Abstract
Acquired renal tubular disorder can be observed in various disease processes, especially autoimmune diseases. Gitelman syndrome is an autosomal recessive disease characterized by hypokalemic metabolic alkalosis, hypomagnesemia, and hypocalciuria. This disorder is caused by mutation in the SLC12A3 gene, which encodes the thiazide-sensitive NaCl cotransporter (NCCT). Acquired Gitelman syndrome has been reported and the majority has been associated with Sjögren's syndrome. The presence of circulating auto-antibodies to NCCT was suggested as a mechanism of acquired Gitelman syndrome. Treatment of acquired Gitelman syndrome was done with supplements of potassium and magnesium and prednisone was effective in some cases. Acquired Gitelman syndrome should be included in the differential diagnosis of renal involvement in patients with autoimmune diseases, especially Sjögren's syndrome.
Keywords: Gitelman syndrome, Sjögren's syndrome, thiazide-sensitive NaCl cotransporter
Introduction
Gitelman syndrome is an autosomal recessive disease characterized by hypokalemic metabolic alkalosis, hypomagnesemia, and hypocalciuria1). This disorder is caused by mutation in the SLC12A3 gene, which encodes the thiazide-sensitive NaCl cotransporter (NCCT). Acquired renal tubular disorder can be observed in various disease processes, especially autoimmune diseases2-10). Acquired Gitelman syndrome associated with autoimmune disease is rare and four cases have been reported in the medical literature8, 10-12). The mechanism underlying acquired Gitelman syndrome associated with autoimmune disease is not clear. Recently, the presence of circulating auto-antibodies against NCCT in a patient with autoimmune disease was reported8).
In this review article, we will summarize the acquired Gitelman syndrome associated with autoimmune disease and discuss the presence of circulating auto-antibodies against tubular transporter as a mechanism of acquired renal tubular disorders.
Inherited Gitelman syndrome
Inherited Gitelman syndrome is caused by mutations in SLC12A3 gene encoding NCCT13). NCCT is expressed at the apical membrane of the distal convoluted tubule (DCT), and loss-of-function mutation in NCCT leads to disruption of Na+ and Cl- reabsorption in the DCT. Decreased Na+ reabsorption in the DCT leads to increased sodium delivery to the collecting tubule resulting in mild volume contraction, which activates the renin-angiotensin-aldosterone system. Aldosterone stimulated secretion of potassium and hydrogen ions finally results in mild hypokalemic metabolic alkalosis. The mechanisms leading to hypomagnesemia and hypocalciuria in Gitelman syndrome remain unclear. Thiazide-induced hypocalciuria and hypomgnesemia have been explained by passive Ca2+ reabsorption in proximal tubules and decreased epithelial Mg2+ channel TRPM614). Hypomagnesemia and hypocalciuria in Gitelman syndrome are suggested to have a similar mechanism of thiazide-induced hypocalciuria and hypomagnesemia because the electrolyte disturbances of Gitelman syndrome resemble those observed with chronic administration of thiazide diuretics.
Acquired Gitelman syndrome
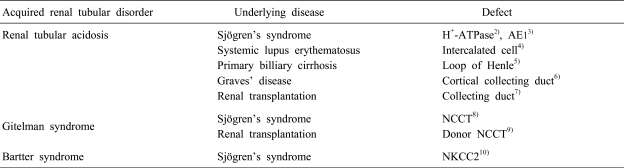
Acquired renal tubular disorder can be observed in various disease processes. Myeloma light chains, amyloidosis and disorder of vitamin D metabolism have been reported as causes of acquired renal tubular disorder, but the most frequent causes of acquired renal tubular disorder are autoimmune diseases such as systemic lupus erythematosus, Sjögren's syndrome, autoimmune thyroiditis and primary biliary cirrhosis. Table 1 shows acquired renal tubular disorder in various autoimmune diseases.
Table 1.
Acquired Renal Tubular Disorders in Various Diseases
AE1, anion exchanger 1; NCCT, thiazide-sensitive NaCl cotransporter; NKCC2, sodium-chloride-potassium cotransporter.
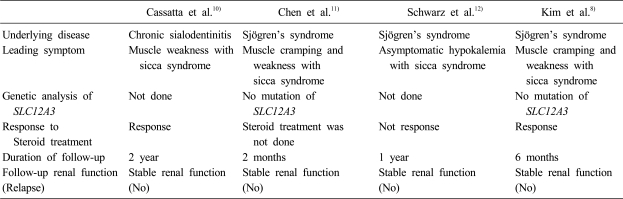
Acquired Gitelman syndrome is rare and five cases have been reported in the English literature. Four cases were associated with autoimmune diseases, Sjögren's syndrome, and one case with renal transplantation. Table 2 shows the clinical features of acquired Gitelman syndrome with Sjögren's syndrome. Cassatta et al. first described acquired Gitelman syndrome in a patient with chronic sialoadenitis10). Unfortunately, this case did not fit the diagnostic criteria for Sjögren's syndrome. Chen et al. reported on a patient with acquired Gitelman syndrome with primary Sjögren's syndrome, which met the criteria for Sjögren's syndrome11). Schwarz et al. added a case of acquired Gitelman syndrome with primary Sjögren's syndrome12). Kim et al. reported a patient with acquired Gitelman syndrome in primary Sjögren's syndrome and showed the presence of circulating auto-antobodies against NCCT8).
Table 2.
Review of Acquired Gitelman Syndrome in Sjögren's Syndrome
In three of four patients, the leading symptoms were muscular weakness and cramping of extremities with sicca syndrome. The diagnosis of acquired Gitelman syndrome in these cases was based upon the clinical and laboratory findings. Chen et al. proved the functional defect in NCCT using thiazide and furosemide testing, with the protocol reported by Colussi et al., Kim et al. first proved the defect in NCCT by immunohistochemical staining of NCCT. All four patients were treated with supplement of potassium and/or magnesium and/or spironolactone. In three of four patients, serum potassium and magnesium levels were improved and clinical symptoms were attenuated with supplement of potassium and/or magnesium. Steroid treatment was done in three patients and was effective in two patients8, 10). The prognosis of acquire Gitelman syndrome with primary Sjögren's syndrome may be good. In three of four patients, normokalemia was maintained during the follow-up period. No patient had renal insufficiency or relapse.
Circulating auto-antibodies and acquired Gitelman syndrome
Some investigations have been done on the pathogenensis of acquired tubular dysfunction associated with autoimmune diseases. Formation of auto-antibodies against various tubular transporters was suspected and much effort was given to detect the auto-antibodies. Walsh et al. documented the immunohistochemical comparison of primary distal RTA versus acquired distal RTA associated with Sjögren's syndrome3). They performed immunohistochemical staining of renal tissue from a patient with a unique SLC4A1 mutation, SA613F encoding the anion exchanger AE1 and tissue from another patient with autoimmiune distal RTA due to Sjögren's syndrome. In primary distal RTA, the expression and location of AE1 and vacuolar H+-ATPase (vH+-ATPase) were altered. However, neither protein could be detected in acquired distal RTA. These findings indicate that the pathogenesis of primary and acquired distal RTA is not the same. Bastini et al in other report detected auto-antibodies against vH+-ATPase in a patient with distal RTA in Sjögren's syndrome4).
For the pathogenesis of acquire Gitelman syndrome with primary Sjögren's syndrome, recently Kim et al. reported the presence of circulating auto-antobodies against NCCT8). They incubated the serum of the patient, who was diagnosed as acquired Gitelman syndrome and primary Sjögren's syndrome, to renal tissue from a normal mouse. They compared the staining pattern of the incubated normal mouse kidney with rabbit polyclonal anti-NCCT antibody. The incubation of the patient's serum with normal mouse kidney tissue showed similar patterns of NCCT in the DCT compared to the incubation of normal mouse kidney with the rabbit polyclonal anti-NCCT antibody. These findings suggest that the presence of the circulating auto-antibodies in the patient's serum that were reactive to normal mouse NCCT.
Summary
Gitelman syndrome is an inherited disease. However, Gitelman syndrome can be acquired in patients with autoimmune diseases, especially Sjögren's syndrome. The presence of circulating auto-antibodies to NCCT was suggested as a mechanism of acquired Gitelman syndrome. The leading symptoms of acquired Gitelman syndrome associated with Sjögren's syndrome were muscular weakness and cramping of extremities with sicca syndrome. Treatment of acquired Gitelman syndrome was done with supplements of potassium and magnesium and prednisone was effective in some cases. The prognosis of acquired Gitelman syndrome appears to be good. Although, until now, only 4 cases of acquired Gitelman syndrome associated with Sjögren's syndrome were reported, we suspect that acquired Gitelman syndrome is underreported. Acquired Gitelman syndrome should be included in the differential diagnosis of renal involvement in patients with autoimmune diseases, especially Sjögren's syndrome.
References
- 1.Gitelman HJ, Graham JB, Welt LG. A new familial disorder characterized by hypokalemia and hypomagnesemia. Trans Assoc Am Physicians. 1966;79:221–235. [PubMed] [Google Scholar]
- 2.Bastani B, Haragsim L, Gluck S, Siamopoulos KC. Lack of H-ATPase in distal nephron causing hypokalaemic distal RTA in a patient with Sjogren's syndrome. Nephrol Dial Transplant. 1995;10:908–909. [PubMed] [Google Scholar]
- 3.Walsh S, Turner CM, Toye A, Wagner C, Jaeger P, Laing C, et al. Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant. 2007;22:807–812. doi: 10.1093/ndt/gfl662. [DOI] [PubMed] [Google Scholar]
- 4.Bastani B, Underhill D, Chu N, Nelson RD, Haragsim L, Gluck S. Preservation of intercalated cell H(+)-ATPase in two patients with lupus nephritis and hyperkalemic distal renal tubular acidosis. J Am Soc Nephrol. 1997;8:1109–1117. doi: 10.1681/ASN.V871109. [DOI] [PubMed] [Google Scholar]
- 5.Chanarin I, Loewi G, Tavill AS, Swain CP, Tidmarsh E. Defect of renal tubular acidification with antibody to loop of Henle. Lancet. 1974;2:317–318. doi: 10.1016/s0140-6736(74)91694-8. [DOI] [PubMed] [Google Scholar]
- 6.Konishi K, Hayashi M, Saruta T. Renal tubular acidosis with autoantibody directed to renal collecting-duct cells. N Engl J Med. 1994;331:1593–1594. doi: 10.1056/NEJM199412083312316. [DOI] [PubMed] [Google Scholar]
- 7.Heering P, Degenhardt S, Grabensee B. Tubular dysfunction following kidney transplantation. Nephron. 1996;74:501–511. doi: 10.1159/000189443. [DOI] [PubMed] [Google Scholar]
- 8.Kim YK, Song HC, Kim WY, Yoon HE, Choi YJ, Ki CS, et al. Acquired Gitelman syndrome in a patient with primary Sjogren syndrome. Am J Kidney Dis. 2008;52:1163–1167. doi: 10.1053/j.ajkd.2008.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Hu DC, Burtner C, Hong A, Lobo PI, Okusa MD. Correction of renal hypertension after kidney transplantation from a donor with Gitelman syndrome. Am J Med Sci. 2006;331:105–109. doi: 10.1097/00000441-200602000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Casatta L, Ferraccioli GF, Bartoli E. Hypokalaemic alkalosis, acquired Gitelman's and Bartter's syndrome in chronic sialoadenitis. Br J Rheumatol. 1997;36:1125–1128. doi: 10.1093/rheumatology/36.10.1125. [DOI] [PubMed] [Google Scholar]
- 11.Chen YC, Yang WC, Yang AH, Lin SH, Li HY, Lin CC. Primary Sjogren's syndrome associated with Gitelman's syndrome presenting with muscular paralysis. Am J Kidney Dis. 2003;42:586–590. doi: 10.1016/s0272-6386(03)00792-3. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz C, Barisani T, Bauer E, Druml W. A woman with red eyes and hypokalemia: a case of acquired Gitelman syndrome. Wien Klin Wochenschr. 2006;118:239–242. doi: 10.1007/s00508-006-0559-4. [DOI] [PubMed] [Google Scholar]
- 13.De Jong JC, Van Der Vliet WA, Van Den Heuvel LP, Willems PH, Knoers NV, Bindels RJ. Functional expression of mutations in the human NaCl cotransporter: evidence for impaired routing mechanisms in Gitelman's syndrome. J Am Soc Nephrol. 2002;13:1442–1448. doi: 10.1097/01.asn.0000017904.77985.03. [DOI] [PubMed] [Google Scholar]
- 14.Nijenhuis T, Vallon V, van der Kemp AW, Loffing J, Hoenderop JG, Bindels RJ. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J Clin Invest. 2005;115:1651–1658. doi: 10.1172/JCI24134. [DOI] [PMC free article] [PubMed] [Google Scholar]