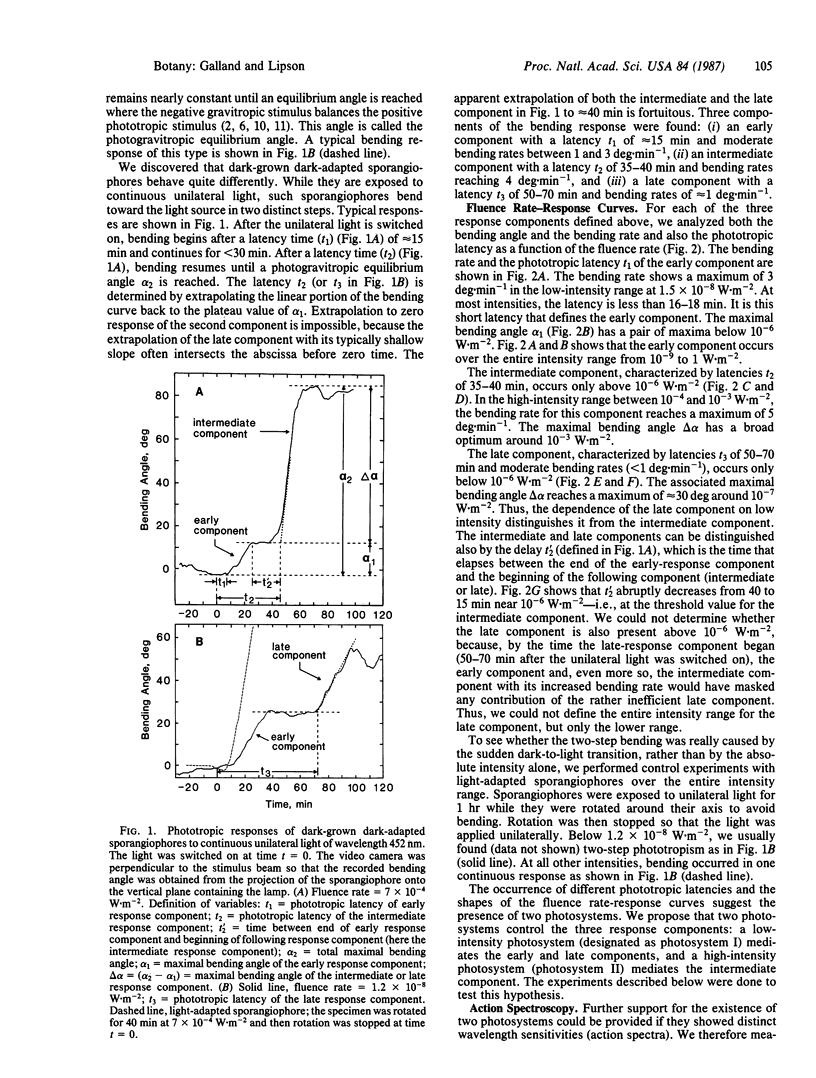
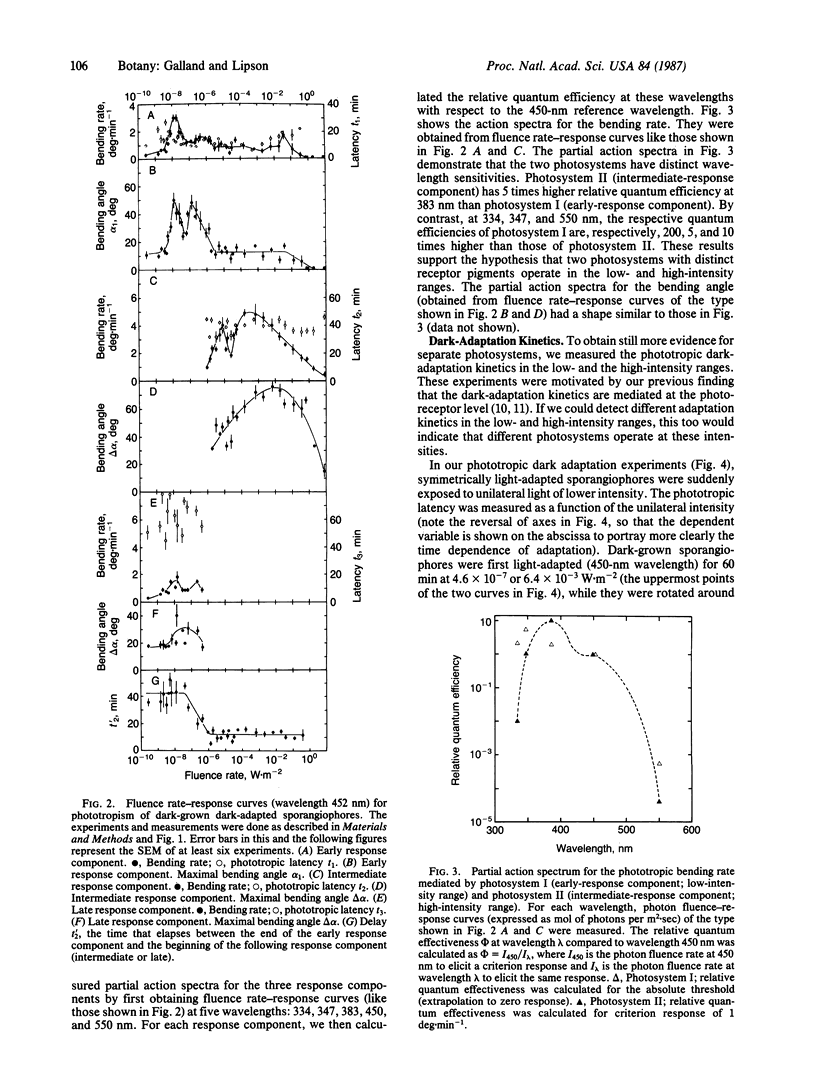
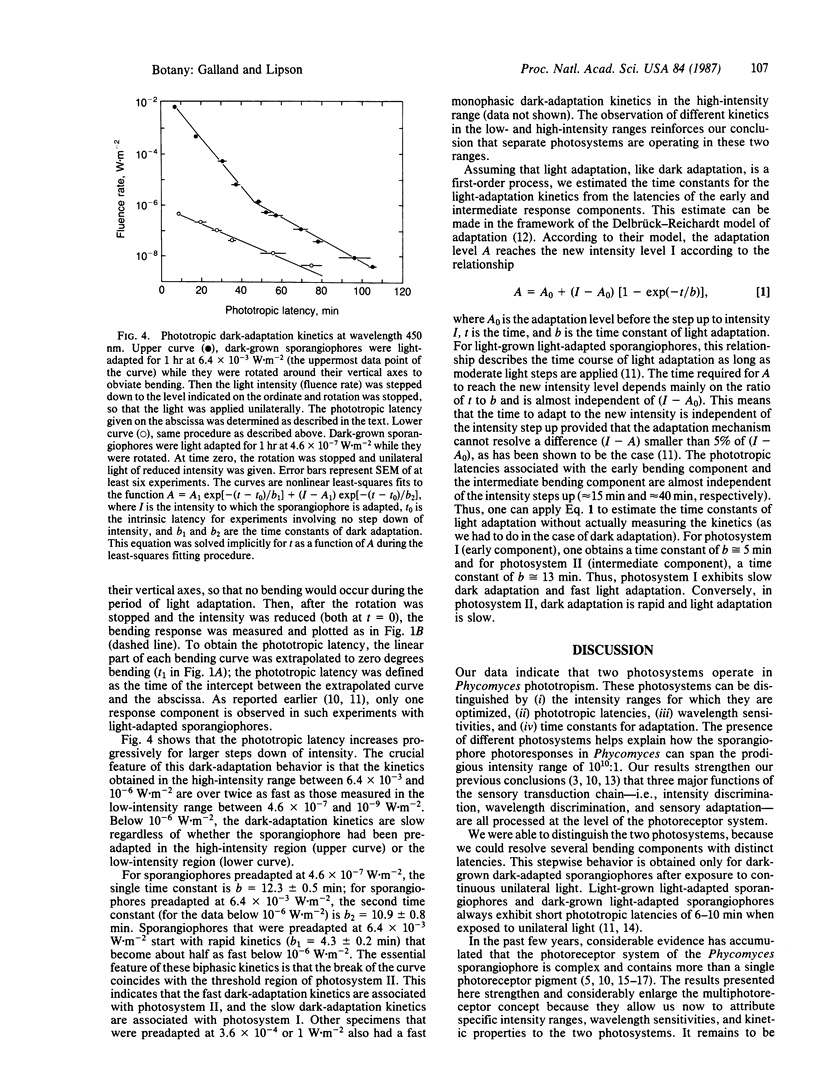
Abstract
Phototropism in the fungus Phycomyces is mediated by two photosystems that are optimized for the low-intensity region (below 10(-6) W X m-2) and the high-intensity region (above 10(-6) W X m-2). These photosystems can be distinguished under special experimental conditions, in which sporangiophores grown in the dark are suddenly exposed to continuous unilateral light. With this treatment, the bending occurs in two steps. Below 10(-6) W X m-2, an early-response component (15-min latency) and a late-response component (50- to 70-min latency) are observed that are mediated by photosystem I. Above 10(-6) W X m-2, the early component is augmented by an intermediate component with a 40-min delay that is mediated by photosystem II. The two photosystems are distinguished further by their wavelength sensitivities and adaptation kinetics. Photosystem I is more effective at 334, 347, and 550 nm than photosystem II, but it is less effective at 383 nm. At wavelength 450 nm, the dark-adaptation kinetics associated with photosystem I are approximately half as fast as those associated with photosystem II. However, the light-adaptation kinetics of photosystem I are approximately equal to 3 times faster than the kinetics associated with photosystem II. The existence of two photosystems clarifies several behavioral features of Phycomyces and helps explain how the sporangiophore can manage the full range of 10 decades.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Briggs W. R. Light Dosage and Phototropic Responses of Corn and Oat Coleoptiles. Plant Physiol. 1960 Nov;35(6):951–962. doi: 10.1104/pp.35.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delbrück M., Shropshire W. Action and Transmission Spectra of Phycomyces. Plant Physiol. 1960 Mar;35(2):194–204. doi: 10.1104/pp.35.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P., Lipson E. D. Action spectra for phototropic balance in Phycomyces blakesleeanus: dependence on reference wavelength and intensity range. Photochem Photobiol. 1985 Mar;41(3):323–329. doi: 10.1111/j.1751-1097.1985.tb03492.x. [DOI] [PubMed] [Google Scholar]
- Galland P., Lipson E. D. Modified action spectra of photogeotropic equilibrium in Phycomyces blakesleeanus mutants with defects in genes madA, madB, madC, and madH. Photochem Photobiol. 1985 Mar;41(3):331–335. doi: 10.1111/j.1751-1097.1985.tb03493.x. [DOI] [PubMed] [Google Scholar]
- Galland P., Pandya A. S., Lipson E. D. Wavelength dependence of dark adaptation in Phycomyces phototropism. J Gen Physiol. 1984 Nov;84(5):739–751. doi: 10.1085/jgp.84.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P., Russo V. E. Light and dark adaptation in Phycomyces phototropism. J Gen Physiol. 1984 Jul;84(1):101–118. doi: 10.1085/jgp.84.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galland P., Russo V. E. Threshold and adaptation in Phycomyces. Their interrelation and regulation by light. J Gen Physiol. 1984 Jul;84(1):119–132. doi: 10.1085/jgp.84.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iino M., Schäfer E. Phototropic response of the stage I Phycomyces sporangiophore to a pulse of blue light. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7103–7107. doi: 10.1073/pnas.81.22.7103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipson E. D., Block S. M. Light and dark adaptation in Phycomyces light-growth response. J Gen Physiol. 1983 Jun;81(6):845–859. doi: 10.1085/jgp.81.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. K., Jayaram M., Hamilton R. M., Delbrück M. Replacement of riboflavin by an analogue in the blue-light photoreceptor of Phycomyces. Proc Natl Acad Sci U S A. 1981 Jan;78(1):266–269. doi: 10.1073/pnas.78.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shropshire W., Withrow R. B. Action Spectrum of Phototropic Tip-Curvature of Avena. Plant Physiol. 1958 Sep;33(5):360–365. doi: 10.1104/pp.33.5.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VARJU D., EDGAR L., DELBRUCK M. Interplay between the reactions to light and to gravity in Phycomyces. J Gen Physiol. 1961 Sep;45:47–58. doi: 10.1085/jgp.45.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman B. K., Briggs W. R. Phototropic Dosage-Response Curves for Oat Coleoptiles. Plant Physiol. 1963 May;38(3):248–253. doi: 10.1104/pp.38.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]



