraction/homogenization buffer [buffer volume used is 1:6 w/v; containing 10 mM cacodylic acid pH 5.0, 0.15 M NaCl, 20 mM ZnCl, 1.5 mM NaN3, and 0.01% Triton X-100 (v/v)] and centrifuged (13 000 g, 10 min, room temperature). The supernatant was collected and protein concentration determined (BCA assay, Cat# 23225, Pierce Thermo Scientific, Rockford, IL, USA). Left ventricular extracts (20 μg protein) and collagen standards (0.25–32 μg), were placed in a 96-well microtiter plate, dried and stained with 0.1% Sirius red (F3BA in picric acid (w/v) for 1 h). The wells were then destained briefly with 250 μL of 10mM HCl, and washed with 250 μL of 0.1 N NaOH for 5 min. The resultant absorbance was determined (540 nm, VersaMax, Molecular Devices, Sunnyvale, CA, USA). Using the standard curve generated from the known collagen standards, the relative absorbance was first converted to absolute collagen content and then expressed as collagen content per wet weight of myocardium (μg/mg wt wt).
Data analysis
Myocardial MMP, TIMP, and cytokine profiles, and collagen values were compared between the adult and paediatric idiopathic DCM groups by using a Student's t-test (Stata Intercooled, v8.0). Results were presented as mean ± SEM. Values of P < 0.05 were considered to be statistically significant.
Results
Myocardial matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase profiles in adult and paediatric dilated cardiomyopathy
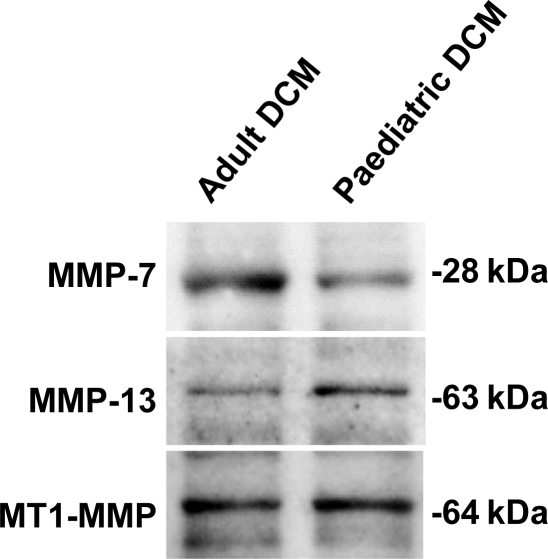
For those analytes that required an immunoblotting approach, representative immunoblots for the myocardial samples obtained from adult and paediatric samples for MMP-7, -13, and MT1-MMP are shown in Figure 1. The quantitative analyses from the immunoblotting approach as well as the MSA approach are summarized in Table 1. This table has divided the MMP profiles based upon the taxonomy classifications. For the collagenases, MMP-8 was significantly higher in the paediatric DCM group. With respect to the gelatinases, MMP-9 levels were significantly increased in paediatric DCM. In marked contrast, stromelysin and matrilysin, MMP-3 and -7, respectively, were significantly reduced in the paediatric DCM group. Membrane type-1 MMP, MT1-MMP levels appeared increased in the paediatric DCM group, but this did not reach statistical significance.
Figure 1.
Immunoblotting of myocardial MMP-13, -7, and MT1-MMP was performed from left ventricular myocardial samples from adult and paediatric idiopathic dilated cardiomyopathy. MMP-7 abundance was lower in the paediatric group, and the quantitative results are summarized in Table 1.
Table 1.
Human left ventricular myocardial MMP and TIMP abundance by multiplex suspension array or immunoblotting
| Family/class | MMP species | Adult DCM | Paediatric DCM | P-value |
|---|---|---|---|---|
| Collagenase | MMP-8 (pg/mg) | 0.45 ± 0.07 | 0.75 ± 0.18 | <0.05 |
| MMP-13 (IOD) | 85.2 ± 7.4 | 100.8 ± 10.4 | 0.11 | |
| Gelatinase | MMP-2 (pg/mg) | 0.93 ± 0.19 | 2.4 ± 1.3 | 0.15 |
| MMP-9 (pg/mg) | 0.54 ± 0.08 | 0.98 ± 0.25 | <0.05 | |
| Stromelysin/matrilysin | MMP-3 (pg/mg) | 0.016 ± 0.003 | 0.003 ± 0.000 | <0.05 |
| MMP-7 (IOD) | 252.3 ± 20.4 | 169.0 ± 16.9 | <0.05 | |
| Membrane type | MT-1-MMP (IOD) | 192.1 ± 27.3 | 247.5 ± 66.9 | 0.18 |
| TIMP | TIMP-1 (pg/mg) | 0.27 ± 0.03 | 0.56 ± 0.16 | <0.05 |
| TIMP-2 (pg/mg) | 4.79 ± 0.84 | 7.50 ± 1.90 | 0.07 | |
| TIMP-3 (pg/mg) | 0.245 ± 0.075 | 0.102 ± 0.044 | <0.05 | |
| TIMP-4 (pg/mg) | 0.012 ± 0.003 | 0.008 ± 0.002 | 0.13 |
MMP-13, -7, and MT1-MMP by immunoblotting, and reported as integrated optical density (IOD).
Myocardial cytokines and collagen content in adult and paediatric dilated cardiomyopathy
In the present study, each of the four known TIMPs were assessed by the quantitative MSA approach and are summarized in Table 1. TIMP-1 abundance was significantly increased in the paediatric DCM group. TIMP-2 appeared increased in the paediatric DCM group, but this did not reach statistical significance. In contrast, myocardial TIMP-3 abundance was significantly lower in the paediatric DCM group. TIMP-4, which has a restricted expression profile, which includes the cardiovascular system,4,16,17 appeared unchanged between adult and paediatric DCM.
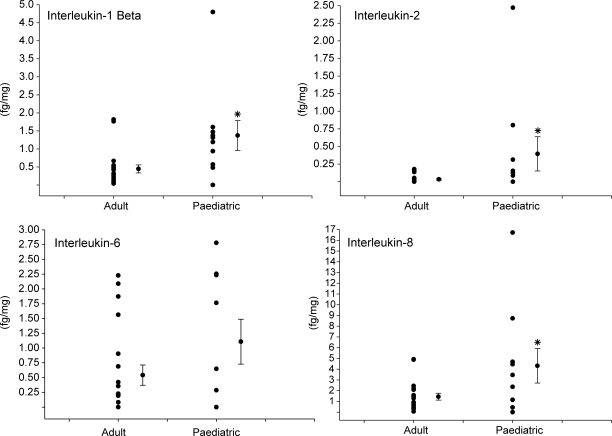
The relative myocardial levels for the interleukins IL-1b, IL-2, IL-6, and IL-8 were determined in the adult and paediatric DCM samples and the results are summarized in Figure 2. There were detectable levels for these interleukins in both the adult and paediatric myocardial samples, but the heterogeneity for the myocardial cytokine levels was more pronounced in the paediatric DCM samples. Nevertheless, the levels for IL-1b, IL-2, and IL-8 were significantly increased in paediatric DCM.
Figure 2.
Left ventricular myocardial levels of the interleukins, IL-1b, IL-2, IL-6, and IL-8 were quantified by high sensitivity multiplex suspension array in both adult and paediatric dilated cardiomyopathy (DCM) samples. Detectable levels were obtained in both sample sets, but IL-1b, IL-2, and IL-8 were significantly increased in paediatric DCM. The distribution of absolute values, in femtograms/mg wet weight of myocardium (fg/mg) is shown along with the mean and standard error of the mean (SEM). *P < 0.05 vs. adult DCM.
Finally, myocardial collagen content was measured in both adult and paediatric DCM samples using a biochemical assay. Left ventricular myocardial collagen content was higher in adult DCM when compared with paediatric DCM (44.9 ± 8.1 vs. 27.8 ± 5.3 μg/mg wt wt, P < 0.05).
Discussion
There have been a large number of studies in adult cardiac disease states that have identified abnormalities in MMP and TIMPs within the myocardium.3–11,16 These previous studies have identified that changes in all 4 classes of MMPs, the collagenases, gelatinases, stromelysin/lysins, and the membrane type MMPs can occur within the myocardium of adults with severe LV dysfunction. Moreover, these past studies have identified that changes in TIMP levels occur in the myocardium, and that changes in the balance between myocardial MMPs and TIMPs which may favour extracellular matrix remodelling.4–6,8,16,18 However, there is limited information on MMP/TIMP profiles in paediatric patients with LV dysfunction, and there has been no previous systematic study which has profiled MMP/TIMP levels between adult and paediatric DCM. The present study addressed this issue through quantitation of MMP/TIMP profiles in both adult and paediatric forms of idiopathic DCM which resulted in two novel observations. First, the collagenase MMP-8 and the gelatinase MMP-9 were significantly increased in paediatric DCM when compared with adult DCM, but MMPs of the stromelysin/lysin class were substantially reduced. Second, TIMP-1 levels were increased, but TIMP-3 levels substantially reduced in paediatric DCM. These findings demonstrated that fundamental differences in myocardial extracellular degradation pathways exist between paediatric and adult DCM, which may explain the tendency for a more accelerated LV remodelling process in paediatric idiopathic DCM.
Differential matrix metalloproteinase profiles in paediatric vs. adult dilated cardiomyopathy
Past studies in adult cardiac disease states, such as DCM, have identified abnormalities in the expression and activity of myocardial MMPs as well as an association between the progression of LV remodelling.3–5,7–11 One of the first reports of abnormalities in MMP profiles in adult DCM was by Gunja-Smith et al.,7 whereby increased MMP zymographic activity was associated with abnormalities in collagen cross-linking and overall matrix structure. Furthermore, increased plasma levels of collagen telopeptides indicative of increased matrix turnover have been reported in adult patients with DCM.19 Finally, a large number of past studies have identified increased levels of certain MMPs such as the gelatinases, the matrilysins, and the membrane-type MMPs in adult DCM.5,9,20–22 While associative, these past clinical studies have suggested that changes in myocardial MMP profiles can potentially accelerate the LV remodelling process, particularly LV dilation which is the architectural milestone in DCM. However, animal model systems of DCM have provided a more robust cause–effect relationship between LV remodelling and myocardial MMP induction.4,6,23 However, it must be recognized that myocardial MMP induction is not uniform, and that specific MMP sub-types can be induced which are specific to the underlying aetiology of the LV remodelling process. For example, different profiles of MMPs exist in viral myocarditis as opposed to idiopathic DCM.5,9 In addition, the magnitude of changes in myocardial MMP types can be different in ischemic vs. non-ischemic DCM.9–11,21,24 Thus, it could be postulated, but yet proven, that the type and magnitude of myocardial MMP profiles would be different in adult when compared with paediatric DCM. The present study provides the first quantitative data to support this postulate in that in idiopathic forms of DCM in children, a much different portfolio of myocardial MMPs emerge when compared with adult idiopathic DCM. In addition, local levels for the cytokines IL-1b, IL-2, and IL-8 were increased in paediatric DCM suggesting a differential localized MMP induction pathway exists when compared with adult DCM. This observation underscores the heterogeneity of MMP expression that can occur within the myocardium, and that uniquely different induction pathways and downstream substrates exist for this diverse family of proteases.
One of the first surprising observations from the present study was the robust increase in the collagenase MMP-8 and the gelatinase MMP-9 in paediatric DCM when compared with adult DCM. This relative increase in these MMP types is unlikely due to differences in the magnitude of LV systolic dysfunction as the LV ejection fractions were equivalent between groups. Rather, this difference in relative MMP-8 and MMP-9 levels likely reflect amplified and/or differential induction pathways for these MMP types exist in the paediatric myocardium. Both MMP-8 and MMP-9 are most often associated with inflammatory processes and have been shown to acutely and robustly increase in adult patients following myocardial infarction and cardiac surgery.13–15,25 However, in the present study, the elevation of these MMP types was unlikely to be an acute inflammatory process due to the chronic nature of the DCM process, and also unlikely to be secondary to a known infectious process, as a full screening panel in these patients was negative. However, despite the absence of any histopathological evidence of an inflammatory response, interleukin levels such as IL-1b, IL-2, and IL-8 were increased within the paediatric DCM samples. Kindermann et al.26 demonstrated that a low-level chronic inflammation can be present in DCM patients with suspected myocarditis. These results suggest that localized cytokine signalling pathways may be upregulated in paediatric DCM, which in turn would cause an amplified induction of these MMP types. There are similarities in transcription factor binding sites on the promoter region of both MMP-8 and MMP-9,3,4,27 which would suggest that upstream signalling pathways could play a predominant role in the robust increase in these MMP types with paediatric DCM. In the present study, the myocardial levels of the gelatinase MMP-2 were similar between adult and paediatric DCM, and the promoter region for MMP-2 is dissimilar to that of MMP-8 and MMP-9.4,25 In addition, MMP-3 and MMP-7 were actually reduced in paediatric DCM. This further underscores that differential induction pathways likely exist for certain MMP types between paediatric and adult DCM. However, it must be recognized that in the present study, the total levels of MMP types were examined in myocardial homogenates, and therefore whether and to what degree changes in certain MMP types may be reflective of net proteolytic activity remains to be established. The present study identified that total myocardial collagen content was lower in paediatric DCM samples when compared with adult DCM. However, whether this is actually reflective of increased degradation remains to be established. The present study did attempt to address this issue through examination of a critical post-translational regulatory point in overall MMP activity—the TIMPs.
Differential tissue inhibitor of matrix metalloproteinase profiles in paediatric vs. adult dilated cardiomyopathy
In the present study, myocardial TIMP levels did not change in a uniform fashion and were distinctly different in paediatric DCM when compared with adult DCM. While the relative binding affinity of TIMPs to different active MMPs can be dependent upon assay conditions, it appears that TIMP-1 binds with less efficiency to MT-MMPs.4,28 Thus, the relative increase in TIMP-1 myocardial levels in paediatric DCM does not necessarily imply a net reduction in overall matrix proteolytic activity. Furthermore, there is now conclusive evidence that TIMPs bind to specific domains on inactive, proMMPs.4,28 One of the most studied with respect to this interaction is that of TIMP-2 binding to proMMP-2.4,27 In this instance, a proMMP-2–TIMP-2 complex is formed which in turn can bind to a MT1-MMP dimer complex. This in turn causes the formation of a tetrameric structure which ultimately leads to proteolytic processing and activation of the bound MMP-2 molecule. Therefore, the relatively increased levels of TIMP-2 which were observed in paediatric DCM may actually promulgate increased MMP activation. Indeed, the reduced LV myocardial collagen content in paediatric DCM when compared with adult DCM which was observed in the present study would favour this postulate. In the present study, TIMP-3 myocardial levels were reduced in paediatric DCM, which may result in several biological effects. First, TIMP-3 binds directly to extracellular matrix proteins and thereby can provide a means for stabilizing MMP–TIMP complexes within the interstitial space.4,28 Indeed transgenic deletion of TIMP-3 in a murine construct caused LV myocardial matrix remodelling and ultimately LV dysfunction.18 TIMP-3 may also interfere with local myocardial cytokine processing, which in turn may influence MMP induction pathways.4,28 Thus, the two-fold reduction in TIMP-3 myocardial levels with paediatric DCM when compared with adult DCM may significantly affect matrix stability as well as local signalling cascades. Indeed, the present study demonstrated that local myocardial levels of IL-1b, IL-2, and IL-8 were increased in the paediatric DCM samples. The reduced TIMP-3 levels coupled by elevated interleukin levels would potentially cause increased local cytokine processing and signalling within the paediatric DCM samples. Finally, TIMPs can affect non-matrix-related processes such as cell growth and survival.4,27,29 Thus, the discordant changes in relative TIMP-1, -2 , -3, and -4 levels observed in paediatric DCM may result in a number of pleiotropic effects on both cellular and extracellular processes which in turn would promulgate adverse LV remodelling.
Limitations and future directions
One fundamental limitation of the present study is that MMP/TIMP comparisons were made between paediatric and adult DCM, and a true referent control group was not included. While we have developed an approach to obtain age-matched ‘normal' LV myocardial samples from adults through myocardial biopsies at the time of elective coronary revascularization,30 obtaining referent normal paediatric LV myocardial samples would be problematic, if not unethical. Thus, comparisons between paediatric DCM and normal referent LV samples were not performed. Tayebjee et al.31 reported a slight negative correlation between plasma TIMP-1 and TIMP-2 with age, and no significant correlation between plasma MMP-2 and MMP-9 with age. Thus, age-dependent changes in MMP and TIMP levels independent of the DCM process cannot be ruled out in the present study. However, this type of analysis was not the main thrust of the study, which is to elucidate a specific, and differential, MMP and TIMP profile in paediatric DCM when compared with the adult form of this disease. The present study did demonstrate unique differences in relative MMP and TIMP levels in paediatric DCM which in and of itself may hold both biological and clinical significance. First, this study demonstrated that certain MMP and TIMP types, which can directly affect structure and function, were altered in a unique pattern in children with DCM from that of adult DCM. Second, it has been demonstrated in adult cardiovascular disease states that plasma MMP/TIMP profiling can be associated with the degree of LV remodelling.25,29,31,32 Future directions of investigation include establishing the longitudinal plasma MMP/TIMP profiles in children with DCM as potential biomarkers for diagnosis and disease progression.
While the clinical screening and histopathological studies excluded major genetic mutations and active myocarditis as the aetiology of the paediatric DCM samples included in the study, this does not exclude the fact that unrecognized mutations which would favour a more aggressive MMP induction may exist. Thus, one potential hypothesis that can be developed based upon the results from the present study is that there is a potential genetic component in those patients with paediatric DCM which would specifically amplify MMP transcription. Indeed, specific MMP polymorphisms have been identified in patients, which result in increased levels of certain MMP types and are associated with cardiovascular disease progression.33 Whether and to what degree specific MMP/TIMP gene polymorphisms occur in paediatric patients with DCM, which in turn would favour a more aggressive matrix proteolytic profile, remains to be established.
Summary
Paediatric and adult idiopathic DCM share a similar disease phenotype: LV dilatation and systolic dysfunction as a result of maladaptive LV myocardial remodelling. However, in addition to an earlier onset of disease, paediatric patients have an aggressive clinical course and poor outcomes. In the present study, paediatric idiopathic DCM patients were found to manifest robust increases in myocardial levels of certain classes of MMPs, which was associated with unique changes in TIMPs when compared with adult DCM. The relative comparisons of MMPs and TIMPs in adult DCM to that of the present study are summarized in Table 2. While additional studies are necessary, these distinct differences in determinants of myocardial matrix structure and function may contribute to the more progressive nature of DCM in children and may be potentially useful as biomarkers for diagnosis and prognosis in paediatric DCM.
Table 2.
Relative magnitude of left ventricular MMP and TIMP levels in adult and paediatric dilated cardiomyopathy
Funding
This work was supported by NIH grants HL059165-09, HL057952-08, a Merit Award from the Veterans’ Affairs Health Administration and the Children's Cardiomyopathy Foundation, USA.
References
- 1.Komajda M, Jais JP, Reeves F, Goldfarb B, Bouhour JB, Juillieres Y, Lanfranchi J, Peycelon P, Geslin P, Carrie D, Grosgogeat Y. Factors predicting mortality in idiopathic dilated cardiomyopathy. Eur Heart J. 1990;11:824–831. doi: 10.1093/oxfordjournals.eurheartj.a059803. [DOI] [PubMed] [Google Scholar]
- 2.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 3.Deschamps AM, Spinale FG. Pathways of matrix metalloproteinase induction in heart failure: bioactive molecules and transcriptional regulation. Cardiovasc Res. 2006;69:666–676. doi: 10.1016/j.cardiores.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 5.Cheung C, Luo H, Yanagawa B, Leong HS, Samarasekera D, Lai JC, Suarez A, Zhang J, McManus BM. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in coxsackievirus-induced myocarditis. Cardiovasc Pathol. 2006;15:63–74. doi: 10.1016/j.carpath.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Coker ML, Thomas CV, Clair MJ, Hendrick JW, Krombach RS, Galis ZS, Spinale FG. Myocardial matrix metalloproteinase activity and abundance with congestive heart failure. Am J Physiol. 1998;274(5 Pt 2):H1516–H1523. doi: 10.1152/ajpheart.1998.274.5.H1516. [DOI] [PubMed] [Google Scholar]
- 7.Gunja-Smith Z, Morales AR, Romanelli R, Woessner JF., Jr Remodeling of human myocardial collagen in idiopathic dilated cardiomyopathy. Role of metalloproteinases and pyridinoline cross-links. Am J Pathol. 1996;148:1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 8.Spinale FG. Matrix metalloproteinases: regulation and dysregulation in the failing heart. Circ Res. 2002;90:520–530. doi: 10.1161/01.res.0000013290.12884.a3. [DOI] [PubMed] [Google Scholar]
- 9.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 10.Tziakas DN, Chalikias GK, Papaioakeim M, Hatzinikolaou EI, Stakos DA, Tentes IK, Papanas N, Kortsaris A, Maltezos E, Hatseras DI. Comparison of levels of matrix metalloproteinase-2 and -3 in patients with ischemic cardiomyopathy vs. nonischemic cardiomyopathy. Am J Cardiol. 2005;96:1449–1451. doi: 10.1016/j.amjcard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 11.Yokoseki O, Yazaki Y, Suzuki J, Imamura H, Takenaka H, Isobe M. Association of matrix metalloproteinase expression and left ventricular function in idiopathic dilated cardiomyopathy. Jpn Circ J. 2000;64:352–357. doi: 10.1253/jcj.64.352. [DOI] [PubMed] [Google Scholar]
- 12.Oliver KG, Kettman JR, Fulton RJ. Multiplexed analysis of human cytokines by use of the FlowMetrix system. Clin Chem. 1998;44:2057–2060. [PubMed] [Google Scholar]
- 13.Dorman BH, Stroud RE, Wyckoff MM, Zellner JL, Botta D, Leonardi AH, Ikonomidis JS, Spinale FG. Differential effects of epsilon-aminocaproic acid and aprotinin on matrix metalloproteinase release in patients following cardiopulmonary bypass. J Cardiovasc Pharmacol. 2008;51:418–423. doi: 10.1097/FJC.0b013e318168400a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joffs C, Gunasinghe HR, Multani MM, Dorman BH, Kratz JM, Crumbley AJ, 3rd, Crawford FA, Jr, Spinale FG. Cardiopulmonary bypass induces the synthesis and release of matrix metalloproteinases. Ann Thorac Surg. 2001;71:1518–1523. doi: 10.1016/s0003-4975(01)02442-0. [DOI] [PubMed] [Google Scholar]
- 15.Schneider S, Gunasinghe H, Sistino J, Blackwell M, Spinale F. Effects of leukocyte depletion filters on matrix metalloproteinase activation in an extracorporeal circulation circuit. J Extra Corpor Technol. 2003;35:139–142. [PubMed] [Google Scholar]
- 16.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 17.Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer. 2008;7:85. doi: 10.1186/1476-4598-7-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassiri Z, Oudit GY, Sanchez O, Dawood F, Mohammed FF, Nuttall RK, Edwards DR, Liu PP, Backx PH, Khokha R. Combination of tumor necrosis factor-alpha ablation and matrix metalloproteinase inhibition prevents heart failure after pressure overload in tissue inhibitor of metalloproteinase-3 knock-out mice. Circ Res. 2005;97:380–390. doi: 10.1161/01.RES.0000178789.16929.cf. [DOI] [PubMed] [Google Scholar]
- 19.Klappacher G, Franzen P, Haab D, Mehrabi M, Binder M, Plesch K, Pacher R, Grimm M, Pribill I, Eichler HG, Glogar HD. Measuring extracellular matrix turnover in the serum of patients with idiopathic or ischemic dilated cardiomyopathy and impact on diagnosis and prognosis. Am J Cardiol. 1995;75:913–918. doi: 10.1016/s0002-9149(99)80686-9. [DOI] [PubMed] [Google Scholar]
- 20.Coker ML, Zellner JL, Crumbley AJ, Spinale FG. Defects in matrix metalloproteinase inhibitory stoichiometry and selective MMP induction in patients with nonischemic or ischemic dilated cardiomyopathy. Ann N Y Acad Sci. 1999;878:559–562. doi: 10.1111/j.1749-6632.1999.tb07726.x. [DOI] [PubMed] [Google Scholar]
- 21.Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, 3rd, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 22.Picard F, Brehm M, Fassbach M, Pelzer B, Scheuring S, Kury P, Strauer BE, Schwartzkopff B. Increased cardiac mRNA expression of matrix metalloproteinase-1 (MMP-1) and its inhibitor (TIMP-1) in DCM patients. Clin Res Cardiol. 2006;95:261–269. doi: 10.1007/s00392-006-0373-z. [DOI] [PubMed] [Google Scholar]
- 23.Spinale FG, Krombach RS, Coker ML, Mukherjee R, Thomas CV, Houck WV, Clair MJ, Kribbs SB, Johnson LL, Peterson JT. Matrix metalloproteinase inhibition during developing congestive heart failure in pigs: effects on left ventricular geometry and function. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 24.Rouet-Benzineb P, Buhler JM, Dreyfus P, Delcourt A, Dorent R, Perennec J, Crozatier B, Harf A, Lafuma C. Altered balance between matrix gelatinases (MMP-2 and MMP-9) and their tissue inhibitors in human dilated cardiomyopathy: potential role of MMP-9 in myosin-heavy chain degradation. Eur J Heart Fail. 1999;1:337–352. doi: 10.1016/s1388-9842(99)00048-3. [DOI] [PubMed] [Google Scholar]
- 25.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients following myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 26.Kindermann I, Kindermann M, Kandolf R, Klingel K, Bültmann B, Müller T, Lindinger A, Böhm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. doi: 10.1161/CIRCULATIONAHA.108.769489. [DOI] [PubMed] [Google Scholar]
- 27.Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003;253:269–285. doi: 10.1023/a:1026028303196. [DOI] [PubMed] [Google Scholar]
- 28.Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: Embracing the MMP-independent-side of the family. J Mol Cell Cardiol. 2010;48:445–453. doi: 10.1016/j.yjmcc.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Lovelock JD, Baker AH, Gao F, Dong JF, Bergeron AL, McPheat W, Sivasubramanian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H461–H468. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- 30.Spruill LS, Lowry AS, Stroud RE, Squires CE, Mains IM, Flack EC, Beck C, Ikonomidis JS, Crumbley AJ, McDermott PJ, Spinale FG. Membrane-type-1 matrix metalloproteinase transcription and translation in myocardial fibroblasts from patients with normal left ventricular function and from patients with cardiomyopathy. Am J Physiol Cell Physiol. 2007;293:C1362–C1373. doi: 10.1152/ajpcell.00545.2006. [DOI] [PubMed] [Google Scholar]
- 31.Tayebjee MH, Lip GY, Blann AD, Macfadyen RJ. Effects of age, gender, ethnicity, diurnal variation and exercise on circulating levels of matrix metalloproteinases (MMP)-2 and -9, and their inhibitors, tissue inhibitors of matrix metalloproteinases (TIMP)-1 and -2. Thromb Res. 2005;115:205–210. doi: 10.1016/j.thromres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 32.McElmurray JH, 3rd, Mukherjee R, New RB, Sampson AC, King MK, Hendrick JW, Goldberg A, Peterson TJ, Hallak H, Zile MR, Spinale FG. Angiotensin-converting enzyme and matrix metalloproteinase inhibition with developing heart failure: comparative effects on left ventricular function and geometry. J Pharmacol Exp Ther. 1999;291:799–811. [PubMed] [Google Scholar]
- 33.Ye S. Influence of matrix metalloproteinase genotype on cardiovascular disease susceptibility and outcome. Cardiovasc Res. 2006;69:636–645. doi: 10.1016/j.cardiores.2005.07.015. [DOI] [PubMed] [Google Scholar]



 OR
OR