Abstract
Background.
To examine the association between obesity history and hand grip strength, and whether the association is partly explained by subclinical inflammation and insulin resistance.
Methods.
Data are from 2,021 men and women aged 55 years and older participating in the representative population-based Health 2000 Survey in Finland. Body mass and body height, maximal hand grip strength, C-reactive protein, and insulin resistance based on homeostasis model assessment (HOMA-IR) were measured in a health examination. Recalled weight at 20, 30, 40, and 50 years of age were recorded to obtain a hierarchical classification of obesity history. Obesity was defined as body mass index ≥ 30 kg/m2.
Results.
Earlier onset of obesity was associated with lower hand grip strength (p < .001) after controlling for age, sex, education, smoking, alcohol use, physical activity, several chronic diseases, and current body weight. Based on adjusted logistic regression models, the odds (95% confidence interval) for very low relative hand grip strength were 2.76 (1.78–4.28) for currently obese, 5.57 (3.02–10.28) for obese since age of 50 years, 6.53 (2.98–14.30) for obese since age of 40 years, and 10.36 (3.55–30.24) for obese since age of 30 years compared with never obese participants. The associations remained highly significant even after adjusting for current C-reactive protein and HOMA-IR, but these variables had only minor role in explaining the association between obesity history and hand grip strength.
Conclusions.
Long-term exposure to obesity is associated with poor hand grip strength later in life. Maintaining healthy body weight throughout the life span may help to maintain adequate muscle strength in old age. Prospective studies with information on prior muscle strength are needed to examine in detail the causal association between obesity history and muscle strength.
Keywords: Muscle strength, Obesity, Inflammation, Insulin resistance, Aging
AN age-related reduction of muscle mass and strength is a major public health concern in older persons because of its important role in the causal pathway leading to functional limitations, increased risk of falls, disability, and mortality (1,2). Beyond the traditional risk factors for impaired muscle strength, such as low physical activity, nutritional deficiencies, and chronic diseases, the interrelationship between fat and muscle tissue has attracted increased interest. It has been suggested that excessive amount of fat tissue may contribute to accelerated loss of muscle mass and strength with aging (3), but no previous studies have examined longitudinally the association between obesity and low muscle strength.
It is now evident that adipose tissue is not just passive energy storage, but rather, an active metabolic tissue that secretes hormones and proteins. For example, in adipose tissue, either adipocytes directly or infiltrating macrophages produce proinflammatory cytokines and adipokines, which upregulate the inflammatory response (4,5). Increased inflammation in turn may lead to catabolism and contribute to muscle mass and strength decline (6,7). Additionally, excess adiposity is associated with insulin resistance and depressed anabolic action of insulin (8). Studies have shown that insulin resistance is an independent correlate of poor muscle strength (9,10) and diabetes predicts accelerated loss of leg muscle strength and quality (11).
We hypothesize that longer exposure to obesity might aggravate the unwanted consequences of obesity, such as increased inflammation and insulin resistance, and lead to lower muscle strength. Thus, the objective of this study was to examine whether early onset of obesity (body mass index [BMI] ≥ 30 kg/m2) is associated with lower hand grip strength using data from the Health 2000 Survey, a representative population-based study from Finland. Additionally, the mediating roles of inflammation and insulin resistance on the association between obesity history and hand grip strength were examined.
METHODS
Study Design and Participants
The study is based on the Health 2000 Survey, a comprehensive nationwide health interview and examination survey carried out in Finland 2000–2001 (12). The two-stage stratified cluster sample comprised 8,028 adults 30 years or older, living in mainland Finland either in the community or in the institutions. Participants 80 years or older were oversampled (2:1) in relation to their proportion in the population. The sample for the present study was limited to participants 55 years or older (N = 3,392), 2,055 women and 1,337 men.
The data were collected in two phases. First, a structured health interview was completed at the participant's home by a trained interviewer for 3,186 participants (94% of the sample). A health examination was carried out for 2,572 participants (76% of the sample) by trained professionals in a nearby study center. In addition, 306 participants (9% of the sample) not able or willing to participate in the study center examinations took part in an abbreviated health examination at their place of living. Only those participants were included in the study who had information on obesity history and hand grip strength and their blood samples were analyzed (N = 2,155). All participants signed informed written consent forms approved by the Ethics Committee for Epidemiology and Public Health in the Hospital District of Helsinki and Uusimaa in Finland.
Obesity History
Current weight was measured to the nearest 100 g and standing height to the nearest 0.5 cm, with participants wearing light clothing and no shoes. In the questionnaire, participants were asked to recall their weight at the ages of 20, 30, 40, and 50 years. Current BMI was calculated as weight (kilograms) divided by the square of height (meters). BMI at ages 20, 30, 40, and 50 years was calculated using recalled weight and current measured height.
Based on BMI at the given ages, participants were classified into nonobese (18.5–29.9 kg/m2) and obese (≥30.0 kg/m2) according to the criteria of the World Health Organization (13). Participants were then categorized into six groups to obtain a hierarchical classification of obesity history: (a) never obese; (b) previously obese, but currently nonobese; (c) currently obese, but nonobese at the ages of 20–50 years; (d) obese since the age of 50 years; (e) obese since the age of 40 years; and (f) obese since the age of 30 years. Those who had been obese since the age of 20 years (n = 5) were merged with the last category. Those 14 persons with a history of weight cycling (ie, obese at the age of 30 or 40 years, nonobese at the age of 50 years, and currently obese) were excluded from the analyses. In addition, participants were asked whether they had gained or lost weight during the last 12 months and whether the weight change was intentional or unintentional. To exclude participants potentially suffering from undernourishment or catabolic state, those who were underweight (BMI < 18.5 kg/m2) or had unintentionally lost 5% or more during the past year (n = 133) and one person with unreliable weight history data were not included in the analyses. Thus, the final sample for this study consisted of 2,021 participants (60% of the total sample).
Hand Grip Strength
Hand grip strength was used as a proxy for overall strength (14,15). Hand grip strength was measured in Newtons (N) using a handheld dynamometer based on strain gauge sensors (Good Strength, IGS01, Metitur Oy, Jyväskylä, Finland). The measurement was taken with the dominant hand with the participant seated, elbow flexed at a 110° angle, wrist in a neutral position, and the interphalangeal joint of the index finger at a 90° angle. The participant was instructed to squeeze the handle with maximal effort for 3–5 seconds and standard encouragement for maximal performance was given. The measurement was repeated after a 30-second pause for recovery. If the two results differed more than 10%, a third attempt was made. The best result was used in the analyses. The reliability of the hand grip strength test, measured using the intraclass correlation coefficient, was excellent (intraclass correlation coefficient = 0.95, n = 265) (16).
Inflammation and Insulin Resistance
Fasting blood samples were drawn during the clinical examination. The samples were centrifuged at the examination site, stored in deep freezers at −20°C, and transferred within 1 week to the National Public Health Institute to be stored in deep freezers at −70°C until used for laboratory analysis. In this study, inflammation was measured with C-reactive protein (CRP). CRP was determined using an ultrasensitive immunoturbidometric test (Orion Diagnostica, Espoo, Finland) with Optima analyzer (Thermo Electron Corporation, Vantaa, Finland). The lowest detection level was 0.20 mg/L. All values below the detection level were coded as 0.10 mg/L. The interassay coefficient of variation was 4.5%. Levels of fasting glucose and insulin were determined using the glucose dehydrogenase method (Diagnostica, Merck, Darmstadt, Germany) and radioimmunoanalysis (Pharmacia, Uppsala, Sweden), respectively. The interassay coefficient of variation was 2.0% for glucose and 5.2% for insulin. The degree of insulin resistance was calculated using data on fasting glucose and insulin according to the homeostasis model assessment (HOMA-IR). HOMA-IR is a good index assessing insulin resistance across a wide range of values and is well correlated with insulin-mediated glucose uptake calculated by euglycemic glucose clamp (17,18).
Covariates
The level of education was classified as basic education (0–9 years), intermediate education (10–12 years), and higher education (13 years or more). For smoking status, participants were categorized into never-smokers (no smoking more than 1 year during their lifetime), former smokers (quit smoking at least 1 month before), and current smokers. Alcohol use was measured with a questionnaire as average weekly consumption (gram per week) based on the consumption of different drink types during the past month and classified as no alcohol use, moderate use, and heavy use. The limit for heavy use was set 280 g/wk in men (eg, 5 alcohol units per day, 7 d/wk) and 140 g/wk in women (19). Leisure time physical activity was determined based on a Gothenburg scale (20) and participants were categorized as inactive (eg, reading, watching television), moderate active (eg, walking, biking, gardening, outdoor recreations at least 4 h/wk), and very active (eg, running, biking, gymnastics at least 3 h/wk).
Waist circumference was measured on naked skin at the end of a light expiration with the participant standing from a half way between the iliac crest and the lowest rib. The physician ascertained medical conditions by using structured uniform diagnostic criteria based on current clinical practice and used medication. Chronic conditions used in this study were type 2 diabetes, hypertension, coronary heart disease (angina pectoris or myocardial infarction), congestive heart disease, asthma, chronic obstructive pulmonary disease, depression, and knee and hip osteoarthritis.
Statistics
The characteristics of study population are reported as mean values and standard deviations for normally distributed continuous variables: median values and interquartile ranges for nonnormally distributed continuous variables and proportions for categorical variables. Differences across obesity history categories were examined with t test or nonparametric Kruskal–Wallis test statistics for continuous variables and chi-square test for categorical variables. The interaction of Sex × Obesity History on hand grip strength was tested with generalized linear models. Because no significant interaction was found (p = .10), the analyses were carried out combining men and women into one group.
To compare hand grip strength across obesity history categories, analysis of covariance and generalized linear model with Tukey post hoc test were used. In addition, the linear trend across categories was examined by entering categorical variables in the model as ordinal variables. Because muscle strength is dependent on the body size, the analyses were adjusted for current body weight in addition to age and sex. Similar analyses were carried out to examine the associations between obesity history and level of CRP, obesity history and HOMA-IR, as well as the association between CRP and hand grip strength and HOMA-IR and hand grip strength.
To test the association between obesity history categories and hand grip strength, a linear regression analysis was utilized by using “never obese” group as a reference group. Six models were fitted including CRP, HOMA-IR, and covariates that were significantly associated with obesity history. In addition, a percentage change in beta coefficients was calculated to estimate the effects of covariates, CRP, and HOMA-IR by using the following formula: (regression coefficientModel1 − regression coefficientModel2/3/4)/regression coefficientModel1 × 100. Finally, logistic regression analysis was used to examine the risk of having very low muscle strength by obesity history categories. Because absolute muscle strength is higher among heavier persons, relative muscle strength was calculated as hand grip strength divided by current body weight. Participants were divided into sex-specific deciles and the lowest decile was chosen to indicate very low relative muscle strength. Same six models were fitted as in linear regression analysis. Similar analysis was carried out for obese participants only.
Statistical analyses were completed using the SAS 9.1 Statistical Package (SAS Institute Inc., Cary, NC). The data were weighted to reduce bias due to nonresponse and to correct the oversampling in the age group of 80 years and older in order to represent the Finnish population. The complex sampling design was taken into account by using SAS survey procedures.
RESULTS
The mean age of the study population was 67 years (range 55–99), 57% were women and 43% men. The descriptive characteristics of the study population by hierarchical classification of obesity history are presented in Table 1.
Table 1.
Characteristics of Study Population by Hierarchical Classification of Obesity History (n = 2,021)
| Never Obese | Previously Obese, but Currently Nonobese (a) | Currently Obese (b) | Obese Since 50 years (c) | Obese Since 40 years (d) | Obese Since 30 years (e) | p* | |
| n (% of sample) | 1,288 (63.7) | 116 (5.7) | 410 (20.3) | 124 (6.1) | 58 (2.9) | 25 (1.2) | |
| Women, % | 51.9 | 47.6 | 63.7 | 56.1 | 63.9 | 45.4 | a, b |
| Age (years), mean (SD) | 65.87 (8.03) | 68.05 (9.36) | 67.08 (7.90) | 62.63 (7.14) | 63.85 (7.07) | 64.05 (8.30) | c |
| Current body mass index (kg/m2), mean (SD) | 25.80 (2.54) | 27.24 (1.99) | 32.38 (2.32) | 34.03 (3.23) | 36.60 (4.63) | 36.87 (6.12) | a, b, c, d, e |
| Current waist circumference (cm), mean (SD) | |||||||
| Men | 96.03 (8.23) | 99.97 (6.73) | 110.84 (5.95) | 114.42 (8.03) | 121.89 (15.03) | 115.15 (14.31) | a, b, c, d, e |
| Women | 86.75 (8.34) | 93.06 (8.80) | 102.80 (8.30) | 106.92 (11.13) | 112.31 (8.34) | 111.59 (12.67) | a, b, c, d, e |
| Current body height (cm), mean (SD) | |||||||
| Men | 173.33 (6.93) | 171.89 (7.33) | 172.55 (6.78) | 174.15 (7.05) | 175.91 (7.86) | 172.68 (6.65) | |
| Women | 160.06 (5.84) | 157.57 (6.00) | 158.90 (5.86) | 159.59 (6.01) | 157.84 (6.58) | 154.10 (6.71) | a, e |
| CRP (mg/L), median (IQR) | 0.84 (0.31–2.09) | 1.01 (0.38–2.75) | 1.50 (0.76–3.24) | 1.97 (0.89–4.24) | 2.54 (1.20–5.50) | 2.69 (0.58–8.59) | b, c, d, e |
| HOMA-IR, median (IQR) | 1.68 (1.11–2.53) | 1.99 (1.18–3.48) | 2.93 (1.96–4.76) | 3.09 (2.06–5.97) | 4.76 (2.80–8.53) | 4.61 (2.36–6.36) | a, b, c, d, e |
| Education, % | a, b, c, d | ||||||
| Basic (0–9 years) | 24.3 | 30.6 | 34.3 | 38.0 | 36.6 | 30.1 | |
| Intermediate (10–12 years) | 49.7 | 55.2 | 50.5 | 45.4 | 50.9 | 60.9 | |
| Higher (≥13 years) | 25.9 | 14.2 | 15.2 | 16.7 | 12.5 | 9.0 | |
| Physical activity, % | a, b, d, e | ||||||
| Inactive | 20.1 | 30.3 | 34.6 | 27.4 | 40.1 | 45.9 | |
| Moderate active | 65.2 | 49.9 | 53.3 | 57.0 | 48.9 | 46.0 | |
| Very active | 14.8 | 19.8 | 12.1 | 15.7 | 11.0 | 8.1 | |
| Smoking, % | c | ||||||
| Currently | 15.4 | 20.1 | 12.1 | 10.3 | 7.4 | 13.2 | |
| Former | 26.7 | 29.0 | 27.8 | 36.3 | 25.1 | 45.3 | |
| Never | 58.0 | 50.9 | 60.2 | 53.4 | 67.5 | 41.5 | |
| Alcohol consumption, % | b, d | ||||||
| Heavy | 5.6 | 6.7 | 4.2 | 5.9 | 9.5 | 4.4 | |
| Moderate | 55.5 | 43.3 | 46.9 | 44.5 | 34.6 | 51.1 | |
| No alcohol | 38.9 | 50.0 | 48.9 | 49.7 | 56.0 | 44.5 | |
| Type 2 diabetes, % | 4.3 | 21.7 | 10.6 | 14.7 | 25.7 | 21.5 | a, b, c, d, e |
| Depression, % | 4.9 | 6.1 | 8.5 | 13.4 | 5.0 | 13.5 | b, c |
| Asthma, % | 7.8 | 7.4 | 9.3 | 6.6 | 10.9 | 10.3 | |
| Chronic obstructive pulmonary disease, % | 3.2 | 6.0 | 2.5 | 2.2 | 5.3 | 4.4 | |
| Congestive heart disease, % | 2.5 | 8.0 | 6.1 | 0.8 | 6.5 | 10.5 | a, b, e |
| Coronary heart disease, % | 15.0 | 17.7 | 19.6 | 15.4 | 10.0 | 16.9 | b, c |
| Hypertension, % | 29.3 | 37.6 | 42.1 | 48.5 | 56.1 | 54.6 | b, d, e |
| Hip osteoarthritis, % | 3.4 | 11.2 | 6.9 | 2.5 | 6.3 | 4.1 | a, b |
| Knee osteoarthritis, % | 4.7 | 14.3 | 12.5 | 8.3 | 16.9 | 19.1 | a, b, d, e |
Notes: CRP = C-reactive protein; HOMA-IR = homeostasis model assessment indicating insulin resistance; IQR = interquartile range. Values are shown in mean (SD) and medians (IQR) for continuous variables and percentages for categorical variables.
Indicates significant (p < .05) difference between “never obese” and other groups in pairwise comparisons.
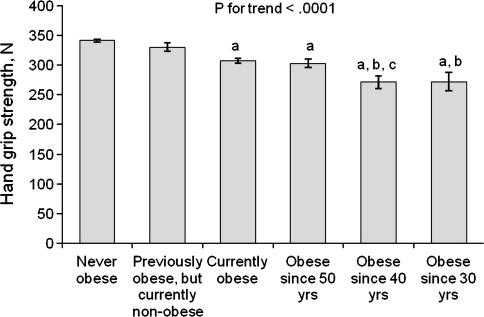
In Figure 1, the mean level of hand grip strength is presented in different obesity history categories. The earlier the obesity onset had been the lower the current hand grip strength was (p for trend <.0001). Furthermore, linear regression analyses showed a significant negative association between CRP and hand grip strength (p < .0001) and HOMA-IR and hand grip strength (p < .0001).
Figure 1.
Mean hand grip strength (standard error) by hierarchical classification of obesity history. Adjusted for age, sex, and current body weight. Letters indicate a statistical significant difference (p < .05) between categories. a = never obese; b = previously obese, but currently nonobese; c = currently obese.
In Table 2, the regression coefficients for the association between obesity history and hand grip strength are provided. After adjusting for education, lifestyle factors, and prevalent chronic diseases (Model 2), regression coefficients decreased 18%–23% among obese compared with Model 1, but the between-group differences remained statistically significant. In Model 2, age, sex, current body weight, physical activity, smoking, alcohol consumption, type 2 diabetes, and depression were independently associated with hand grip strength (p for all <.05). The mediating role of inflammation in the association between obesity history and hand grip strength was examined by adjusting for CRP (Model 3). All the differences in comparison to “never obese” groups remained statistically significant except for the “previously obese” and the regression coefficient decreased 17%–32% among obese compared with Model 1. CRP remained significant in the model (p < .0001). Similarly, the mediating role of insulin resistance in the association between obesity history and hand grip strength was examined by adjusting for HOMA-IR (Model 4). There were no major changes in group comparisons, the regression coefficient decreased 20%–26% among obese compared with Model 1 and HOMA-IR remained significant in the model (p = .002).
Table 2.
Regression Coefficients (With SE) of Hand Grip Strength by Hierarchical Classification of Obesity History
| Never Obese | Previously Obese, but Currently Nonobese |
Currently Obese |
Obese Since 50 years |
Obese Since 40 years |
Obese Since 30 years |
||||||
| Regression Coefficient (SE) | p | Regression Coefficient (SE) | p | Regression Coefficient (SE) | p | Regression Coefficient (SE) | p | Regression Coefficient (SE) | p | ||
| Model 1 | Reference | −9.16 (7.51) | 0.22 | −33.25 (5.15) | <.0001 | −37.90 (7.80) | <.0001 | −67.03 (11.18) | <.0001 | −68.83 (15.45) | <.0001 |
| Model 2 | Reference | 0.41 (7.49) | 0.96 | −27.17 (5.11) | <.0001 | −29.07 (7.74) | 0.0002 | −53.38 (11.11) | <.0001 | −56.30 (15.22) | 0.0002 |
| % Change* | 105 | 18 | 23 | 20 | 18 | ||||||
| Model 3 | Reference | 0.54 (7.83) | 0.95 | −24.58 (5.14) | <.0001 | −25.74 (7.81) | 0.0010 | −47.99 (11.05) | <.0001 | −57.19 (15.39) | 0.0002 |
| % Change* | 106 | 26 | 32 | 28 | 17 | ||||||
| Model 4 | Reference | −0.31 (7.46) | 0.97 | −25.86 (5.11) | <.0001 | −28.19 (7.71) | 0.0003 | −51.80 (11.08) | <.0001 | −55.10 (15.17) | 0.0003 |
| % Change* | 97 | 22 | 26 | 23 | 20 | ||||||
Notes: SE = standard error. Model 1: adjusted for age, sex, and current body weight; Model 2: Model 1 + adjusted for education, physical activity, smoking, alcohol use, and diseases (diabetes, hypertension, depression, knee osteoarthritis); Model 3: Model 2 + adjusted for log C-reactive protein (CRP); Model 4: Model 2 + adjusted for log homeostasis model assessment (HOMA-IR).
Percentage change in regression coefficients from Model 1 is computed by (regression coefficientModel1 − regression coefficientModel2/3/4/5/6)/regression coefficientModel1 × 100.
In Table 3, the odds ratios for very low relative muscle strength, defined here as the lowest decile of relative hand grip strength, are presented by obesity history categories. In comparison to “never obese” group, the odds for very low relative muscle strength increased the earlier the obesity onset had been. After adjusting for education, lifestyle factors, and chronic diseases (Model 2), the odds (95% confidence interval) were 2.76 (1.78–4.28) for currently obese, 5.57 (3.02–10.28) for obese since age of 50 years, 6.53 (2.98–14.30) for obese since age of 40 years, and 10.36 (3.55–30.24) for obese since age of 30 years. Further adjustments for CRP and HOMA-IR slightly decreased odds ratios (Models 3 and 4). Separate analysis was carried out among obese persons using “currently obese” as a reference group. Earlier onset of obesity was associated with 2.0–3.9 times higher odds for very low relative muscle strength compared with recently acquired obesity. Due to the small number of participants, the confidence intervals of odds ratios overlapped in comparison of participants who had been obese since age of 50, 40, and 30 years and no significant group differences were observed.
Table 3.
OR (95% CI) of Very Low Relative Muscle Strength* According to Hierarchical Classification of Obesity History
| Never Obese | Previously Obese, but Currently Nonobese |
Currently Obese |
Obese Since 50 years |
Obese Since 40 years |
Obese Since 30 years |
||||||
| OR | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Model 1 | |||||||||||
| All | 1.00 | 0.95 | 0.36–2.55 | 3.45 | 2.27–5.24 | 6.97 | 3.86–12.60 | 9.55 | 4.50–20.28 | 15.53 | 5.73–42.11 |
| Only obese | 1.00 | 2.02 | 1.10–3.73 | 2.73 | 1.27–5.86 | 4.60 | 1.67–12.65 | ||||
| Model 2 | |||||||||||
| All | 1.00 | 0.69 | 0.25–1.90 | 2.76 | 1.78–4.28 | 5.57 | 3.02–10.28 | 6.53 | 2.98–14.30 | 10.36 | 3.55–30.24 |
| Only obese | 1.00 | 2.09 | 1.10–3.96 | 2.49 | 1.11–5.60 | 3.96 | 1.31–11.99 | ||||
| Model 3 | |||||||||||
| All | 1.00 | 0.46 | 0.14–1.49 | 2.50 | 1.60–3.91 | 4.74 | 2.54–8.84 | 5.11 | 2.31–11.29 | 9.19 | 3.03–27.89 |
| Only obese | 1.00 | 2.02 | 1.06–3.84 | 2.31 | 1.02–5.20 | 3.94 | 1.28–12.18 | ||||
| Model 4 | |||||||||||
| All | 1.00 | 0.68 | 0.24–1.90 | 2.42 | 1.53–3.84 | 4.83 | 2.57–9.08 | 5.41 | 2.41–12.13 | 8.87 | 3.01–26.17 |
| Only obese | 1.00 | 2.06 | 1.08–3.91 | 2.36 | 1.05–5.29 | 3.91 | 1.29–11.87 | ||||
Notes: CI = confidence interval; OR = odds ratio. Model 1: adjusted for age and sex; Model 2: Model 1 + adjusted for education, physical activity, smoking, alcohol use, and diseases (diabetes, hypertension, depression, knee osteoarthritis); Model 3: Model 2 + adjusted for log C-reactive protein (CRP); Model 4: Model 2 + adjusted for log homeostasis model assessment (HOMA-IR).
Very low relative muscle strength is defined as the lowest decile of relative hand grip strength (hand grip strength/current body weight).
DISCUSSION
Among persons aged 55 years and older, obesity history was associated with current hand grip strength and this influence went beyond the effects of current body weight, lifestyle factors, and chronic conditions. The earlier the obesity onset had been the lower was hand grip strength in old age. A positive dose–response effect of obesity duration on CRP and HOMA-IR was also observed. To our knowledge, this is the first attempt to estimate the effect of obesity duration on muscle strength. Our results contribute to understanding the variation in hand grip strength in older age. Moreover, the results of this study confirm earlier findings about obesity duration on physical performance (21) and mobility disability (22,23).
There are several pathways through which an extended exposure to obesity might influence muscle strength, independent of the level of BMI achieved. In this study, the mediating roles of inflammation and insulin resistance were examined. Earlier obesity onset correlated with higher levels of CRP and HOMA-IR that, in turn, correlated with lower hand grip strength. Nevertheless, higher levels of CRP and HOMA-IR explained only a small proportion of lower hand grip strength gradient with increasing obesity duration.
However, when interpreting the findings, it must be emphasized that we had longitudinal information only on body weight. The level of hand grip strength, inflammation, or insulin resistance earlier in life is not known. Therefore, it is also unknown whether obesity duration, per se, and its metabolic consequences caused poor hand grip strength or if some persons had low strength already before becoming obese, for example, due to sedentary lifestyle, which in turn can facilitate weight gain. However, current literature supports our hypothesis because both subclinical inflammation and insulin resistance are known consequences of obesity and risk factors for low muscle strength (7,9,10,24). In addition, a number of studies have shown that the duration of obesity is a risk factor for insulin resistance (25) and type 2 diabetes (26–29).
In the present study, obesity duration was defined based on BMI values across the adult life span. BMI was used as an indicator of overall obesity, but it does not reflect body composition or body fat distribution. Unfortunately, we did not have longitudinal data on any other obesity indicators, such as waist circumference or waist-to-hip ratio. Abdominal obesity is particularly interesting because adipose tissue in the visceral area is known to produce high amounts of inflammatory cytokines and adipokines (5,30) and is also closely related to insulin resistance (8). Also other features of unfavorable body fat distribution such as increased muscle fat infiltration can provide explanation for decreased hand grip strength among persons with long obesity history through decreased muscle quality, increased subclinical inflammation, and insulin resistance (31,32).
In the future, prospective longitudinal studies are needed to examine more closely the biologic mechanisms that underlie the association between obesity duration and decreased muscle strength. In addition to inflammation and insulin resistance, especially the role of physical activity, diabetes, and depression should be examined because they showed independent association with hand grip strength and were associated with obesity duration. For example, early onset of overweight and obesity may lead to reduced physical activity contributing to decreased muscle strength. Unfortunately, we did not have information on physical activity history; thus, the role of physical activity in younger years on the association between obesity history and hand grip strength could not be evaluated in this study. Recognizing these intermediate factors is important in terms of timely interventions. If obesity or the obesity-related intermediate consequences are not intervened, extended duration of obesity may lead to other negative health consequences, such as metabolic syndrome (33), knee osteoarthritis (34), functional limitations (21–23), and hospitalization (35).
Because the prevalence of overweight and obesity have increased also in the younger population, growing number of people will be suffering from obesity for decades. The results of this study suggest that a long-lasting obesity may predispose to decreased muscle strength, thus potentially endangering to an imbalance between fat and muscle mass or strength. This condition has recently been termed as sarcopenic obesity (3,36,37) or dynapenic obesity (38) and is especially associated with poor physical functioning in older adults (38,39).
Some limitations of the present study should be recognized. First, weights at age of 20, 30, 40, and 50 years were obtained retrospectively and may therefore reduce validity of the findings. However, several studies have examined the accuracy of recalled weight information and it has shown to be good (40,41). Factors that might influence on the recall accuracy, include current body weight, sex, race, and recall period (40,41), were controlled in our analyses (except for race). Second, we did not have information on body height at age of 30, 40, and 50 years, therefore objectively measured current height was used to calculate BMI. Because body height decreases with age, this might have caused slight overestimation of BMI at earlier years. Third, because hand grip strength was used as a proxy for overall muscle strength (14,15), future studies should examine whether the results apply also when using lower extremity strength, which is directly affected by body weight. Finally, the only available measure of low-grade inflammation was CRP and insulin resistance was not measured directly, but was estimated based on the HOMA-IR. Our results should be confirmed by using other measures of inflammation and insulin resistance.
In conclusion, long-term exposure to obesity is associated with poor hand grip strength later in life in a representative population-based sample of persons aged 55 years and older. Given the increasing prevalence of overweight and obesity at younger ages, the number of years that obese older adults will live with obesity is also increasing. This may lead to health risks associated with obesity as well as to increase the number of sarcopenic obese persons among the future generations of older adults. Thus, maintaining healthy body weight throughout the life span may help to prevent or delay muscle strength decline in old age. Further prospective studies with information on physical activity history and prior muscle strength are needed to examine in detail the causal association between obesity history and muscle strength.
FUNDING
This study was supported in part by a grant from the Juho Vainio Foundation, Finland, and in part by the Intramural Research Program of the National Institutes of Health, National Institute on Aging.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Acknowledgments
The authors would like to thank the volunteers and the research staff of the Health 2000 Survey.
References
- 1.Rantanen T, Harris T, Leveille SG, et al. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci. 2000;55A:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Penninx BWJH, Volpato S, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–888. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 4.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- 5.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008;32:772–779. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 6.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-α with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57A:M326–M332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 7.Schaap LA, Pluijm SM, Deeg DJ, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–1189. doi: 10.1093/gerona/glp097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 9.Abbatecola AM, Ferrucci L, Ceda G, et al. Insulin resistance and muscle strength in older persons. J Gerontol A Biol Sci Med Sci. 2005;60A:1278–1282. doi: 10.1093/gerona/60.10.1278. [DOI] [PubMed] [Google Scholar]
- 10.Barzilay JI, Cotsonis GA, Walston J, et al. Insulin resistance is associated with decreased quadriceps muscle strength in nondiabetic adults aged >or=70 years. Diabetes Care. 2009;32:736–738. doi: 10.2337/dc08-1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the Health, Aging, and Body Composition study. Diabetes Care. 2007;30:1507–1512. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 12.Aromaa A, Koskinen S, editors. Health and Functional Capacity in Finland. Baseline Results of the Health 2000 Health Examination Survey. Helsinki, Finland: Publications of the National Public Health Institute B12/2004; 2004. http://www.terveys2000.fi/julkaisut/baseline.pdf. Accessed May 10, 2010. [Google Scholar]
- 13.World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Geneva, Switzerland: WHO Technical Report Series 894; 2000. Report of a WHO Consultation. [PubMed] [Google Scholar]
- 14.Lauretani F, Russo C, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 15.Rantanen T, Era P, Kauppinen M, Heikkinen E. Maximal isometric muscle strength and socioeconomic status, health, and physical activity in 75-year-old persons. J Aging Phys Act. 1994;2:206–220. [Google Scholar]
- 16.Sainio P, Koskinen S, Natunen S. Functional capacity. In: Heistaro S, editor. Methodology Report. Health 2000 Survey. Helsinki, Finland: Publications of the National Health Institute B26/2008; 2008. pp. 92–105. http://www.terveys2000.fi/doc/methodologyrep.pdf. Accessed May 10, 2010. [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 19.Yearbook of Alcohol and Drug Statistics. Helsinki, Finland: National Research and Development Centre for Welfare and Health (STAKES); 2003. [Google Scholar]
- 20.Wilhelmsen L, Tibblin G, Werko L. A primary preventive study of Gothenburg, Sweden. Prev Med. 1972;1:153–160. doi: 10.1016/0091-7435(72)90082-5. [DOI] [PubMed] [Google Scholar]
- 21.Houston DK, Ding J, Nicklas BJ, et al. The association between weight history and physical performance in the Health, Aging and Body Composition study. Int J Obes (Lond) 2007;31:1680–1687. doi: 10.1038/sj.ijo.0803652. [DOI] [PubMed] [Google Scholar]
- 22.Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the Health, Aging and Body Composition Study. Am J Epidemiol. 2009;169:927–936. doi: 10.1093/aje/kwp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenholm S, Rantanen T, Alanen E, Reunanen A, Sainio P, Koskinen S. Obesity history as a predictor of walking limitation at old age. Obesity. 2007;15:929–938. doi: 10.1038/oby.2007.583. [DOI] [PubMed] [Google Scholar]
- 24.Schrager MA, Metter EJ, Simonsick E, et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919–925. doi: 10.1152/japplphysiol.00627.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlsson S, Persson PG, Alvarsson M, et al. Weight history, glucose intolerance, and insulin levels in middle-aged Swedish men. Am J Epidemiol. 1998;148:539–545. doi: 10.1093/oxfordjournals.aje.a009679. [DOI] [PubMed] [Google Scholar]
- 26.Wannamethee SG, Shaper AG. Weight change and duration of overweight and obesity in the incidence of type 2 diabetes. Diabetes Care. 1999;22:1266–1272. doi: 10.2337/diacare.22.8.1266. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai Y, Teruya K, Shimada N, et al. Association between duration of obesity and risk of non-insulin-dependent diabetes mellitus. The Sotetsu Study. Am J Epidemiol. 1999;149:256–260. doi: 10.1093/oxfordjournals.aje.a009800. [DOI] [PubMed] [Google Scholar]
- 28.Pontiroli AE, Galli L. Duration of obesity is a risk factor for non-insulin-dependent diabetes mellitus, not for arterial hypertension or for hyperlipidaemia. Acta Diabetol. 1998;35:130–136. doi: 10.1007/s005920050117. [DOI] [PubMed] [Google Scholar]
- 29.Everhart JE, Pettitt DJ, Bennett PH, Knowler WC. Duration of obesity increases the incidence of NIDDM. Diabetes. 1992;41:235–240. doi: 10.2337/diab.41.2.235. [DOI] [PubMed] [Google Scholar]
- 30.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56:1010–1013. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 31.Goodpaster BH, Krishnaswami S, Resnick H, et al. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 32.Delmonico MJ, Harris TB, Visser M, et al. Longitudinal study of muscle strength, quality, and adipose tissue infiltration. Am J Clin Nutr. 2009;90(6):1579–1585. doi: 10.3945/ajcn.2009.28047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Janssen I, Katzmarzyk PT, Ross R. Duration of overweight and metabolic health risk in American men and women. Ann Epidemiol. 2004;14:585–591. doi: 10.1016/j.annepidem.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Manninen P, Riihimäki H, Heliövaara M, Suomalainen O. Weight changes and the risk of knee osteoarthritis requiring arthroplasty. Ann Rheum Dis. 2004;63:1434–1437. doi: 10.1136/ard.2003.011833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schafer MH, Ferraro KF. Obesity and hospitalization over the adult life course: does duration of exposure increase use? J Health Soc Behav. 2007;48:434–449. doi: 10.1177/002214650704800407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr Metab Cardiovasc Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 37.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity—definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouchard DR, Janssen I. Dynapenic-obesity and physical function in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:71–77. doi: 10.1093/gerona/glp159. [DOI] [PubMed] [Google Scholar]
- 39.Stenholm S, Alley D, Bandinelli S, et al. The effect of obesity combined with low muscle strength on decline in mobility in older persons: results from the InCHIANTI Study. Int J Obes (Lond) 2009;33:635–644. doi: 10.1038/ijo.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perry GS, Byers TE, Mokdad AH, Serdula MK, Williamson DF. The validity of self-reports of past body weights by U.S. adults. Epidemiology. 1995;6:61–66. doi: 10.1097/00001648-199501000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Tamakoshi K, Yatsuya H, Kondo T, et al. The accuracy of long-term recall of past body weight in Japanese adult men. Int J Obes Relat Metab Disord. 2003;27:247–252. doi: 10.1038/sj.ijo.802195. [DOI] [PubMed] [Google Scholar]