Abstract
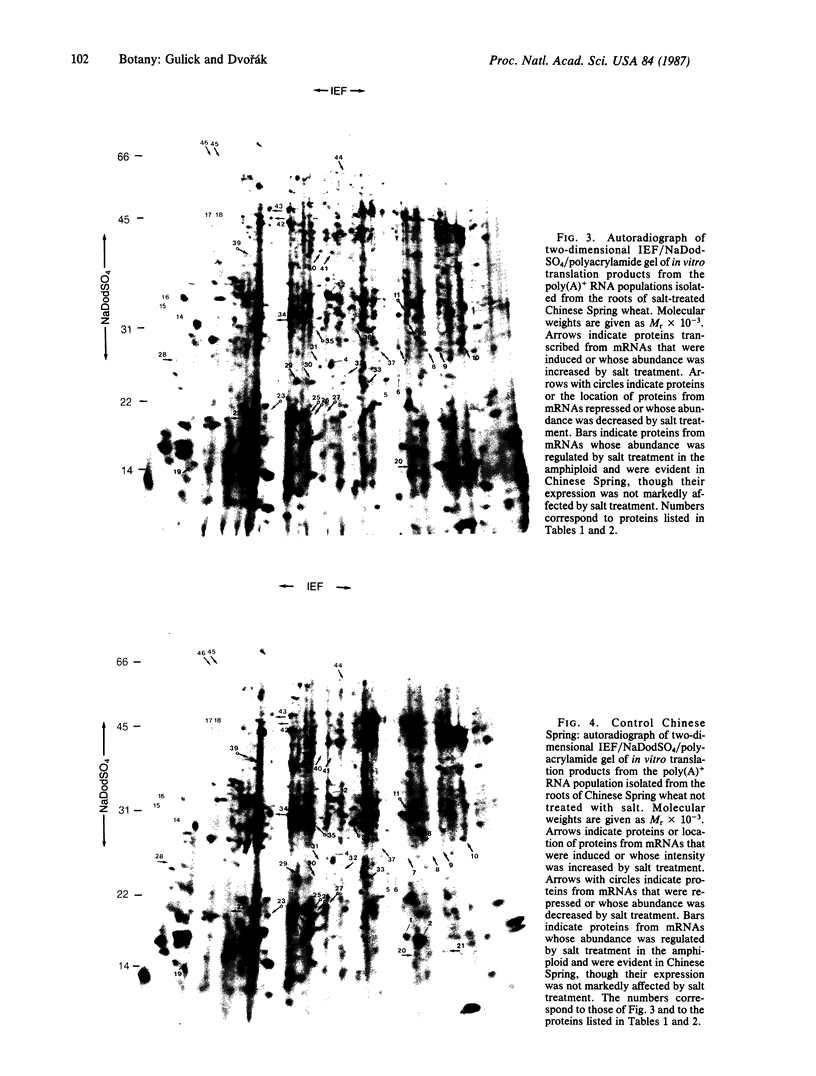
An artificial amphiploid from a cross between salinity-sensitive bread wheat cultivar Chinese Spring and highly tolerant Elytrigia elongata (Host) Nevski (= Agropyron elongatum Host) shows enhanced salinity tolerance relative to Chinese Spring. Poly(A)+ RNA was isolated from roots, expanding leaves, and old leaves from amphiploid and Chinese Spring plants prior to and after acclimation to high levels of NaCl in solution cultures. Two-dimensional gel electrophoresis of the in vitro translation products was used to compare these mRNA populations. The amphiploid had 10 mRNA species induced or enhanced and 8 species repressed in root tissue during acclimation to saline growth conditions. These 18 transcripts affected by salt treatment were also detected in wheat roots, but only 4 of these were similarly regulated. In Chinese Spring the acclimation to saline stress resulted in a marked change in the level of expression of 34 transcripts in root tissue; of these, 26 were detected in the amphiploid and only 6 were regulated as in the amphiploid. No differences were seen in gene expression between salt-treated and control plants in leaves and meristematic crowns and unexpanded leaves of the amphiploid.
Keywords: gene expression, mRNA, in vitro translation, stress tolerance, interspecific hybrid
Full text
PDF


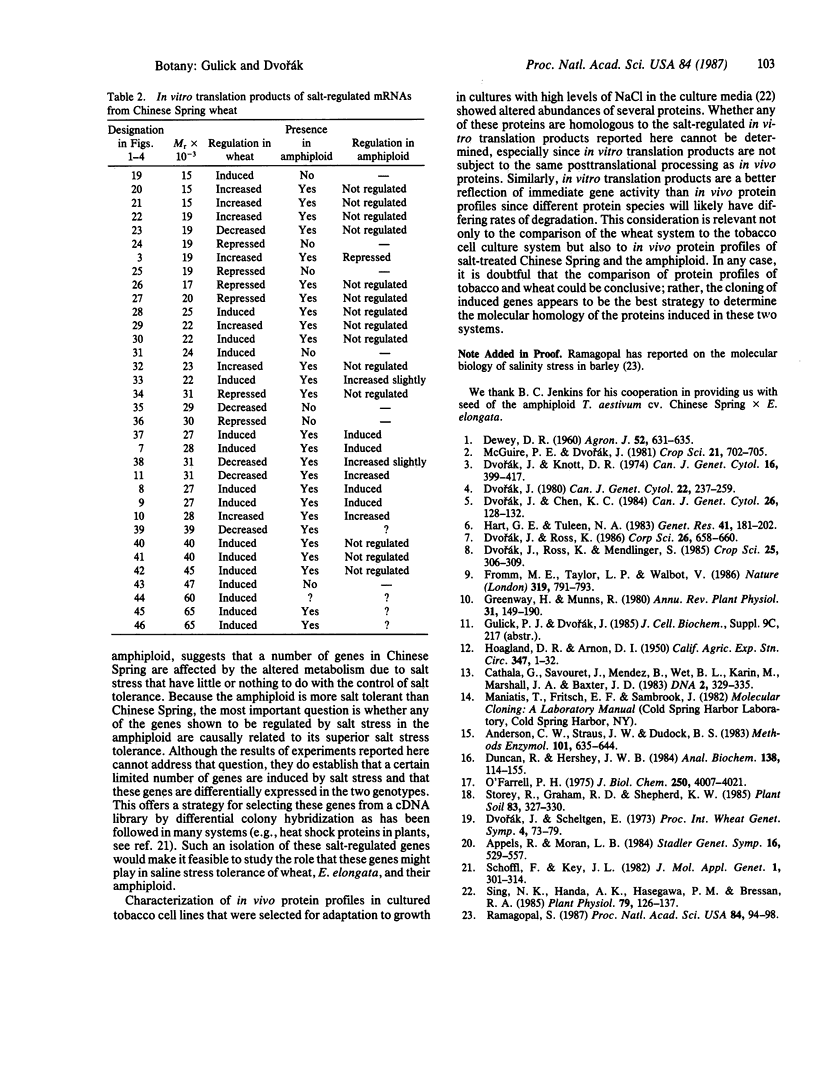
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Straus J. W., Dudock B. S. Preparation of a cell-free protein-synthesizing system from wheat germ. Methods Enzymol. 1983;101:635–644. doi: 10.1016/0076-6879(83)01044-7. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Duncan R., Hershey J. W. Evaluation of isoelectric focusing running conditions during two-dimensional isoelectric focusing/sodium dodecyl sulfate-polyacrylamide gel electrophoresis: variation of gel patterns with changing conditions and optimized isoelectric focusing conditions. Anal Biochem. 1984 Apr;138(1):144–155. doi: 10.1016/0003-2697(84)90783-8. [DOI] [PubMed] [Google Scholar]
- Fromm M. E., Taylor L. P., Walbot V. Stable transformation of maize after gene transfer by electroporation. 1986 Feb 27-Mar 5Nature. 319(6056):791–793. doi: 10.1038/319791a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ramagopal S. Differential mRNA transcription during salinity stress in barley. Proc Natl Acad Sci U S A. 1987 Jan;84(1):94–98. doi: 10.1073/pnas.84.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöffl F., Key J. L. An analysis of mRNAs for a group of heat shock proteins of soybean using cloned cDNAs. J Mol Appl Genet. 1982;1(4):301–314. [PubMed] [Google Scholar]
- Singh N. K., Handa A. K., Hasegawa P. M., Bressan R. A. Proteins Associated with Adaptation of Cultured Tobacco Cells to NaCl. Plant Physiol. 1985 Sep;79(1):126–137. doi: 10.1104/pp.79.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]