Abstract
Background.
Leukocyte telomere length (LTL) is considered a biomarker of human aging and based on cross-sectional studies it shortens with age. However, longitudinal studies reported that many adults display LTL lengthening.
Methods.
Using Southern blots, we compared cross-sectional rates of age-related LTL change across a ∼20 year age range with those based on longitudinal evaluations in three surveys (S1, S2, and S3) with three time intervals: S1–S2 (5.8 years), S2–S3 (6.6 years), and S1–S3 (12.4 years). Hierarchical linear modeling was used to explore LTL dynamics using LTL data from S1, S2, and S3.
Results.
Cross-sectionally, mean LTL shortenings were 24.6, 25.4, and 23.6 bp/y at S1, S2, and S3, respectively. Longitudinally, more variation was observed in the rate of LTL change during the shorter than longer follow-up periods. Furthermore, using simple differences in LTL, 14.4% and 10.7% of individuals displayed LTL lengthening during S1–S2 and S2–S3, respectively, but only 1.5% during S1–S3 (p < 0.001). The estimated mean rate of LTL shortening based on averaging empirical Bayes’ estimates of LTL from a parsimonious hierarchical linear modeling model was 31 bp/y with a range from 23 to 47 bp/y with none of the participants showing LTL lengthening over the average 12.4 years of follow-up.
Conclusions.
As aging displays a unidirectional progression, it is unlikely that LTL elongates with age. LTL elongation in longitudinal studies primarily reflects measurement errors of LTL in relation to the duration of follow-up periods.
Keywords: Leukocyte telomere length, Aging, Longitudinal, Cross-sectional, African Americans, Whites, body mass index, sex
A body of research has focused on the relation between leukocyte telomere length (LTL) and indices of human aging, principally those linked to the aging or disease status of the cardiovascular system (reviewed in 1–3). Moreover, recent studies in elderly twins apparently resolved the controversy about whether LTL predicts survival, as they showed that the co-twin with the shorter LTL was more likely to die first (4,5). Thus, LTL might serve as a biomarker of human aging.
Most studies of the relation between LTL and indices of human health and aging have used the convenient cross-sectional design (6–11). The cross-sectional design can only infer the pace of age-dependent LTL shortening based on the LTL of individuals of different ages; this estimated age association may be influenced by factors such as the cohort age range and survival effects. There is considerable interindividual variation in LTL at any age, commencing at birth (12,13). Thus, shortened LTL, for instance, might stem from shorter LTL at birth, an accelerated rate of LTL shortening afterward (the pace of which is much faster during the formative years than during adult life), or both. Accordingly, longitudinal information about LTL attrition in the individual should augment understanding of the role of LTL dynamics in human aging.
Only a few studies reported LTL dynamics based on longitudinal analyses and the cohorts examined were followed-up for less than 15 years (14–19). These studies reported no change in LTL and even LTL elongation in a subset of individuals. Two questions arise: First, is LTL gain a true biologic phenomenon? Second, what is the duration of follow-up required for reliably detecting LTL attrition for a given LTL measurement error? This error, which can be estimated from the interassay coefficient of variation (CV), impacts on determination of the follow-up duration.
Theoretical considerations suggest that the longer duration of the follow-up period and the smaller the CV, the more credible are the findings of the change in LTL over time (20) and the greater the likelihood of detecting determinants of LTL change if they exist in the underlying population. We tested this concept with empirical observations in the Bogalusa Heart Study (BHS).
MATERIALS AND METHODS
Participants
The BHS is a long-term investigation of the natural history of cardiovascular disease (21). Between 1995 and 2009, three surveys (S1, S2, and S3) were conducted. LTL data were available for the current study in 271 participants, who had blood samples collected in all three surveys, that is, S1 (age = 19.9–41.5 y), S2 (age = 25.7–46.3 y), and S3 (age = 31.5–49.9 y). The study protocol was approved by Institutional Review Board of Tulane University.
LTL Measurements
We used Southern blots of the terminal restriction fragments, generated by HinfI/RsaI restriction enzymes to measure LTL (22). The IDs of the three samples (S1, S2, and S3) from each of the 271 donors identified them as belonging to the same individual. Besides the IDs, the laboratory that measured LTL was blinded with respect to the sequence of S1, S2, and S3 and to other characteristics of the subjects.
Assessment of LTL Measurement Error
Two strategies were employed. The first was to examine the relation between blind duplicates and the second was to examine the relation between laboratory duplicates measured on different occasions.
The assessment of the measurement error of the blind duplicates was performed as follows: During S3, blood samples drawn from 33 randomly selected individuals were divided into two aliquots in the field center. One vial was labeled with a blind duplicate identification (ID) number and the other was labeled with the true ID number. The two sets of vials were randomly distributed among all other vials and shipped to the laboratory for LTL measurements. The within-subject CV (in percent) was used as an index of the LTL measurement error.
Statistical Analysis
Analyses were performed using SAS version 9.1 (SAS Institute, Inc., Cary, NC). Comparisons for continuous variables were made using t tests or when nonnormal using Wilcoxon rank sum tests and for binary variables using chi-square tests.
The yearly rate of change (loss or gain) in LTL, expressed in bp/y, was computed as the difference between LTL measured in the pairs of surveys, that is, S1–S2, S2–S3, and S1–S3.
Analysis of covariance was performed using general linear models to test for differences in LTL between African Americans (AfAs) and whites and between men and women. The age-related trends of LTL in cross-sectional analyses in S1, S2, and S3 were derived separately in linear regression models.
Hierarchical linear models (HLMs) using PROC MIXED utilized data from all three surveys to estimate the absolute LTL over time, allowing each participant to have his or her own intercept and slope. Fixed effects for age (at S1, S2, and S3), race, baseline smoking status, baseline body mass index (BMI) and interactions with age were evaluated. Participant-specific rates of change in the LTL use the empirical Bayes’ estimates of the slopes. Although the range of intervals between actual visits varied, we used the estimated distribution for differences in LTL measured 6 and 12 years apart to estimate the expected percentage of subjects to show apparent elongation using the estimated mean changes in LTL for each covariate combination and the empirical distribution of covariates in this cohort.
The intraclass correlation coefficient was calculated as the ratio of between and total variance from the HLM. The within-subject CV is the ratio of the mean to the within-subject standard deviation from the HLM.
Unless otherwise indicated, data are presented as mean (standard deviation).
RESULTS
Cohort Characteristics
Age and follow-up periods of the study cohort are summarized in Table 1. The average age was 31.1 (4.7) years, 36.8 (4.3) years, and 43.5 (4.4) years for S1, S2, and S3, respectively. The mean S1–S2 interval was 5.8 (1.5) years and that of S2–S3 was 6.6 (1.0) years. White men had a higher BMI than white women, whereas AfA men had a lower BMI than AfA women.
Table 1.
General Characteristics in S1, S2, and S3
| Whites |
African Americans |
p for race difference |
||||
| Males (n = 73) | Females (n = 131) | Males (n = 19) | Females (n = 48) | Males | Females | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age at S1 | 31.8 (4.2) | 30.8 (4.8) | 31.8 (4.4) | 30.4 (5.0) | 0.973 | 0.466 |
| Age at S2 | 37.5 (4.0) | 36.6 (4.4) | 37.3 (3.8) | 36.4 (4.7) | 0.923 | 0.557 |
| Age at S3 | 44.0 (4.1) | 43.3 (4.5) | 44.0 (3.7) | 42.9 (4.5) | 0.942 | 0.498 |
| Interval S1–S2 | 5.7 (1.5) | 5.8 (1.5) | 5.6 (1.9) | 5.9 (1.6) | 0.791 | 0.622 |
| Interval S2–S3 | 6.5 (1.0) | 6.7 (0.9) | 6.7 (0.6) | 6.6 (1.3) | 0.422 | 0.818 |
| Interval S1–S3 | 12.1 (1.9) | 12.5 (1.7) | 12.3 (2.2) | 12.6 (1.9) | 0.429 | 0.939 |
| BMI at S1 | 28.0 (5.3) | 25.1 (5.6)** | 27.6 (8.7) | 30.4 (8.8) | 0.307 | <0.001 |
| BMI at S2 | 29.6 (6.0) | 26.7 (5.9)** | 29.1 (9.5) | 32.0 (8.6) | 0.385 | <0.001 |
| BMI at S3 | 30.4 (6.0) | 27.8 (6.2)** | 30.4 (9.7) | 33.9 (8.8) | 0.345 | <0.001 |
| N (%) | N (%) | N (%) | N (%) | Males | Females | |
| Smokers at S1 | 18 (25) | 41 (31) | 9 (47) | 15 (31) | 0.053 | 0.995 |
| Smokers at S2 | 14 (19) | 31 (24) | 5 (26) | 14 (29) | 0.494 | 0.452 |
| Smokers at S3 | 12 (16) | 31 (24) | 6 (32) | 10 (21) | 0.138 | 0.690 |
Notes: Testing performed using Wilcoxon rank sum tests for age, interval, and body mass index (BMI) and chi-square tests for current smoking status. Sex difference within races: *p < 0.05 (none), **p < 0.01. SD = standard deviation.
Intraclass Correlations and CVs of Duplicate Measurements of LTL
The intraclass correlation coefficient of LTL between field blind duplicate samples was 0.947 (95% confidence intervals: 0.899–0.973). The intraclass correlation coefficient of LTL between laboratory duplicates was 0.94 (95% CI: 0.93–0.95). The CV for the 33 field blind duplicates was 2.4% (95% confidence intervals: 1.9–3.1) and 2.4% (95% confidence intervals: 2.3–2.5) for the laboratory duplicates.
LTL Dynamics in the Cross-sectional Design
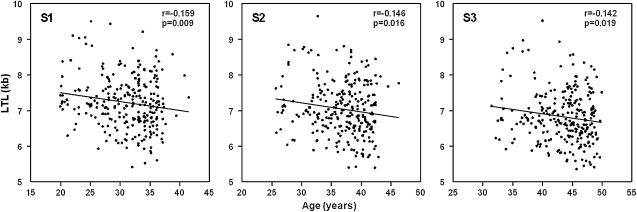
Figure 1 displays the cross-sectional data of LTL as a function of age at S1, S2, and S3. LTL significantly shortened with age (24.6, 25.4, and 23.6 bp/y of age at S1, S2, and S3, respectively).
Figure 1.
Cross-sectional analysis of leukocyte telomere length (LTL) versus age in the three surveys (S1, S2, and S3).
Table 2 presents LTL data by race and sex for S1, S2, and S3. AfAs consistently displayed a longer LTL than did whites across the duration of the follow-up. However, there was no significant sex effect on LTL in this modest-sized cohort.
Table 2.
Leukocyte Telomere Length (LTL, kb) in Three Surveys (S1, S2, and S3)
| Whites |
African Americans |
p for race difference |
||||
| Males (n = 73) | Females (n = 131) | Males (n = 19) | Females (n = 48) | Males | Females | |
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| LTL at S1 | 7.005 (0.617) | 7.114 (0.699) | 7.713 (0.656) | 7.650 (0.732) | <0.001 | <0.001 |
| LTL at S2 | 6.844 (0.645) | 6.938 (0.711) | 7.525 (0.688) | 7.450 (0.769) | <0.001 | <0.001 |
| LTL at S3 | 6.612 (0.582) | 6.749 (0.711) | 7.196 (0.634) | 7.215 (0.792) | <0.001 | <0.001 |
Note: Testing performed using a t test assuming equal variances. No significance sex differences within race were found. SD = standard deviation.
Table 3 displays a regression model assessing the individual (partial R2) and joint effects (model R2) of age, race, sex, BMI, and smoking on LTL at S1, S2, and S3. As the age range for S1, S2, and S3 in the cross-sectional model was restricted to two decades, age explained only 2.01–2.54% of the variance of LTL. However, race explained 9.03–12.10% of the variance. Sex, as indicated earlier, had little effect of LTL, but BMI and smoking status did exert discernable effects: BMI explained 0.97–2.05% of the LTL variation (all statistically significant) and smoking status explained 0.64–1.23% (marginally or borderline significance for S1 and S3). Overall, the joint effects of age, race, sex, BMI, and smoking explained 13.5–16.7% of the LTL variation in the cross-sectional design.
Table 3.
Regression of Leukocyte Telomere Length (LTL, bp) in Three Surveys (S1, S2, and S3) on Age, Race, Sex, BMI, and Smoking
| LTL at S1 |
LTL at S2 |
LTL at S3 |
||||||||||
| Independent variable | β | p | Partial R2 | Model R2 | β | p | Partial R2 | Model R2 | β | p | Partial R2 | Model R2 |
| Intercept | 7,472.7 | <0.001 | — | — | 7,663.8 | <0.001 | — | — | 7,363.4 | <0.001 | — | — |
| Age | −21.8 | 0.013 | 2.54 | — | −24.0 | 0.015 | 2.14 | — | −22.1 | 0.022 | 2.01 | — |
| Race | 641.6 | <0.001 | 12.10 | — | 628.9 | <0.001 | 10.62 | — | 553.3 | <0.001 | 9.03 | — |
| BMI | −13.6 | 0.034 | 1.00 | — | −17.7 | 0.006 | 2.05 | — | −12.3 | 0.041 | 0.97 | — |
| Smoking | 177.8 | 0.049 | 1.23 | 16.96 | −144.6 | 0.157 | 0.64 | 15.50 | −192.1 | 0.060 | 1.16 | 13.55 |
Notes: A negative regression coefficient denotes a shorter LTL and a positive coefficient denotes a longer LTL. African American = 1, white = 0; smoking yes = 1, no = 0. BMI = body mass index.
LTL Dynamics in the Longitudinal Design
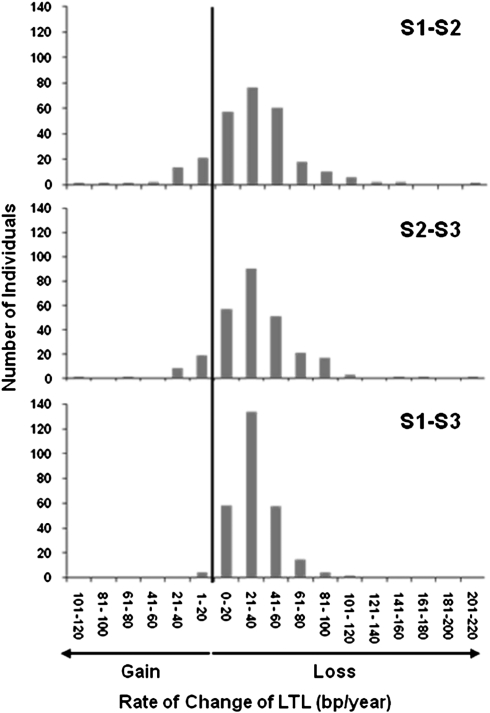
Figure 2 displays the distribution of the rate of change in LTL among participants for S1–S2, S2–S3, and S1–S3 based on the subtracting LTL values at the two measurement time points. The mean rates of LTL change, which also included a subset that gained LTL during follow-up, were a shortening by 31.4, 33.5, and 32.2 bp/y for S1–S2, S2–S3, and S1–S3, respectively. There was more variation, evident as a wider spread, in the rate of LTL change during S1–S2 and S2–S3 than during S1–S3 (Figure 2). Furthermore, 14.4% and 10.7% of individuals displayed LTL lengthening during intervals S1–S2 and S2–S3, respectively, but only 1.5% in interval S1–S3 (p < 0.001 for the decline in proportion with longer follow-up).
Figure 2.
Distribution of yearly rate of change in leukocyte telomere length (LTL) for the two consecutive follow-up periods, S1–S2, S2–S3, and the two periods combined, S1–S3.
The correlation coefficient of the rate of change in LTL between S1–S2 and S2–S3, adjusted for baseline age, was −0.390 (p < 0.0001). When adjusted for both baseline age and LTL, the correlation coefficient of rates of change between the two periods was −0.399 (p < 0.0001). To gain further insight into this association, we compared the rate of change during S2–S3 in individuals who demonstrated LTL elongation (N = 39) during S1–S2 versus those who showed LTL shortening (N = 232) during this period. The rate of LTL shortening during S2–S3 in individuals who gained telomeres in S1–S2 was significantly faster (59.7 (53.2) bp/y) compared with their peers who showed LTL shortening during S1–S2 (29.1 (29.4) bp/y; p < 0.0001). We then compared the rate of LTL shortening during S1–S2 in individuals with LTL elongation during S2–S3 (N = 29) versus those who showed LTL shortening during that time period (N = 242). Again, the rate of LTL shortening during S1–S2 in those individuals who showed LTL lengthening during S2–S3 was significantly faster (52.7 (37.0) bp/y) than in their peers who showed LTL shortening during that period (28.8 (35.6) bp/y; p = 0.0011).
Among 67 individuals who displayed LTL elongation in the original analysis, 66 individuals (N = 37 for S1–S2, N = 28 for S2–S3, and N = 1 for S1–S3) had sufficient DNA to repeat the measurements 7 months after original measurements. The correlation between the first and second sets of measurements (each performed in duplicate) was r = 0.932, p = 0.0001 (Supplementary Figure 1).
For these individuals, all of whom displayed LTL elongation in the original analysis on repeated LTL measurements 44 individuals showed LTL shortening and 22 showed LTL elongation (mean rate of LTL shortening = 17.2 bp/y).
We used HLMs, which provided mean and participant-specific estimates of LTL change, to gain a further insight into LTL dynamics over the entire duration of the follow-up. Table 4 displays fixed-effects results from two HLMs, which include main effects and interaction effects with age. The interaction effects in Model 1 show the estimated effects of race, sex, smoking, and BMI on the rate of change in LTL. Model 2 is a more parsimonious model in which the interaction between age and BMI is omitted.
Table 4.
Estimated Fixed Effects from Hierarchical Linear Models Using Leukocyte Telomere Length (LTL, bp) from S1 to S3 as the Dependent Variable, with Random Effects for the Intercept and Slope
| Independent variable | β | p Value |
| Model 1 | ||
| Intercept | 6,834.2 | <0.001 |
| Age | −31.7 | <0.001 |
| Race | 588.4 | <0.001 |
| Sex | 41.8 | 0.63 |
| Baseline smoking | −218.3 | 0.017 |
| Baseline BMI | −13.1 | 0.042 |
| Age × Race | −5.4 | 0.028 |
| Age × Sex | 4.5 | 0.044 |
| Age × Baseline Smoking | −4.7 | 0.040 |
| Age × Baseline BMI | 0.12 | 0.45 |
| Model 2 | ||
| Intercept | 6,834.4 | <0.001 |
| Age | −31.6 | <0.001 |
| Race | 590.0 | <0.001 |
| Sex | 41.6 | 0.63 |
| Baseline smoking | −220.0 | 0.016 |
| Baseline BMI | −13.6 | 0.034 |
| Age × Race | −5.0 | 0.038 |
| Age × Sex | 4.3 | 0.052 |
| Age × Baseline Smoking | −5.0 | 0.026 |
Notes: Age and BMI are centered so the intercept corresponds to age 40 years and BMI of 27 kg/m2 for Model 1 and age of 40 years for Model 2. Race is coded: AfA = 1, white = 0; sex coded women = 1, men = 0; smoking coded yes = 1, no = 0. Unstructured covariances were used when fitting the models. The βs estimate the increase in LTL for a 1 unit increase in the covariate, with a negative coefficient denoting a shorter LTL. For example, for nonsmoking white men, the β for age in Model 2 estimates a decrease of 31.6 bp/y. Tests of interactions with age are used to evaluate differences in rates of LTL change by covariates, for example, the β for the Age × Race interaction in Model 2 indicates AfAs have a 5.0 bp/y greater rate of LTL shortening. AfA = African American; BMI = body mass index.
The mean LTL shortening based on averaging empirical Bayes’ estimates from Model 2 was 31 bp/y, whereas the individual-specific rate of change declined between 23 and 47 bp/y. No participant was estimated to display LTL lengthening based on the HLM. The individual slopes (empirical Bayes’ estimates) generally fit the observed data for both those whose LTL shortened between surveys and those who seemed to elongate their LTL. Supplementary Figure 2 shows a stratified random sample of 12 individuals’ LTLs over time; 6 who had consecutive shortening in observed LTL values over time and 6 who did not.
Examining the interaction effects in Model 1 showed that AfAs displayed a faster rate of LTL shortening than whites (by 5.4 bp/y, p = 0.028), women had a slower rate of LTL shortening than men (by 4.5 bp/y, p = 0.044), and those that reported smoking at S1 had a faster rate of shortening than those who did not (by 4.7 bp/y, p = 0.040). BMI was not associated with a faster rate of LTL shortening (p = 0.45). Although the lack of a significant Age × BMI interaction in Model 1 suggests that having a higher BMI at baseline did not increase the rate of LTL shortening, those persons with higher BMI had a shorter LTL (13.6 bp shorter LTL per unit higher BMI in Model 2, p = 0.034).
Besides age, race was the single most important determinant of LTL with AfAs having a 590 bp longer LTL at age 40 years based on Model 2. Because of the significant interaction with age, the race difference increases by 5.0 bp for each year younger than 40 and diminishes by 5.0 bp for each year older than 40.
Expected Percentage of Subjects with Apparent Elongation between Visits
Using the results from Model 2, the expected mean (standard deviation) difference in LTL measurements 6 and 12 years apart is a decrease ranging from 185 (201) to 199 (201) bp and 375–389 (212) bp, respectively, and we would expect that 17.2% of individuals would have apparent LTL elongation between interval visits of 6 years and 3.7% between interval visits of 12 years. The observed percentages were actually lower, 10.7%–14.4% for S1–S2 and S2–S3, which had a mean interval of approximately 6 years, and 1.5% for S1–S3, which had a mean interval of approximately 12 years.
DISCUSSION
Researchers have often suggested that a longitudinal evaluation might provide a better understanding than the cross-sectional strategy about the connection between LTL dynamics and a host of biologic and environmental indices (22–26). But, as shown here, technical shortcomings in the determination of LTL need to be addressed.
On average, LTL shortening in cross-sectional analyses of the adult population at large has been reported to be less than 40 bp/y and the reported CV of LTL measurement by different methods range between 0.9% and 28% (27–29). But as shown in the present study, even a relatively low measurement error, expressed in a CV of ∼2.5% can result in sufficient misclassification.
The error in LTL measurement probably explains why during S1–S2 and S2–S3 many more individuals displayed LTL elongation than during S1–S3. The negative correlation of rates of LTL shortening between S1–S2 and S2–S3 suggests that individuals with a relatively low rate of LTL shortening in one period were prone to display a relatively high rate of LTL shortening prior to or after that period. This expression of regression to the mean may be a reflection of laboratory error. Support for this conclusion was provided by the repeat LTL measurements in individuals who showed LTL elongation. Measurement errors should primarily affect results derived from individuals with a relatively slower rate of LTL shortening, that is, those below the mean rate of LTL shortening. Repeating LTL measurements in samples from these individuals confirmed this supposition, showing that the majority of them showed LTL shortening upon reexamination. The HLMs further strengthened the tenet that LTL elongation was mainly, if not solely, the outcome of telomere measurement error. These models showed that after removing unexplained errors based on all LTL measurements during the follow-up period, no individual displayed LTL elongation over time.
Although a number of potential biologic causes for LTL elongation have been offered to explain the wide swings in the rate of LTL change (16) in the general population, some of the findings might simply indicate the presence of a large measurement error of LTL relative to the magnitude of the LTL attrition (ie, a too short follow-up) rather than an expression of a true biologic phenomenon.
Any longitudinal study that examines age-dependent LTL shortening provides a slice―a small one under most circumstances―of the life span of the individual. In contrast, at any given age, the value of LTL represents LTL attrition during the entire life course of the individual from birth onward, only that the considerable interindividual variation in birth LTL renders it difficult to fully appreciate that attrition without knowing the individual's LTL at birth. It follows that both the cross-sectional data and longitudinal data of LTL provide incomplete but complementary perspectives on the relation between LTL dynamics and biologic phenomena of interest. Based on cross-sectional and longitudinal evaluations (30–32) and a series of simulations (33), the rate of LTL shortening is very rapid during infancy and early childhood. In contrast, the rate of LTL shortening during adulthood, on the basis of both the cross-sectional and longitudinal data, is much slower than during early life.
The effect of LTL shortening with age in a cross-sectional design depends on the age range and interindividual variation in LTL of the sample (28,34). The 2.5% or less contribution of age to variation in LTL in our cross-sectional analysis reflects the relatively narrow age range of our cohort at S1, S2, and S3, which was ∼20 years, and the studied period within the life course (young to mid-adulthood). In that light, the effects of BMI, smoking, and particularly race on LTL based on the cross-sectional analysis (Table 3) were considerable.
At birth, LTL is equivalent in boys and girls (13,14), but studies in adults have observed, with few exceptions (34,35), that LTL is longer in women than in men (4,7,36–40). The first cited exception applied to a cross-sectional study, which examined healthy aging in highly selected participants (age range 16–104 years old) who were free of any apparent disease (34). The second cited exception was a cross-sectional evaluation of LTL in BHS participants (35). Nevertheless, in the latter study, a longer LTL was observed in women than in men using HphI/MnII instead of HinfI/RsaI restriction enzymes to generate the terminal restriction fragments (35). If newborn boys and girls have equivalent LTL but on average, women have a longer LTL, one would anticipate that either during maturation or adult life, the rate of LTL shortening would be slower in women than in men. But a number of cross-sectional analyses and a longitudinal evaluation more than 6 years (14) were unable to show such sex-related differences in adults. Bekaert and colleagues (7) did find in a cross-sectional study that the rate of LTL shortening was slower in women than men and the present study confirms this finding. HLM Models 1 and 2 showed modestly slower mean rates (4.5 and 4.3 bp/y) of shortening among women compared with men (p = 0.044 and p = 0.052, respectively). As both effects were marginal, they need confirmation in larger studies.
An inverse relation between BMI and LTL was observed before (41–44). Paradoxically, although higher baseline BMI was associated with a shorter LTL, it was not associated with accelerated shortening in this study. We were also limited in how we could analyze the BMI, as the change in BMI was relatively small across the follow-up period. Again, larger studies with follow-up periods during different phases of the human life span with a broader range of BMI values are necessary to more fully understand the effect of age and BMI on the rate of LTL shortening.
Several cross-sectional evaluations of different cohorts, including the BHS, observed that smokers had a shorter LTL than nonsmokers (16,37,38,41) but did not show that smokers displayed an accelerated LTL shortening during a 6-year longitudinal evaluation in BHS participants (16). Applying HLM to data from three measurement time points that spanned approximately 12.4 years, we were able to show that individuals reporting smoking at baseline have shorter LTL and have an accelerated LTL shortening, but again, this requires confirmation in large-scale longitudinal studies.
Finally, the HLM models showed that the racial difference in LTL narrowed with age in line with the faster LTL shortening in adult AfAs than whites, a phenomenon we observed in a large cross-sectional study of age range that covers seven decades (35).
In conclusion, longitudinal evaluation of LTL provides an important additional dimension to our understanding of LTL dynamics. Our data strongly suggest that LTL elongation with age is an unlikely occurrence and is probably a measurement artifact. Analysis of longitudinal data should use statistical methods that estimate effects after accounting for unexplained error. Moreover, researchers need to recognize the limitation of current methods of telomere length measurements. That being said, well-designed, properly powered studies using techniques that minimize measurement error of LTL can provide valuable insight into the role of telomere biology in human aging.
FUNDING
This work was supported by grants HD-061437 and HD-062783 from the National Institute of Child Health and Human Development and AG-16592 from the National Institute on Aging.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomed.gerontologyjournals.org/.
References
- 1.Samani NJ, van der Harst P. Biological ageing and cardiovascular disease. Heart. 2008;94:537–539. doi: 10.1136/hrt.2007.136010. [DOI] [PubMed] [Google Scholar]
- 2.Fuster JJ, Andrés V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–1180. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 3.Aviv A. Telomeres and human somatic fitness. J Gerontol A Biol Sci Med Sci. 2006;61:871–873. doi: 10.1093/gerona/61.8.871. [DOI] [PubMed] [Google Scholar]
- 4.Kimura M, Hjelmborg JB, Gardner JP, et al. Short leukocyte telomeres forecast mortality: a study in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakaysa SL, Mucci LA, Slagboom PE, et al. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6:769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- 6.Njajou OT, Cawthon RM, Damcott CM, et al. Telomere length is paternally inherited and is associated with parental lifespan. Proc Natl Acad Sci U S A. 2007;104:12135–12139. doi: 10.1073/pnas.0702703104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bekaert S, De Meyer T, Rietzschel ER, et al. Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- 8.Spyridopoulos I, Erben Y, Brummendorf TH, et al. Telomere gap between granulocytes and lymphocytes is a determinant for hematopoetic progenitor cell impairment in patients with previous myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:968–974. doi: 10.1161/ATVBAHA.107.160846. [DOI] [PubMed] [Google Scholar]
- 9.Brouilette SW, Moore JS, McMahon AD, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 10.van der Harst P, van der Steege G, de Boer RA, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell CJ, Demissie S, Kimura M, et al. Leukocyte telomere length and carotid artery intimal medial thickness: The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008;28:1165–1171. doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okuda K, Bardeguez A, Gardner JP, et al. Telomere length in the newborn. Pediatr Res. 2002;52:377–381. doi: 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Akkad A, Hastings R, Konje JC, et al. Telomere length in small-for-gestational-age babies. BJOG. 2006;113:318–323. doi: 10.1111/j.1471-0528.2005.00839.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Ruiz CM, Gussekloo J, van Heemst D, et al. Telomere length in white blood cells is not associated with morbidity or mortality in the oldest old: a population-based study. Aging Cell. 2005;4:287–290. doi: 10.1111/j.1474-9726.2005.00171.x. [DOI] [PubMed] [Google Scholar]
- 15.Ehrlenbach S, Willeit P, Kiechl S, et al. Influences on the reduction of relative telomere length over 10 years in the population-based Bruneck Study: introduction of a well-controlled high-throughput assay. Int J Epidemiol. 2009;38(6):1725–1734. doi: 10.1093/ije/dyp273. [DOI] [PubMed] [Google Scholar]
- 16.Aviv A, Chen W, Gardner JP, et al. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Lenner P, Roos G. The individual blood cell telomere attrition rate is telomere length dependent. PLoS Genet. 2009;5(2):e1000375. doi: 10.1371/journal.pgen.1000375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farzaneh-Far R, Lin J, Epel E, et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the heart and soul study. PloS One. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farzaneh-Far R, Lin J, Epel E, Harris WS, Blackburn EH, Whooley MA. Association of marine omega-3 fatty acid levels with telomeric aging in patients with coronary heart disease. JAMA. 2010;303:250–257. doi: 10.1001/jama.2009.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–983. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- 21.Berenson GS, McMahan CA, Voors AW, et al. Cardiovascular Risk Factors in Children—The Early Natural History of Atherosclerosis and Essential Hypertension. New York: Oxford University Press; 1980. pp. 47–123. [Google Scholar]
- 22.Kimura M, Stone RC, Hunt SC, et al. Measurement of telomere length by the southern blot analysis of the terminal restriction fragment lengths. Nature Protocols. 2010;5:1596–1607. doi: 10.1038/nprot.2010.124. [DOI] [PubMed] [Google Scholar]
- 23.Kuh D. A life course perspective on telomere length and social inequalities in aging. Aging Cell. 2006;5:579–580. doi: 10.1111/j.1474-9726.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 24.Xu Q, Parks CG, DeRoo LA, et al. Multivitamin use and telomere length in women. Am J Clin Nutr. 2009;89:1857–1863. doi: 10.3945/ajcn.2008.26986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Movérare-Skrtic S, Svensson J, Karlsson MK, et al. Serum insulin-like growth factor-I concentration is associated with leukocyte telomere length in a population-based cohort of elderly men. J Clin Endocrinol Metab. 2009;94:5078–584. doi: 10.1210/jc.2009-1450. [DOI] [PubMed] [Google Scholar]
- 26.Lansdorp PM. Stress, social rank and leukocyte telomere length. Aging Cell. 2006;5:583–584. doi: 10.1111/j.1474-9726.2006.00247.x. [DOI] [PubMed] [Google Scholar]
- 27.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35:1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 29.Shen J, Terry MB, Gurvich I, et al. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67:5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- 30.Rufer N, Brümmendorf TH, Kolvraa S, et al. Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood. J. Exp. Med. 1999;190:157–167. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeichner SL, Palumbo P, Feng Y, et al. Rapid telomere shortening in children. Blood. 1999;93:2824–2830. [PubMed] [Google Scholar]
- 32.Frenck RW, Jr., Blackburn EH, Shannon KM. The rate of telomere sequence loss in human leukocytes varies with age. Proc Natl Acad Sci U S A. 1998;95:5607–5610. doi: 10.1073/pnas.95.10.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sidorov I, Kimura M, Yashin A, et al. Leukocyte telomere dynamics and human hematopoietic stem cell kinetics during somatic growth. Exp Hematol. 2009;37:514–524. doi: 10.1016/j.exphem.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Barbieri M, Paolisso G, Kimura M, et al. Higher circulating levels of IGF-1 are associated with longer leukocyte telomere length in healthy subjects. Mech Ageing Dev. 2009;130:771–776. doi: 10.1016/j.mad.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Hunt SC, Chen W, Gardner JP, et al. Leukocyte telomeres are longer in African Americans than in whites: the National Heart, Lung, and Blood Institute Family Heart Study and the Bogalusa Heart Study. Aging Cell. 2008;7:451–458. doi: 10.1111/j.1474-9726.2008.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jeanclos E, Schork NJ, Kyvik KO, et al. Telomere length inversely correlates with pulse pressure and is highly familial. Hypertension. 2000;36:195–200. doi: 10.1161/01.hyp.36.2.195. [DOI] [PubMed] [Google Scholar]
- 37.Vasan RS, Demissie S, Kimura M, et al. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system: the Framingham Heart Study. Circulation. 2008;117:1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nawrot TS, Staessen JA, Gardner JP, et al. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick AL, Kronmal RA, Gardner JP, et al. Leukocyte telomere length and cardiovascular disease in the Cardiovascular Health Study. Am J Epidem. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 40.Benetos A, Okuda K, Lajemi M, et al. Telomere length as indicator of biological aging: the gender effect and relation with pulse pressure and pulse wave velocity. Hypertension. 2001;37(part 2):381–385. doi: 10.1161/01.hyp.37.2.381. [DOI] [PubMed] [Google Scholar]
- 41.Valdes A, Andrew T, Gardner JP, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;66:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 42.Kim S, Parks CG, DeRoo LA, et al. Obesity and weight gain in adulthood and telomere length. Cancer Epidemiol Biomarkers Prev. 2009;18:816–820. doi: 10.1158/1055-9965.EPI-08-0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nordfjäll K, Eliasson M, Stegmayr B, Lundin S, Roos G. Nilsson PM Increased abdominal obesity, adverse psychosocial factors and shorter telomere length in subjects reporting early ageing; the MONICA Northern Sweden Study. Scand J Public Health. 2008;36:744–752. doi: 10.1177/1403494808090634. [DOI] [PubMed] [Google Scholar]
- 44.Gardner JP, Li S, Srinivasan SR, et al. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.