Abstract
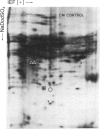
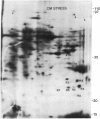
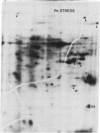
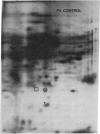
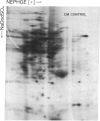
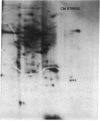
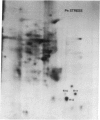
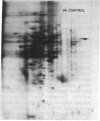
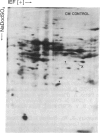
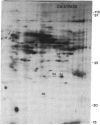
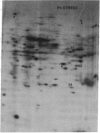
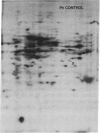
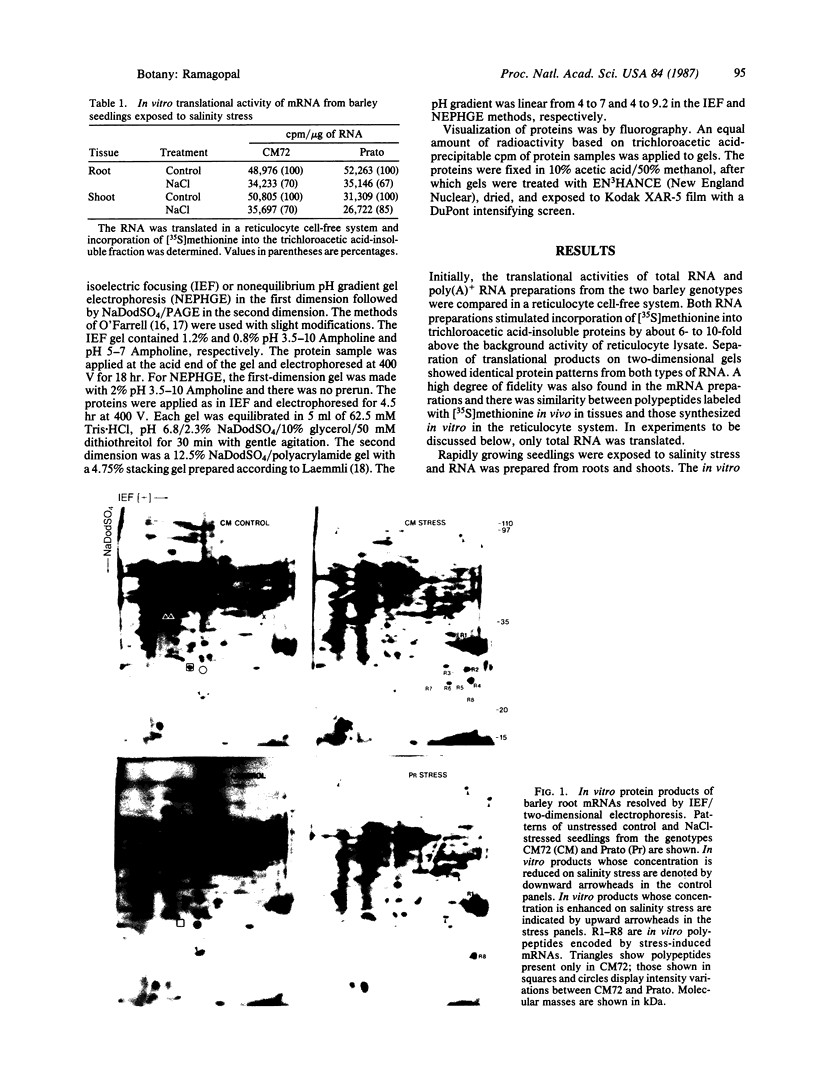
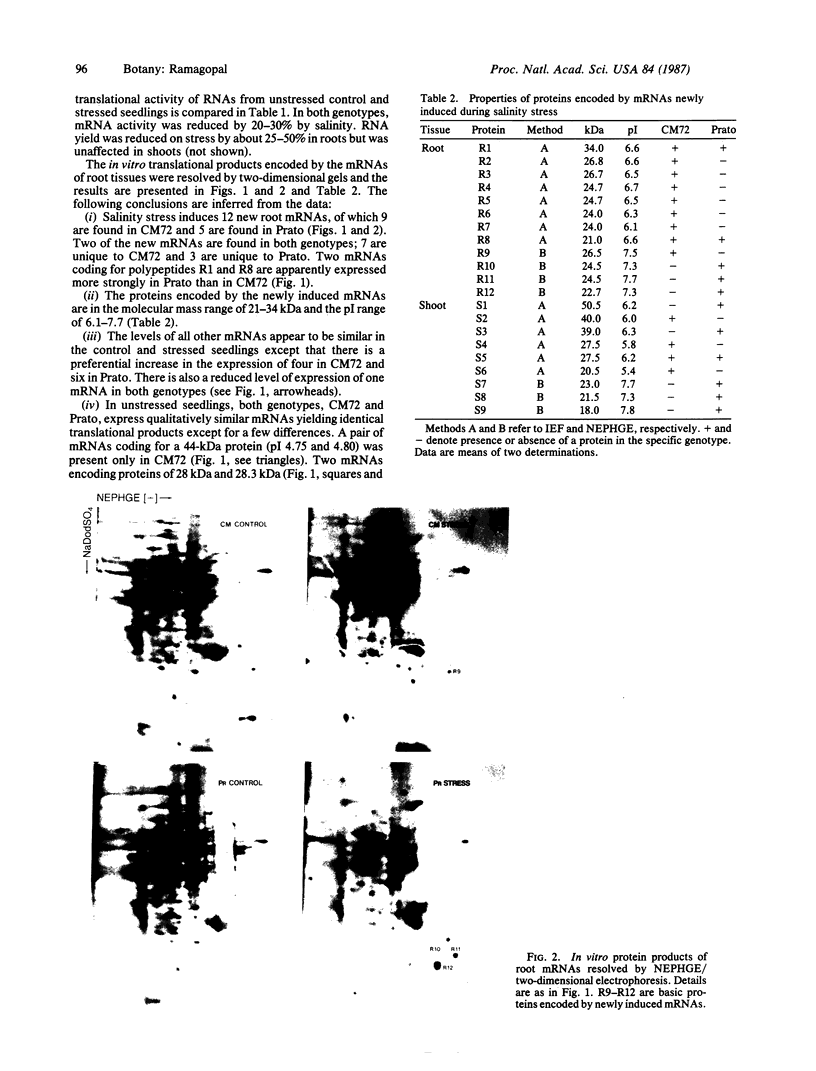
The molecular and genetic bases of salinity tolerance in plants are not understood. Gene expression at the mRNA level was investigated in a salt-tolerant and a salt-sensitive genotype of barley. Seedlings were exposed to NaCl stress and translatable mRNAs were isolated from root and shoot tissues. A reticulocyte cell-free system was programed with barley mRNAs and the in vitro products were resolved on two-dimensional polyacrylamide gels following isoelectric focusing or nonequilibrium pH gradient gel electrophoresis in the first dimension. The functional mRNAs in unstressed seedlings were qualitatively almost indistinguishable in the two genotypes. However, salinity stress triggered differential transcription of specific mRNAs depending upon genotype and tissue. In roots, 12 new mRNAs were induced that encoded proteins of 21-34 kDa, with a pI range of 6.1-7.7. In shoots, the 9 new mRNAs coded for proteins of 18-50.5 kDa, with a pI range of 5.4-7.8. These new stress mRNAs represented one of two main classes. Class I consisted of mRNAs shared by both genotypes. Class II represented mRNAs specific to each genotype; unique mRNAs of roots accumulated preferentially in the salt-tolerant genotype, whereas those of shoots accumulated in the salt-sensitive genotype. The findings suggest that transcriptional as well as posttranscriptional mechanisms regulate gene expression in barley during salinity stress.
Keywords: salt tolerance, gene expression, genotypic variation, tissue specificity
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ericson M. C., Alfinito S. H. Proteins Produced during Salt Stress in Tobacco Cell Culture. Plant Physiol. 1984 Mar;74(3):506–509. doi: 10.1104/pp.74.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick P., Dvorák J. Gene induction and repression by salt treatment in roots of the salinity-sensitive Chinese Spring wheat and the salinity-tolerant Chinese Spring x Elytrigia elongata amphiploid. Proc Natl Acad Sci U S A. 1987 Jan;84(1):99–103. doi: 10.1073/pnas.84.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy C. L., Niemi K. J., Brambl R. Altered gene expression during cold acclimation of spinach. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3673–3677. doi: 10.1073/pnas.82.11.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key J. L., Lin C. Y., Chen Y. M. Heat shock proteins of higher plants. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3526–3530. doi: 10.1073/pnas.78.6.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsbury R. W., Epstein E., Pearcy R. W. Physiological responses to salinity in selected lines of wheat. Plant Physiol. 1984 Feb;74(2):417–423. doi: 10.1104/pp.74.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Ramagopal S., Ennis H. L. Ribosomal protein synthesis during spore germination and vegetative growth in Dictyostelium discoideum. J Biol Chem. 1982 Jan 25;257(2):1025–1031. [PubMed] [Google Scholar]
- Rush D. W., Epstein E. Genotypic Responses to Salinity: Differences between Salt-sensitive and Salt-tolerant Genotypes of the Tomato. Plant Physiol. 1976 Feb;57(2):162–166. doi: 10.1104/pp.57.2.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]