Abstract
Mutations in genes encoding ion channels, transporters, exchangers, and pumps in human tissues have been increasingly reported to cause hypokalemia. Assessment of history and blood pressure as well as the K+ excretion rate and blood acid-base status can help differentiate between acquired and inherited causes of hypokalemia. Familial periodic paralysis, Andersen's syndrome, congenital chloride-losing diarrhea, and cystic fibrosis are genetic causes of hypokalemia with low urine K+ excretion. With respect to a high rate of K+ excretion associated with faster Na+ disorders (mineralocorticoid excess states), glucoricoid-remediable aldosteronism and congenital adrenal hyperplasia due to either 11β-hydroxylase and 17α-hydroxylase deficiencies in the adrenal gland, and Liddle's syndrome and apparent mineralocorticoid excess in the kidney form the genetic causes. Among slow Cl- disorders (normal blood pressure, low extracellular fluid volume), Bartter's and Gitelman's syndrome are most common with hypochloremic metabolic alkalosis. Renal tubular acidosis caused by mutations in the basolateral Na+/HCO3- cotransporter (NBC1) in the proximal tubules, apical H+-ATPase pump, and basolateral Cl-/HCO3- exchanger (anion exchanger 1, AE1) in the distal tubules and carbonic anhydroase II in both are genetic causes with hyperchloremic metabolic acidosis. Further work on genetic causes of hypokalemia will not only provide a much better understanding of the underlying mechanisms, but also set the stage for development of novel therapies in the future.
Keywords: acid-base equilibrium, aldosterone, blood pressure, genes, hypokalemia, mutation, renin, urine electrolyte
Introdution
Potassium (K+), a major intracellular cation, is critically important in maintaining the osmotic equilibrium of the cell and electrical gradient across the cell membrane. Most of the cellular K+ in the body resides in the intracellular fluid (ICF) (~50 mEq/kg, 98%), largely in the skeletal muscles (3,000 mEq), red blood cells (300 mEq), and liver (200 mEq). In contrast, the total K+ content in the extracellular fluid (ECF) is very low (<1 mEq/kg, approximately 2%) and similar to the daily dietary intake and renal excretion of K+. Plasma K+ concentration is a function of total body K+ and the distribution between intracellular and extracellular stores. Hypokalemia, defined as plasma K+<3.5 mEq/L, is the most common electrolyte abnormality encountered in clinical practice and usually arises from redistribution of K+ from ECF to ICF stores and/or total body K+ depletion. The most significant effects of hypokalemia are cardiovascular, neuromuscular, renal, and metabolic, thus associated with higher morbidity and mortality1). Prompt diagnosis with appropriate management of hypokalemia avoids unnecessary testing and potential dire complications. The treatment of hypokalemia involves weighing the degree and timing of hypokalemia with clinical manifestations, underlying causes, associated conditions, and risks during therapy.
With the unprecedented progress in molecular and genetic analysis, many previously confusing phenotypic features of the inherited disorders can now be understood2). Of note, although hypokalemia in these genetic disorders may be the foremost finding, one should understand that hypokalemia per se is not a specific disease but an associated finding in a large number of different diseases3). An accurate diagnosis will help determine the molecular defect if the basis for hypokalemia is a genetic disorder. In this paper, our approach to hypokalemia is introduced and the genetic causes of hypokalemia are discussed to provide some insights in the field.
K+ homeostasis
K+ concentration in the ICF is close to 35-fold greater than in the ECF and daily K+ intake is approximately equal to the amount of K+ in the ECF. Maintaining normal serum K+ concentration in the ECF requires tight regulation of the distribution of K+ between the ICF and ECF (internal K+ balance) and renal K+ excretion (external balance)4). Derangements in either the internal balance (e.g. K+ shift) or external balance (e.g. K+ wasting) can result in hypokalemia.
1. Regulation of K+ between ICF and ECF
1) Driving force
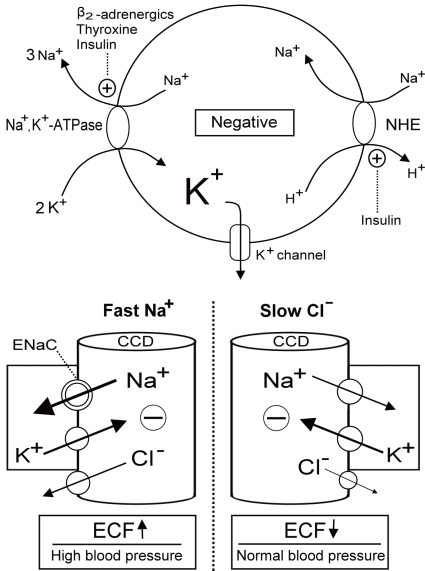
The force driving K+ shift into cells is the more negative voltage in cells. This is created by the Na+,K+-ATPase. Na+,K+-ATPase extrudes three sodium ions (Na+) for every two K+ ions that enter cells, thus producing a net export of positively charged ions. The main hormones that increase the activity of Na+,K+-ATPase include β2-adrenergic agonists, insulin, and thyroid hormone5) (Fig. 1). Increases in the concentration of Na+ inside cells can also activate the Na+,K+-ATPase. Insulin stimulates a membrane Na+/H+ exchanger (NHE), thus helping to prevent hyperkalemia when K+ is ingested in carbohydrate-rich food. Metabolic alkalosis also affects the NHE and causes K+ shift into cells.
Fig. 1.
Regulation of K+ redistribution in cells and K+ secretion in the cortical collecting duct (CCD). The circle depicts the cell membrane (upper panel). Na+,K+-ATPase, Na+/H+ exchanger (NHE), and K+ channels are three major elements controlling K+ shift. Na+,K+-ATPase is activated by β2-adrenergics, insulin and thyroid hormone. NHE, which causes the electroneutral entry of Na+ into cells and thus the net exit of positive voltage via the Na+,K+-ATPase, is also activated by insulin. K+ channels which permit K+ exit is responsible for generating the majority of the resting membrane potential and blocked by barium. The barrel shaped structures represent the terminal CCD (lower panel). The reabsorption of Na+ faster than Cl- (right) or Cl- slower than Na+ (left) in the CCD creates the lumen negative voltage that drives the net secretion of K+. Fast Na+ disorders cause extracellular fluid (ECF) volume expansion and high blood pressure, whereas, slow Cl- disorders lead to diminished ECF volume and low to normal blood pressure. ENaC, epithelial Na+ channels.
2) K+ channels
K+ channels are a diverse and ubiquitous family of membrane-spanning proteins that selectively conduct K+ ions across the cell membrane along its electrochemical gradient in both excitable and non-excitable cells6). There are several different types of K+ channels. These K+ channels share the common features of a water-filled permeation pore allowing K+ ions to flow across the cell membrane, a selectivity filter that specifies K+ as the permeant ion species, and a gating mechanism that serves to switch between open and closed channel conformations. Because K+ ions do not reach diffusion equilibrium, control of the open-probability of K+ channel regulates the transmembrane voltage. K+ channels are inhibited by barium and other drugs6). Closure or opening may not only alter the membrane potential, but also acutely affect the plasma K+ concentration.
2. Renal handling of K+
Most of the filtered K+ is reabsorbed by the proximal tubule and the loop of Henle. The final amount of K+ excreted in the urine is primarily controlled by the late distal convoluted tubule (DCT), the connecting tubule, and the cortical collecting duct (CCD)7). Two factors influence the rate of K+ excretion: the flow rate in the terminal CCD and the net secretion of K+ by principal cells in the CCD which raise the luminal concentration of K+ ([K+]CCD) (Fig. 1). There is an interplay between the magnitudes of the flow rate in the CCD and the [K+]CCD which permits the kidney to excrete all the K+ that is ingested in a steady state8).
1) The flow rate in the CCD
The flow rate in the CCD is directly proportional to the rate of excretion of osmoles and can be expressed as the urine osmole excretion rate divided by plasma osmolality (unrine osmolality × volume/plasma oslmolality) because urine osmolality in the terminal CCD is equal to the plasma osmolality when anti-diuretic hormone (ADH) is present8). While the rate of flow in the CCD is high during a water diuresis, the rate of K+ excretion need not be elevated because ADH must be present to have high rates of K+ secretion. The flow rate in the CCD can also be indirectly represented by urine osmolality/creatinine.
2) [K+]CCD
K+ secretion in the CCD depends on the K+ channel conductance of the apical membrane (primarily by renal outer medullary K+ Channel, ROMK) driven by the electrogenic reabsorption of Na+ (a lumen-negative transepithelial voltage) via epithelial Na+ channels (ENaC)9). Blood aldosterone and luminal bicarbonate (HCO3-) are two major factors enhancing K+ secretion in the CCD10). Aldosterone action leads to an increase in the activity of the ENaC and an alkaline luminal pH appears to exert a decrease in the apparent permeability of chloride (Cl-) and/or an increase in open probability of ROMK. There are two ways to generate more negativity in the lumen of the CCD and thus drive K+ secretion. When Na+ is reabsorbed faster than Cl- (fast Na+ disorders) or Cl- is reabsorbed slower than Na+ (slow Cl- disorders) (Fig. 1), the lumen of the CCD becomes more negatively charged (electrogenic) and drives K+ secretion via the ROMK channel. The [K+] in the CCD can be estimated by calculating the transtubular K+ gradient [TTKG=(urine/plasma [K+])/(urine/plasma osmolality)]. A TTKG greater than 3 in the presence of hypokalemia indicates fast Na+ or slow Cl- disorders11).
Aproach to the genetic causes of hypokalemia
Several acquired extrarenal and renal disorders can have the same findings of hypokalemia as genetic causes. An understanding of the genetic causes of hypokalemia will help determine the molecular defect and avoid inappropriate and cumbersome genetic testing. A detailed history and measurement of blood pressure (BP), K+ excretion rate, and blood acid-base status can help discriminate between the diverse acquired and genetic causes of hypokalemia. Historical clues such as prior hypokalemia, associated organ abnormalities in family members, use of drugs affecting hypokalemia-diuretics, licorice, and aminoglycosides-onset of age, and nephrolithiasis or nephrocalcinosis must be carefully obtained1). The finding of concomitant hypertension and hypokalemia without the use of diuretics suggest the presence of a mineralocorticoid excess state (MES). A major branch point in hypokalemic pathophysiology is the renal K+ excretion response during hypokalemia. The 24-hour urine and/or spot urine have been used to assess renal K+ excretion rate. We prefer a spot (timed) urine collection prior to therapy as a fast and practical alternative. Four spot urine indices of renal response to hypokalemia have been used: fractional excretion of K+, TTKG, urine K+/creatinine ratio and urine osmolality/creatinine1, 12). A 24-hour urine, if collected properly, can provide additional information such as how much K+ is needed to replace the K+ deficit and the state of K+ balance from calculation of K+ input and output. Because K+ deficits usually accompany HCO3- or Cl- loss regardless of the route of K+ loss (gastrointestinal, sweat and renal), either metabolic acidosis or alkalosis is common13). In contrast, conditions of intracellular shift usually exhibit relatively normal acid-base balance. Accordingly, the simultaneous assessment of blood acid-base state is also very crucial in patients with hypokalemia. Therefore, the etiology of hypokalemia can simply be divided into two groups: those with a low K+ excretion rate and those with a high K+ excretion rate.
1. Disorders with a low urine K+ excretion rate
Disorders in the gastrointestinal tract, excessive sweating and conditions with increased shift of extracellular K+ into cells can lead to a low urine K+ excretion. Generally, an increased shift of extracellular K+ into cells can occur acutely in a number of highly stressful conditions associated with increased catecholamines or hyperinsulinemia via the activation of membrane Na+,K+-ATPase or NHE activity. Barium intoxication and chloroquine overdose cause hypokalemia via the direct closure of cellular membrane K+ channels. One should separate the disorders with hypokalemic periodic paralysis (HPP) due to acute shift of K+ into cells from non-HPP, where there is a large total body deficit of K+14). Within the HPP subgroups, the most common are thyrotoxic periodic paralysis (TPP) and sporadic or idiopathic periodic paralysis (SPP) in Asia and familial periodic paralysis (FPP) in Western countries15).
1) Genetic hypokalemia due to increased K+ shift
(1) Familial hypokalemia periodic paralysis (FPP)
It is an autosomal dominant disorder, which is accompanied by muscle weakness or paralysis and occurs more frequently in males, with incomplete penetrance and later onset observed in affected females16). Some cases may present as sporadic because of the incomplete penetrance of the disease, mostly in women. Attacks can be induced by rest after exercise, carbohydrate-rich meals, exposure to cold, or the administration of glucose or insulin or glucocorticoid. Acetazolamide, a carbonic anhydrase inhibitor, can reduce the frequency of attacks in FPP with unclear mechanisms. The molecular lesions affect ion channel genes encoding the dihydropyridine-sensitive voltage-gated Ca2+ channel α1-subunit (CACNA1S) (FPP type I) and tetrodotoxin-sensitive voltage-gated Na+ channel α-subunit (SCN4A) (FPP type II) of skeletal muscle17). The Na+ channel α-subunit shares primary and secondary structure with the Ca2+ channel α-subunit including segments spanning the membrane (S1-S6). The S4 segment contains a high density of positively charged amino acids, with every third one being lysine or arginine, and acts as the voltage sensor in voltage-dependent channel activation. To date, five point mutations affecting arginine substitutions in segment S4 of domains II, III and IV (R528G/H, R900S, R1239G/H) of CACNA1S and eleven point mutations in segment S4 of domains I, II and III of SCN4A (R222W, R669H, R672G,/H/C/S, R675G/Q/W, R1132Q, R1135H) have been found in patients with FPP18). Why mutations in segment S4 of CACNA1S and SCN4A cause episodic hypokalemia remains unclear.
(2) Andersen-Tawil syndrome
It is an autosmal-dominant channelopathy resulting in episodic attacks of muscle weakness (mainly acute hypokalemia, but can be normo- or hyperkalemia), cardiac arrhythmia (ventricular arrhythmias and QT prolongation) and distinctive physical features19). Mutations in the gene (KCNJ2) encoding a pore-forming subunit of the inward rectifier K+ channel protein, Kir2.1, which is expressed in skeletal muscles and heart, lead to this syndrome19). The majority of patients with this syndrome have missense mutations that inhibit channel function through a dominant negative effect. Like FPP, some cases may present as sporadic because of the incomplete penetrance of the disease or have de novo mutation. Furthermore, a wide range of phenotypic severity exists, which can make the correct diagnosis difficult. In contrast to FPP, where cardiac arrhythmias provoked by severe hypokalemia during attacks normalize upon recovery, persistent electrocardiogram abnormalities between attacks is likely in this syndrome20).
2) Genetic hypokalemia where the defect is in the intestinal tract
Congenital chloride-losing diarrhea (CLD) is a rare autosomal recessive disorder characterized by watery diarrhea, hypokalemia and hypochloremic metabolic alkalosis with high fecal content of Cl- (>90 mEq/L)21). It is caused by an inactivating mutation in the downregulated in adenoma (DRA) gene encoding a Cl-/OH- (HCO3-) exchanger expressed in the apical membranes of the colon and ileum with functional similarities with the anion exchange proteins22) Like acquired intestinal inflammation with reduced DRA expression, mutated DRA causes the reabsorption of Cl- and secretion of HCO3- to fall. Proton-pump inhibition of gastric chloride secretion is helpful in diminishing the delivery of Cl- to the colon and in lessening the degree of hypokalemia and metabolic alkalosis in CLD.
3) Genetic hypokalemia where the defect is in exocrine glands
Cystic fibrosis (CF) is an exocrine disease affecting multiple organ systems. The defect in the cystic fibrosis transmembrane regulator (CFTR), acting primarily as a Cl- channel, is associated with CF23). Hypokalemia is not uncommon in patients with CF, especially in tropical or subtropical areas. Defective chloride reabsorption by the dysfunctional CFTR in the sweat ducts of CF patients is responsible for excessive Cl- and Na+ loss in sweat. ECF volume depletion with secondary hyperaldosteronism not only causes Na+ reabsorption and K+ secretion in the CCD, but may also augment K+ secretion in sweat ducts and thereby contribute to the hypokalemia.
2. Disorders with a high urine K+ excretion rate
Hypokalemia due to renal K+ wasting is chronic and usually related to disorders with either increased flow rate to the CCD or increased K+ in the CCD (fast Na+ or slow Cl-).
1) Increased urine flow rate to CCD
Flow rate to the CCD is enhanced when osmole excretion rate is increased. Increased osmole excretion rate can be caused by increased excretion of electrolytes (diuretics or tubular defects) or non-electrolytes (mannitol, glucose, urea).
2) Increased [K+] in the CCD
(1) Fast Na+ disorders
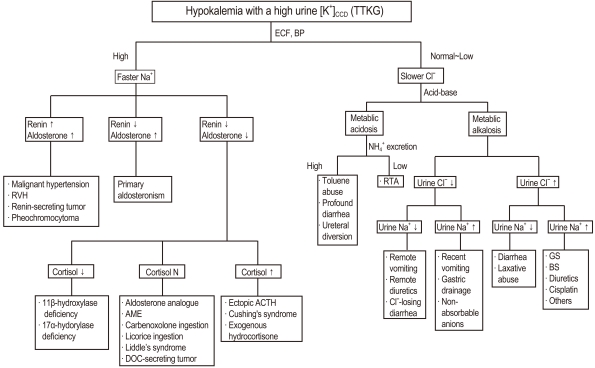
Because Na+ reabsorption in the CCD is augmented, ECF volume is usually expanded and thus BP is high, as in states of mineralocorticoid excess1). In this setting, measurement of plasma renin activity, aldosterone and cortisol concentration help to narrow the differential diagnosis of fast Na+ disorders (Fig. 2). Plasma renin activity and aldosterone levels are high in patients with renin-secreting tumor, renal vascular stenosis, malignant hypertension, and pheochromocytoma. High plasma aldosterone levels but low plasma renin activity indicates primary hyperaldosteronism which can be caused by bilateral adrenal hyperplasia, adrenal adenoma/carcinoma, and glucocorticoid-remediable aldosteronism (GRA). In contrast, low plasma aldosterone level and renin activity represent pseudohyperaldosteronism. Based on the plasma cortisol concentration, three subgroups can be readily divided. Patients with high plasma cortisol concentrations may have ectopic adrenocorticotrophic hormone (ACTH), Cushing's syndrome or exogenous hydrocortisone administration. Low plasma cortisol concentration suggests the diagnosis of congenital adrenal hyperplasia (CAH) due to either 11β-hydroxylase or 17α-hydroxylase deficiencies (11β-OHD and 17α-OHD). A normal plasma cortisol level can be found in 11-deoxycorticosterone (DOC) producing adenoma, Liddle's syndrome, apparent mineralocorticoid excess, and chronic licorice ingestion.
Fig. 2.
Algorithm for the approach to hypokalemia with a high urine [K+]CCD (TTKG). CCD, cortical collecting duct; TTKG, transtubular K+ gradient; ECF, extracellular fluid; BP, blood pressure; RVH, right ventricular hypertrophy; AME, Apparent mineralocorticoid excess; DOC, 11-deoxycorticosterone; ACTH, adrenocorticotrophic hormone; RTA, renal tubular acidosis; GS, Gitelman's syndrome; BS, Bartter's syndrome
a. Genetic hypokalemia associated with mineralocorticoid excess state
The mineralocorticoid receptor (MR), the major regulator of ENaC activity, is normally activated by aldosterone. Genetic mutations causing abnormalities in aldosterone secretion or production of other steroids that activate the MR will lead to hypertension and hypokalemia24).
i. Glucorticoid-remediable aldosteronism
GRA is an autosomal dominant form of hypertension caused by a chimeric gene duplication arising from unequal crossing over between two closely related genes involved in adrenal steroid biosynthesis25). These two genes are 11β-hydroxylase (CYP11B1) encoding aldosterone synthase for aldosterone biosynthesis in the adrenal glomerulosa regulated by angiotensin II and aldosterone synthase (CYP11B2) encoding 11β-hydroxylase for cortisol biosynthesis in the adrenal fasciculata regulated by ACTH. The unequal crossover between CYP11B1 and CYP11B2 genes results in CYP11B2 that allows aldosterone synthesis to be under the control of regulatory promoter sequences of CYP11B1 by ACTH rather than its normal regulator, angiotensin II. Aldosterone secretion thus becomes linked to cortisol secretion, leading to clinical and laboratory features of primary aldosteronism. Exogenous glucocorticoids diminish the ACTH-regulated chimeric genes, in turn suppressing the secretion of aldosterone, leading to reversal of the features of GRA.
ii. CAH due to 11β-OHD and 17α-OHD
Two forms of CAH have hypertension and hypokalemia accompanying a characteristic phenotype with abnormal androgen production and sexual differentiation26). They are 11β-OHD and 17α-OHD caused by CYP11B1 and CYP17 mutations, respectively. Increased ACTH levels in 11β-OHD leads to elevation of 11-deoxycortisol (compound S) and DOC, causing MES. Cortisol precursors are diverted through the 17,20-lyase pathway and this results in hyperandrogenemia, causing acne, hyperpigmentation, and an enlarged phallus in males and prenatal virilization of genitalia in the female newborn27). 17α-OHD has both defective 17α-hydroxylase and 17,20-lyase activities of the 17α-hydroxylase essential for the synthesis of cortisol and gonadal hormones. Lack of 17,20-lyase activity in 17α-OHD prevents androgen synthesis and results in undervirilization in males and failure of spontaneous pubertal development in females. Steroid therapy ameliorates the hypertension and hypokalemia as well as the associated signs and symptoms.
iii. Liddle's syndrome
It is characterized by autosomal dominant transmission of early onset hypertension associated with hypokalemia, metabolic alkalosis, suppressed plasma renin activity, and extremely low plasma aldosterone levels28). This disease is caused by mutations in either the β or the γ subunit of ENaC that delete or alter their cytoplasmic C termini. The mutated ENaC are not internalized (clathrin-coated pits pathway) or degraded (Nedd4 pathway), and instead remain in an activated form on the cell surface. The channel is amiloride and triamterene sensitive provided their concentrations are high enough in luminal fluid, explaining the efficacy of these K+-sparing diuretics in the syndrome.
iv. Apparent mineralocorticoid excess (AME)
AME is a rare but potentially fatal autosomal recessive form of hypertension and hypokalemic metabolic alkalosis associated with hyporeninemia and hypoaldosteronemia and an abnormal ratio of urinary metabolites of cortisol with a high tetrahydrocortisol:tet rahydrocortisone (THF:THE) ratio29). AME is caused by mutations in the gene (HSD11B2) encoding renal-specific 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2). 11β-HSD2 is responsible for converting cortisol to cortisone in the principal cells of distal tubules and crucial for protecting the MR from being occupied by cortisol. The hallmark features in AME resemble those in licorice ingestion because licorice contains glycyrrhetinic acid, which inhibits 11β-HSD230).
(2) Slow Cl- disorders
Because Cl- reabsorption in the CCD is diminished, ECF volume is usually contracted and thus BP is relatively low to normal. Because extracellular K+ loss is often accompanied by ECF HCO3- or Cl- loss, slow Cl- disorders can have either hyperchloremic metabolic acidosis or hypochloremic metabolic alkalosis.
a. Hypochloremic metabolic alkalosis
Metabolic alkalosis is diagnostically useful in patients with severe KCl depletion. An assessment of urine Na+ and Cl- may reveal the basis for renal tubular electrolyte disorders and distinguish it from non-renal Na+ loss (Fig. 2). Low excretion of Na+ and Cl- indicate remote vomiting, remote or yesterday's diuretics, Cl--losing diarrhea (e.g. congenital chloridorrhea), or excessive sweating. Low urine Na,+ but high Cl- excretion, in the presence of hypokalemia and metabolic alkalosis suggests laxative abuse or some chronic diarrhea states with chronic stimulation of renal NH4+ excretion. Conditions of high Na+ excretion, but low Cl-, suggests the presence of an anion that is not reabsorbed. If the urine is alkaline (pH>7), vomiting and/or ingestion of bases are the likely causes. If the urine is not alkaline, intake or generation of anions that are poorly reabsorbed by the kidney is likely31). A high urine Na+ and Cl- excretion is indicative of the recent use of diuretics, intrinsic renal disease, or lack of signaling to stimulate NaCl reabsorption. Inherited or acquired renal tubular disorders such as Gitelman's syndrome (GS), Bartter's syndrome (BS), and related drug-induced toxic disorders, such as from aminoglycosides, cisplatin, diuretics, and foscarnet, are often the diagnoses32). The evaluation of urine divalent Mg2+ and Ca2+ excretion helps localize the exact tubular defect. High urine Ca2+ and Mg2+ excretion is universally present in lesions of the loop of Henle (LOH) whereas low urine Ca2+ and high Mg2+ excretion is invariably found in lesions of the DCT33).
i. Bartter's syndrome (BS) and Gitelman's syndrome (GS)
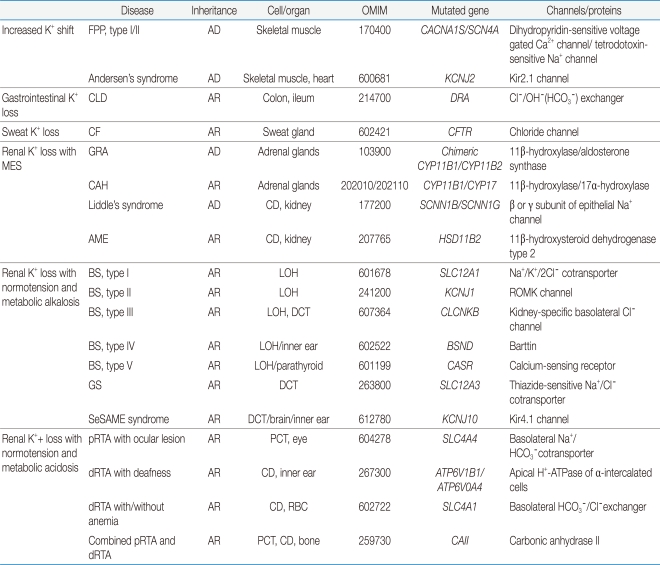
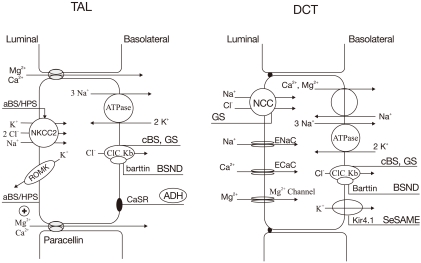
BS and GS are autosomal recessive renal tubular disorders characterized by chronic hypokalemia with renal K+ wasting, metabolic alkalosis, renal salt wasting with low to normal BP and secondary hyperreninemia and hyperaldosteronism. BS results from defective reabsorption of NaCl in the LOH whereas GS is secondary to defective reabsorption of NaCl in the DCT. At the molecular level, GS is mostly due to inactivating mutations in the SLC12A3 gene, which encodes the thiazide-sensitive Na+/Cl- cotransporter (NCC) on the apical membrane of the DCT34). The five subtypes of BS arise from inactivation mutations in genes encoding the Na+/K+/2Cl- cotransporter (NKCC2), K+ channel (ROMK), kidney-specific Cl- channel (CLCNKB), barttin (BSND) and calcium-sensing receptors (CaSR)35, 36) (Table 1, Fig. 3). Recently, a complex syndrome consisting of a combination of epilepsy, ataxia, sensorineural deafness and renal tubulopathy featuring laboratory findings of GS (EAST or SeSAME syndrome) has been described, due to inactivating mutations in the gene encoding Kir4.1, which is also highly expressed in the basolateral membrane of the DCT37, 38). Loss of Kir4.1 function may reduce Na+,K+-ATPase and accumulate intracellular Na+, leading to reduced salt reabsorption in the DCT, similar to GS.
Table 1.
Genetic Mutations in Inherited Hypokalemia
PCT, proximal convoluted tubule; LOH, loop of Henle; DCT, distal convoluted tubule; CD, collecting duct; AD, autosomal dominant; AR, autosomal r ecessive; RBC, red blood cell; GRA, glucocorticoid-remediable aldosteronism; FPP, familial periodic paralysis; CLD, congenital chloride-losing diarrhea; CF, cystic fibrosis; CAH, congenital adrenal hyperplasia; AME, apparent mineralocorticoid excess; BS, Bartter's syndrome; GS, Gitelman's syndrome; pRTA, proximal renal tubular acidosis; dRTA, distal renal tubular acidosis; MES, mineralocorticoid excess state; SeSAME, seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance.
Fig. 3.
Transport proteins in the thick ascending limb (TAL) of loop of Henle (LOH) (left panel) and distal convoluted tubule (DCT) (right panel) affected by gene mutations. Mutations that inactivate Na+/K+/2Cl- cotransporter (NKCC2) or renal outer medullary K+ channel (ROMK) lead to antenatal Bartter syndrome/hyperprostaglandin E syndrome (aBS/HPS) (BS type I and II) and mutations inactivating ClCKb cause classic Bartter syndrome (cBS) (BS type III). Mutations in barttin (chloride channel βsubunit) cause antenatal BS with sensorineural deafness (BSND) (BS type IV). Mutations that activate the calcium-sensing receptors (CaSR) occur in patients with autosomal dominant hypoparathyroidism (ADH) (BS type V). Mutations inactivating Na+/Cl- cotransporter (NCC) or basolateral Cl- channel (ClC-Kb) can cause Gitelman's syndrome (GS). Mutations in Kir4.1 channel cause seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME) syndrome. ADH, anti-diuretic hormone
A maternal history of polyhydramnios, age of onset, neurologic symptoms, deafness, presence of nephrocalcinosis or renal stones, and serum divalent concentration with their urine excretion rates help distinguish among the subtypes of BS and GS. Because Cl- channels are expressed in both the basolateral membrane of LOH and DCT, some patients with classical BS may have clinical profiles similar to that of GS. Unlike BS, non-steroid anti-inflammatory drugs (NSAIDs) are usually not effective in patients with GS due to the relatively normal urinary prostaglandin E2 excretion.
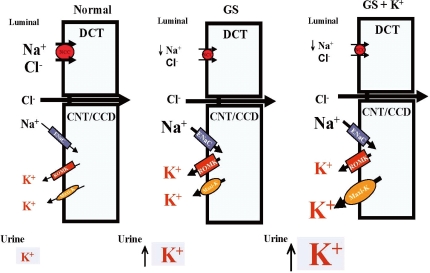
The degree of chronic hypokalemia has been reported to be milder in GS than BS. In fact, profound hypokalemia is not uncommon in patients with GS and is also difficult to correct even with high K+ supplementation and the use of K+-sparing agents. Besides ROMK channels, Ca2+-activated maxi-K+ channels (BKCa), a large-conductance channel stimulated by increased distal renal tubule flow, play an important role in K+ secretion when the flow rate to the CCD is increased39). The importance of maxi-K+ channels in renal K+ secretion is further supported by the finding that BS patients with ROMK mutations do not have hyperkalemia but hypokalemia. Mutations of ROMK decrease NaCl and flow reabsorption in the LOH and result in increased distal flow delivery to the CCD where flow-stimulated K+ secretion via maxi-K+ channels are enhanced despite the loss of functional ROMK40). We have found that both ROMK1 and maxi-K+ expression were increased in the distal renal tubules of GS-causing knockin mice on normal K+ diet, suggesting that both channels are involved in renal K+ wasting in GS (unpublished data). The mice on a high K+ diet still had hypokalemia, indicating that increased intake was matched by increased excretion. Although ROMK1 expression was unchanged between normal and high K+ diets, maxi-K+ was further increased, suggesting that ROMK1 expression had reached a ceiling, but not maxi-K+ expression, which may also be stimulated by K+ repletion and aldosterone. Over-expression/activation of maxi-K+ may be responsible for the persistent hypokalemia in GS patients despite K+ supplementation (Fig. 4). These findings raise the possibility of maxi-K+ inhibitors as an alternate therapeutic target in managing chronic refractory hypokalemia.
Fig. 4.
Mechanisms for persistent hypokalemia in Gitelman's syndrome (GS). Enhanced epithelial Na+ channels (ENaC), renal outer medullary K+ channel (ROMK)1 and Maxi-K expression leads to hypokalemia (middle). High K+ supplement stimulates more accentuated maxi-K expression, accounting for persistent hypokalemia (left). DCT, distal convoluted tubule; CNT, cortical connecting tubules; CCD, cortical colleting duct.
b. Hyperchloremic metabolic acidosis
Hyperchloremic metabolic acidosis provides an important diagnostic clue for the presence of a pathophysiologic process involving both K+ depletion and direct or indirect loss of HCO3-. Estimating the urine NH4+ concentration unveils the basis of metabolic acidosis associated with K+ depletion41). The excretion of NH4+ is low in patients with renal tubular acidosis (RTA), whereas, this excretion is not depressed in simple gastrointestinal loss of NaHCO3. An indirect estimate of NH4+ can be obtained from the urine anion gap (Na++K+-Cl-) or osmolal gap (measured-calculated urine osmolality)/2. The causes for patients who do not have a low NH4+ excretion rate include gastrointestinal loss of HCO3-, chronic toluene abuse, treatment of diabetic acidosis with insulin, and ureteral diversion. Positive urine net charge and osmolal gap less than 100 mOsm/kg H2O are indicative of low urine NH4+ excretion, pointing to the diagnosis of RTA. RTA can be proximal or distal in origin. Intravenous NaHCO3 loading at a rate of 2-3 mEq/kg/hour can be administered to separate proximal from distal RTA.
i. Inherited isolated proximal RTA
Most of the filtered HCO3- is reabsorbed in the proximal convoluted tubules (PCT) as a result of H+ secretion via the electroneutral Na+/H+ exchanger 3 (NHE3) in the luminal membrane and HCO3- exit via the electrogenic Na+/HCO3- cotransporter (NBC1) in the basolateral membrane. Defects in NHE3 or NBC1 can theoretically reduce the reabsorption of filtered HCO3- and cause proximal RTA (pRTA). Mutations in the NHE3 gene have not been identified in patients with proximal RTA to date. On the other hand, mutations in the NBC1 gene (SLC4A4) have been reported in patients with autosomal recessive isolated proximal RTA42). Because NBC1 is also expressed in the ocular tissues and brain, patients with inactivating mutations in NBC1 have ocular abnormalities (glaucoma, band keratopathy, and cataracts) as well as calcifications of the basal ganglia. Of note, hypokalemia is almost always present and can be very severe when a high dose of oral NaHCO3 is given.
ii. Inherited distal RTA
Apical H+-ATPase pump and basolateral Cl-/HCO3- exchanger (anion exchanger 1, AE1) in the late distal tubule (predominantly in α-intercalated cells) participate in the excretion of NH4+, which adds new HCO3- to the body. Genes encoding two H+-ATPase subunits specific to intercalated cells have been identified as ATP6V1B1 and ATP6V0A43). Because ATP6V1B1 is also expressed in endolymphatic sac epithelia, mutations in ATP6V1B1 cause distal RTA with sensorineural hearing loss, whereas, distal RTA with preserved hearing is caused by mutations in ATP6V0A4. AE1 (SLC4A1) is also present in erythrocytes and inactivating mutation in AE1 causes distal RTA and possible anemia44). Hypokalemia in hereditary distal RTA is often severe. First, low distal H+ secretion reduces luminal HCO3- reabsorption and this leads to bicarbonaturia. Second, the mistargeting of AE1 due to the second mutation in AE1 leads to HCO3 secretion. Reminiscent of alkalinized intercalated cells in Sjogren's syndrome with distal RTA, high luminal HCO3- may lead to augmented K+ secretion and hypokalemia.
iii. Inherited proximal and distal RTA
Carbonic anhydrase II (CAII) is known to be expressed in both proximal and distal nephron segments and participates in H+ secretion in both the proximal and distal nephron; CAII is also expressed in brain and osteoclasts. Mutations in CAII can lead to an autosomal recessive proximal and distal RTA with osteopetrosis and cerebral calcification45). The bone thinning effect of the coexistent metabolic acidosis in CAII deficiency may protect the osteopetrotic bones from the excessive thickening generated by osteoclast dysfunction.
Therapeutic strategy in genetic hypokalemia
Genetically engineered mice are often used as animal models of human diseases and are vital tools in investigating molecular pathogenesis of disease and developing and testing novel therapies. It is worthy to emphasize that knock-out mice typically represent null mutations and could be used to study only the effects of the loss of a gene, not a specific mutation46). Knockin strategies, where homologous recombination in embryonic stem (ES) cells is used to replace the endogenous gene with a mutant variant without any other disruption of the gene, can be used to address the effects of specific sequence changes on gene and protein function47). Therefore, knockin mice accurately represent human diseases caused by specific mutations, such as missense, nonsense, or deletion mutations. More importantly, mutation-specific approaches according to the different classes of mutations are currently under investigation to provide a new alternative therapy in genetic disorders. We believe that the application of these diseases-causing knock-in mice may address more specific questions regarding the pathogenesis and new directions in rescue therapies; for instance, rational application of aminoglycosides or premature termination codon (PTC) 124 for nonsense mutations and pharmacologic chaperones for missense mutations48).
Conclusion
Apart from a detailed history and careful physical examination, the measurement of urine K+ excretion rate by spot and/or 24 hour urine, and assessment of blood acid-base status helps discriminate the causes of hypokalemia. Once the clinical diagnosis suggests a likely molecular basis for the disorder, genetic analysis may prove the diagnosis. Genetic causes of hypokalemia are shown in Table 1. Creation and investigation of disease-causing knockin mice as a model of genetic hypokalemia will not only provide a much better understanding of the underlying mechanisms; but also set the stage for the development of novel therapies in the future.
Acknowledgements
This work was supported by a grant from the Research Fund of Tri-Service General Hospital (TSGH-C-98-81) and from the Teh-Tzer Study Group for the Human Medical Research Foundation.
References
- 1.Lin SH, Halperin ML. Hypokalemia: a practical approach to diagnosis and its genetic basis. Curr Med Chem. 2007;14:1551–1565. doi: 10.2174/092986707780831050. [DOI] [PubMed] [Google Scholar]
- 2.Scheinman SJ, Guay-Woodford LM, Thakker RV, Warnock DG. Genetic disorders of renal electrolyte transport. N Engl J Med. 1999;340:1177–1187. doi: 10.1056/NEJM199904153401507. [DOI] [PubMed] [Google Scholar]
- 3.Gennari FJ. Hypokalemia. N Engl J Med. 1998;339:451–458. doi: 10.1056/NEJM199808133390707. [DOI] [PubMed] [Google Scholar]
- 4.Sterns RH, Cox M, Feig PU, Singer I. Internal potassium balance and the control of the plasma potassium concentration. Medicine. 1981;60:339–354. doi: 10.1097/00005792-198109000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Clausen T. Clinical and therapeutic significance of the Na+, K+ pump. Clin Sci. 1998;95:3–17. [PubMed] [Google Scholar]
- 6.Shieh CC, Coghlan M, Sullivan JP, Gopalakrishnan M. Potassium channels: molecular defects, diseases, and therapeutic opportunities. Pharmacol Rev. 2000;52:557–594. [PubMed] [Google Scholar]
- 7.Giebisch G, Krapf R, Wagner C. Renal and extrarenal regulation of potassium. Kidney Int. 2007;72:397–410. doi: 10.1038/sj.ki.5002288. [DOI] [PubMed] [Google Scholar]
- 8.Halperin ML, Kamel KS. Potassium. Lancet. 1998;352:135–140. doi: 10.1016/S0140-6736(98)85044-7. [DOI] [PubMed] [Google Scholar]
- 9.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev. 2005;85:319–371. doi: 10.1152/physrev.00051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin SH, Cheema-Dhadli S, Gowrishankar M, Marliss EB, Kamel KS, Halperin ML. Control of excretion of potassium: lessons from studies during prolonged total fasting in human subjects. Am J Physiol. 1997;273:F796–F800. doi: 10.1152/ajprenal.1997.273.5.F796. [DOI] [PubMed] [Google Scholar]
- 11.Kamel KS, Quaggin S, Scheich A, Halperin ML. Disorders of potassium homeostasis: an approach based on pathophysiology. Am J Kidney Dis. 1994;24:597–613. doi: 10.1016/s0272-6386(12)80220-4. [DOI] [PubMed] [Google Scholar]
- 12.Lin SH, Lin YF, Chen DT, Chu P, Hsu CW, Halperin ML. Laboratory tests to determine the cause of hypokalemia and paralysis. Arch Intern Med. 2004;164:1561–1566. doi: 10.1001/archinte.164.14.1561. [DOI] [PubMed] [Google Scholar]
- 13.Alazami M, Lin SH, Cheng CJ, Davids MR, Halperin ML. Unusual causes of hypokalaemia and paralysis. QJM. 2006;99:181–192. doi: 10.1093/qjmed/hcl011. [DOI] [PubMed] [Google Scholar]
- 14.Lin SH, Lin YF, Halperin ML. Hypokalaemia and paralysis. QJM. 2001;94:133–139. doi: 10.1093/qjmed/94.3.133. [DOI] [PubMed] [Google Scholar]
- 15.Lin SH. Thyrotoxic periodic paralysis. Mayo Clin Proc. 2005;80:99–105. doi: 10.1016/S0025-6196(11)62965-0. [DOI] [PubMed] [Google Scholar]
- 16.Lin SH, Hsu YD, Cheng NL, Kao MC. Skeletal muscle dihydropyridine-sensitive calcium channel (CACNA1S) gene mutations in chinese patients with hypokalemic periodic paralysis. Am J Med Sci. 2005;329:66–70. doi: 10.1097/00000441-200502000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Venance SL, Cannon SC, Fialho D, et al. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129:8–17. doi: 10.1093/brain/awh639. [DOI] [PubMed] [Google Scholar]
- 18.Matthews E, Labrum R, Sweeney MG, et al. Voltage sensor charge loss accounts for most cases of hypokalemic periodic paralysis. Neurology. 2009;72:1544–1547. doi: 10.1212/01.wnl.0000342387.65477.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andelfinger G, Tapper AR, Welch RC, Vanoye CG, George AL, Jr, Benson DW. KCNJ2 mutation results in Andersen syndrome with sex-specific cardiac and skeletal muscle phenotypes. Am J Hum Genet. 2002;71:663–668. doi: 10.1086/342360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontaine B, Fournier E, Sternberg D, Vicart S, Tabti N. Hypokalemic periodic paralysis: a model for a clinical and research approach to a rare disorder. Neurotherapeutics. 2007;4:225–232. doi: 10.1016/j.nurt.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg C, Perheentupa J, Launiala K. Colonic electrolyte transport in health and in congenital chloride diarrhea. J Clin Invest. 1975;56:302–310. doi: 10.1172/JCI108094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoglund P, Haila S, Socha J, et al. Mutations of the Downregulated in adenoma (DRA) gene cause congenital chloride diarrhoea. Nat Genet. 1996;14:316–319. doi: 10.1038/ng1196-316. [DOI] [PubMed] [Google Scholar]
- 23.Bates CM, Baum M, Quigley R. Cystic fibrosis presenting with hypokalemia and metabolic alkalosis in a previously healthy adolescent. J Am Soc Nephrol. 1997;8:352–355. doi: 10.1681/ASN.V82352. [DOI] [PubMed] [Google Scholar]
- 24.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 25.Lifton RP, Dluhy RG, Powers M, et al. A chimaeric 11 beta-hydroxylase/aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature. 1992;355:262–265. doi: 10.1038/355262a0. [DOI] [PubMed] [Google Scholar]
- 26.New MI. Diagnosis and management of congenital adrenal hyperplasia. Annu Rev Med. 1998;49:311–328. doi: 10.1146/annurev.med.49.1.311. [DOI] [PubMed] [Google Scholar]
- 27.Zachmann M. Defects in steroidogenic enzymes. Discrepancies between clinical steroid research and molecular biology results. J Steroid Biochem Mol Biol. 1995;53:159–164. doi: 10.1016/0960-0760(95)00030-4. [DOI] [PubMed] [Google Scholar]
- 28.Palmer BF, Alpern RJ. Liddle's syndrome. Am J Med. 1998;104:301–309. doi: 10.1016/s0002-9343(98)00018-7. [DOI] [PubMed] [Google Scholar]
- 29.Mune T, Rogerson FM, Nikkilä H, Agarwal AK, White PC. Human hypertension caused by mutations in the kidney isozyme of 11 beta-hydroxysteroid dehydrogenase. Nat Genet. 1995;10:394–399. doi: 10.1038/ng0895-394. [DOI] [PubMed] [Google Scholar]
- 30.Lin SH, Yang SS, Chau T, Halperin ML. An unusual cause of hypokalemic paralysis: chronic licorice ingestion. Am J Med Sci. 2003;325:153–156. doi: 10.1097/00000441-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 31.Kamel KS, Ethier J, Levin A, Halperin ML. Hypokalemia in the "beautiful people". Am J Med. 1990;88:534–536. doi: 10.1016/0002-9343(90)90436-h. [DOI] [PubMed] [Google Scholar]
- 32.Chou CL, Chen YH, Chau T, Lin SH. Acquired Bartter-like syndrome associated with gentamicin administration. Am J Med Sci. 2005;329:144–149. doi: 10.1097/00000441-200503000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Bettinelli A, Bianchetti MG, Girardin E, et al. Use of calcium excretion values to distinguish two forms of primary renal tubular hypokalemic alkalosis: Bartter and Gitelman syndromes. J Pediatr. 1992;120:38–43. doi: 10.1016/s0022-3476(05)80594-3. [DOI] [PubMed] [Google Scholar]
- 34.Lin SH, Shiang JC, Huang CC, Yang SS, Hsu YJ, Cheng CJ. Phenotype and genotype analysis in Chinese patients with Gitelman's syndrome. J Clin Endocrinol Metab. 2005;90:2500–2507. doi: 10.1210/jc.2004-1905. [DOI] [PubMed] [Google Scholar]
- 35.Peters M, Jeck N, Reinalter S, et al. Clinical presentation of genetically defined patients with hypokalemic salt-losing tubulopathies. Am J Med. 2002;112:183–190. doi: 10.1016/s0002-9343(01)01086-5. [DOI] [PubMed] [Google Scholar]
- 36.Gamba G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol Rev. 2005;85:423–493. doi: 10.1152/physrev.00011.2004. [DOI] [PubMed] [Google Scholar]
- 37.Bockenhauer D, Feather S, Stanescu HC, et al. Epilepsy, ataxia, sensorineural deafness, tubulopathy, and KCNJ10 mutations. N Engl J Med. 2009;360:1960–1970. doi: 10.1056/NEJMoa0810276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scholl UI, Choi M, Liu T, et al. Seizures, sensorineural deafness, ataxia, mental retardation, and electrolyte imbalance (SeSAME syndrome) caused by mutations in KCNJ10. Proc Natl Acad Sci U S A. 2009;106:5842–5847. doi: 10.1073/pnas.0901749106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pluznick JL, Sansom SC. BK channels in the kidney: role in K(+) secretion and localization of molecular components. Am J Physiol Renal Physiol. 2006;291:F517–F529. doi: 10.1152/ajprenal.00118.2006. [DOI] [PubMed] [Google Scholar]
- 40.Bailey MA, Cantone A, Yan Q, et al. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int. 2006;70:51–59. doi: 10.1038/sj.ki.5000388. [DOI] [PubMed] [Google Scholar]
- 41.Kamel KS, Briceno LF, Sanchez MI, et al. A new classification for renal defects in net acid excretion. Am J Kidney Dis. 1997;29:136–146. doi: 10.1016/s0272-6386(97)90021-4. [DOI] [PubMed] [Google Scholar]
- 42.Igarashi T, Inatomi J, Sekine T, et al. Mutations in SLC4A4 cause permanent isolated proximal renal tubular acidosis with ocular abnormalities. Nat Genet. 1999;23:264–266. doi: 10.1038/15440. [DOI] [PubMed] [Google Scholar]
- 43.Karet FE. Inherited distal renal tubular acidosis. J Am Soc Nephrol. 2002;13:2178–2184. doi: 10.1097/01.asn.0000023433.08833.88. [DOI] [PubMed] [Google Scholar]
- 44.Wrong O, Bruce LJ, Unwin RJ, Toye AM, Tanner MJ. Band 3 mutations, distal renal tubular acidosis, and Southeast Asian ovalocytosis. Kidney Int. 2002;62:10–19. doi: 10.1046/j.1523-1755.2002.00417.x. [DOI] [PubMed] [Google Scholar]
- 45.Borthwick KJ, Kandemir N, Topaloglu R, et al. A phenocopy of CAII deficiency: a novel genetic explanation for inherited infantile osteopetrosis with distal renal tubular acidosis. J Med Genet. 2003;40:115–121. doi: 10.1136/jmg.40.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Manis JP. Knock out, knock in, knock down--genetically manipulated mice and the Nobel Prize. N Engl J Med. 2007;357:2426–2429. doi: 10.1056/NEJMp0707712. [DOI] [PubMed] [Google Scholar]
- 47.Kohan DE. Progress in gene targeting: using mutant mice to study renal function and disease. Kidney Int. 2008;74:427–437. doi: 10.1038/ki.2008.146. [DOI] [PubMed] [Google Scholar]
- 48.Le Roy F, Charton K, Lorson CL, Richard I. RNA-targeting approaches for neuromuscular diseases. Trends Mol Med. 2009;15:580–591. doi: 10.1016/j.molmed.2009.10.005. [DOI] [PubMed] [Google Scholar]